Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 3
Trimethyl Phosphate Mediated Synthesis of Benzothiazepine Derivatives
Chaitramallu M*Chaitramallu M, Department of Chemistry, University of Mysore, Yuvaraja’s College, Mysore, Karnataka, India,
Received: 08-May-2024, Manuscript No. DPC-24-136170; Editor assigned: 13-May-2024, Pre QC No. DPC-24-136170 (PQ); Reviewed: 17-May-2024, QC No. DPC-24-136170; Revised: 01-Jun-2024, Manuscript No. DPC-24-136170 (R); Published: 29-Jun-2024, DOI: 10.4172/0975-413X.16.3. 339-343
Abstract
By using standard techniques to condense substituted chalcones with 3, 4, 5-trimethoxybenzaldehyde in the presence of trimethyl phoshate, a number of new 1, 5-benzothiazepine derivatives were created. The newly synthesized compounds' structures were verified using elemental analysis, mass spectrum data, 1H and 13C NMR, FT-IR, and other methods. The compounds' antibacterial and antioxidant properties were examined.
Keywords
Benzothiazepine derivatives; Trimethyl phosphate; Chalcones; 3, 4, 5-trimethoxybenzaldehyde
Introduction
Benzothiazepine is a bicyclic heterocycle made up of a thiazepine ring fused to a benzene unit. Different nomenclatures may have different meanings depending on where the heteroatom is located on the ring. For these heterocyclic scaffolds, the most prevalent structural isomers are 1,4- and 1,5-benzothiazepines. The chemistry of benzothiazepines has advanced significantly over the past 50 years, resulting in the synthesis of several analogues and the identification of their biological functions [1-2]. Benzodiazepines are examples of heterocyclic compounds with nitrogen and sulphur that have drawn a lot of interest lately. Numerous medicinal properties, including antibacterial, anti-HIV, anti-cytotoxic, anti-inflammatory and anti-cancer properties, have been attributed to benzothiazepines [3-4]. Consequently, a number of traditional green chemistry techniques have been developed for the synthesis of benzothiazepine derivatives in the presence of trimethyl phosphate, owing to their extensive range of biological and synthetic uses. TMP addition causes the reaction to finish in two hours (Figure 1).
Materials and Methods
All of the chemicals and reagents were purchased without additional purification. Melting points are uncorrected and were measured in open capillary tubes. Silica gel plates (60F254) pre-coated by E. Merck are used for Thin Layer Chromatography (TLC). Column chromatography uses 60-120 mesh Acme, India silica gel. Perkin-Elmer model 683 spectrometers were used to record IR spectra in KBr. Using Tetramethyl Silane (TMS) as an internal reference, 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were acquired on a Bruker spectrometer. Analyses of elements were conducted using a Perkin-Elmer 2400. Utilizing the water-Q-TOF ultima spectrometer, mass spectra were acquired. Elemental vario EL III was used to collect micro analytical data [5,6].
General methods
General procedure for the synthesis of chalcones: A solution of sodium hydroxide (0.01) dissolved in ethanol was mixed forcefully with aceto phenone (0.01) and 3, 4, 5-trimethoxybenzaldehyde (0.01). After freezing the reaction mixture in an ice bath, the product was created. From ethanol, it was filtered and then recrystallized. An 89.5% yield of the pale yellow crystalline substance was achieved.
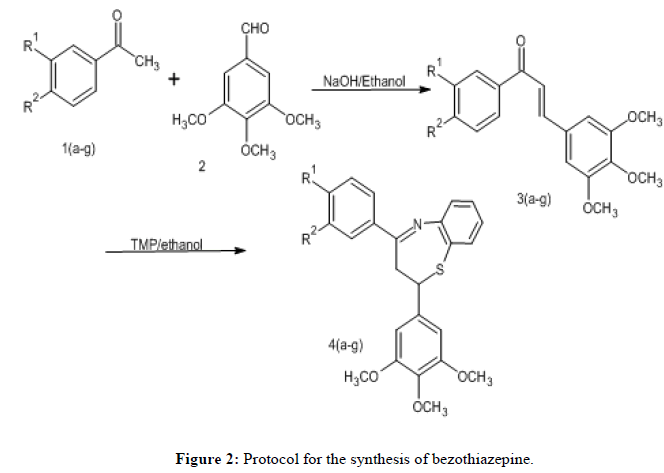
General procedure for the preparation of benzothiazepines: TMP was added to 50 millilitres of ethanol along with one chalcones (0.01 mol). This was mixed with 0.01 mol of 2-aminothiophenol, and the reaction mixture was heated for two hours. Next, 5-6 drops of glacial acetic acid were added to the mixture and the heating was carried out for an additional 3-4 hours. Following cooling, the material was transferred into crushed ice, filtered and then methanol was used to recrystallize it (Figure 2 and Table 1).
| Compound | R1 | R2 |
|---|---|---|
| 4a | OH | H |
| 4b | OCH3 | H |
| 4c | Cl | H |
| 4d | NH2 | H |
| 4e | OH | OH |
| 4f | Br | H |
| 4g | SH | H |
Table 1: Derivatives of bezothiazepine.
DPPH radical scavenging assay
An antioxidant substance that can give hydrogen and be reduced reacts with the DPPH radical. An antioxidant reacts with DPPH to produce diphenyl picrylhydrazine, which is identified by the change from purple to pale yellow. This DPPH radical scavenging activity was carried out using the Shone et al., technique. In short, various chemical concentrations were combined with 1 millilitre of DPPH solution (0.1 mM) in 95% ethanol, shaken and allowed to sit at room temperature for 20 minutes. The absorbance at 517 nm was then measured in comparison to a blank. DPPH absorbance was used as a proxy for radical scavenging activity, which was computed using the following formula:
Scavenging effect (%)=A control (540 nm)-A sample (540 nm)/A control (540 nm) × 100.
Nitric oxide radical scavenging assay
Sodium nitroprusside was used to produce nitric oxide, which was then detected using the Griess reaction. In an aqueous solution at physiological pH, sodium nitroprusside spontaneously produces nitric oxide. This oxide then interacts with oxygen to produce nitric ions, which may be measured spectrophotometrically at 540 nm using the Griess reagent. Because nitric oxide scavengers compete with oxygen for available oxygen, nitric oxide synthesis is decreased. The compounds were combined with 5 mM sodium nitroprusside in phosphate buffer saline and the mixture was incubated at 25°C for 120 minutes. The Griess reagent was used to react the samples mentioned above. The chromophore form's absorbance was measured at 540 nm during the diazotization of nitrate with sulphonyl amide and the coupling with naphthyl ethylene diamine. This absorbance was compared to BHT absorbance standard solutions that were similarly treated with Griess reagent. The following equation was used to calculate the radical scavenging activity.
Scavenging effect (%)=A control (540 nm)-A sample (540 nm)/A control (540 nm) × 100
Anti-microbial activity
Using the disc diffusion method on nutritional agar medium, the antibacterial activity of the synthesized compounds was assessed against both gram-positive (Bacilus subtilius, Streptococcus) and gram-negative (Escherichia coli, Proteus) bacteria in DMF. Each petriplate's sterile medianutrient agar medium, 15 ml was evenly covered with cultures of both gram-positive and gram-negative bacteria. 10 mm diameter sterile discs (Hi- Media) were inserted into the petriplates along with varying drug doses (20 μg, 40, 80, and 100 μg/disc) of the various synthesized compounds. Gentamycin served as a comparative positive control. Three duplicates of each therapy were kept on file. The zone of inhibition was identified after the plates were incubated for 24 hours at 37°C [7-9].
Results and Discussion
By employing sodium hydroxide in the presence of ethanol, we tried to create a gentle and effective cross-Aldol condensation technique for the synthesis of bezothiazopine derivatives. The cross aldol condensation process is employed due to its efficiency in producing α, β-unsaturated carbonyl compounds, also known as chalcones. To increase yields and reaction times, it can be applied successfully to the synthesis of basecatalyzed processes.
To obtain the cyclized products 4a-g, the first step involves condensation of substituted chalcones (3a-g) with 3, 4, 5-trimethoxybenzaldehyde (scheme illustrated in Figure 2). The ideal conditions for the synthesis of bezothiazopine derivatives were determined by examining a variety of reaction parameters, including temperature, solvent and time. It was observed that at room temperature, the reaction involving compounds 1d, 1e, and 1g did not occur. We then conducted reactions at 40°C-50°C and discovered that 450°C is the optimal temperature to provide the derivative in high yields. It was also discovered that the reaction time has dropped to 55 minutes. Product creation began at 40°C and achieved its maximum yield at 50°C. At that point, applying higher temperatures caused the yield to start to drop and the reaction completion time to noticeably shorten. The remaining designed compounds were synthesized and submitted to physicochemical and spectral tests by utilizing the optimized reaction conditions. The second stage was allowing the synthesized chalcones to react with TMP in the presence of ethanol, resulting in the derivatives of benzothiazepine. In order to obtain derivatives with a high yield, the reaction was run for an hour at various temperatures. It was discovered that 60°C is the optimal temperature. After the reaction mixture was obtained, it was added to ice cold water, filtered, dried, and purified using methanol-induced recrystallization. Because of the Ar-C-H of benzothiazepine, the synthesized compounds' IR spectra displayed distinctive absorption bands in the area of 3060 cm-1-3040 cm-1. The -OH stretching produced the absorption band at 3150 cm-1-3350 cm-1, while the C=N stretching produced the band at 1610-1590 cm-1. There was an absorption band C-N at 3550 cm-1-3580 cm-1. When C-Br was present in any of the compounds, it caused a stretching band to form at 600 cm-1-700 cm−1, whereas the C-Cl functional group produced a stretching band at 680-800 cm-1. A low intensity S-H stretching band about 2500-2558 cm-1 confirmed the production of the products, while a strong and powerful absorption around 1690 cm-1 proved the presence of the chalcone -C=O group. Benzothiazepines' -C=N characteristic band was clearly visible between 1610 and 1590 cm-1, demonstrating their structural integrity. The distinct peak of the benzothiazepine ring was visible in the 1H-NMR spectra of the produced compounds at δ 2.84-2.90 and δ 5.20-5.3 ppm (J=9.78, 1.68), respectively. This was caused by the two non-equivalent magnetically equivalent protons of the methylene benzothiazepine ring at positions two and three. Every piece of information regarding these peaks matched what was written in the literature. Trimethyl phosphate has been shown to yield favourable results as an alternate catalyst for the synthesis of benzodiazepines. Next, we experimented with various concentrations of Trimethyl Phosphate (TMP) in water. It is significant that the current protocol supports the earlier synthesis approach [10-12].
DPPH radical scavenging assay
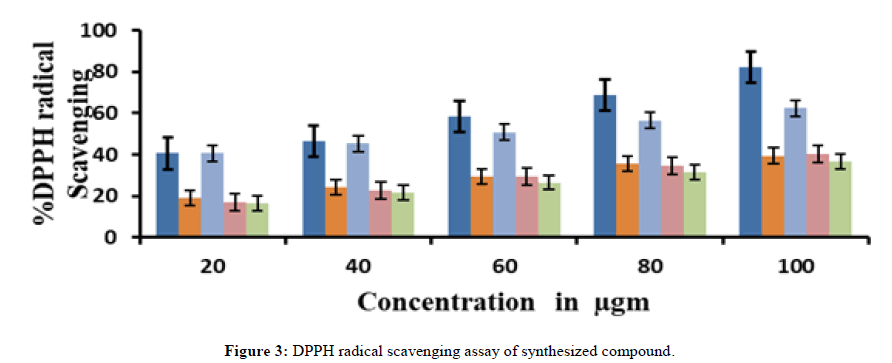
When comparing the benzothiazepine derivatives to the reference molecule BHT, all of the compounds shown a notable ability to scavenge DPPH radicals. Strong DPPH scavenging action was demonstrated by compounds 4a and 4c, with IC50 values of 16.89 μg/mL, 17.24 μg/mL, 18.12 μg/mL, 18.15 μg/mL, and 18.75 μg/mL. There was decreased DPPH scavenging activity in compound 4b.
Nitric oxide radical scavenging assay
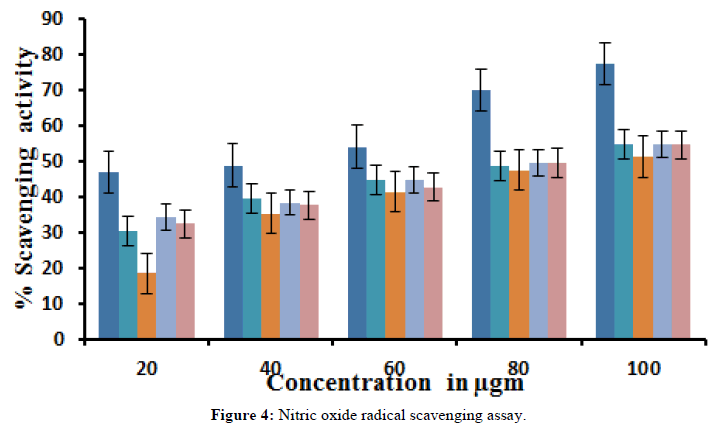
All the benzothiazepine derivatives showed the scavenging of nitric oxide radicals, scavenging is not significant compared to the reference compound BHT 6.4μg/mL (Tables 2 and 3) (Figures 3 and 4).
| Compounds | IC50 (µg/ml) |
|---|---|
| 4a | 30.62 |
| 4b | 35 |
| 4c | 30.62 |
| 4d | 36 |
| 4e | 31.25 |
| BHT | 6.4 |
Table 2: Invitro DPPH radical scavenging assay of synthesized compounds.
| Compounds | IC50 µg/ml |
|---|---|
| 4a | 17.24 |
| 4b | 35.62 |
| 4c | 15.25 |
| 4d | 25.83 |
| 4e | 24.31 |
| BHT | 37.4 |
Table 3: Invitro nitric oxide radical scavenging assay of synthesized compounds.
Anti-microbial activities
The synthesised compounds were examined using an antimicrobial assay. The compounds' antibacterial activity was evaluated in vitro against strains of a yeast-like fungus called Candida albicans as well as two strains of bacteria and two strains of negative bacteria. As reference medications, commercial antibiotics like Gentamycin and flucanozole were utilized. The above table shows the comparison between the outcomes and reference medications. With MIC values ranging from 1.2 μg to 7 μg. Table 4 shows that compounds 4a, 4b, and 4d are the most powerful of all those studied. Compounds 4c were not affecting B. subtilis or other Gram-positive bacteria. In contrast to the reference compounds, the benzothiazepine derivatives exhibit notable activity.
| Compounds | Minimum inbitory concentration (µg) | ||||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | Proteus | C. albicans | |
| 4a | 3 | 7 | 4 | 3 | 1.4 |
| 4b | 0.3 | 0.5 | 0.4 | 0.3 | 0.3 |
| 4c | 0.5 | 0.6 | 1.5 | 0.4 | 0.5 |
| 4d | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 |
| 4e | 0.6 | 0.5 | 0.2 | 0.9 | 0.7 |
| 4f | 0.35 | 0.4 | 0.4 | 0.4 | 0.5 |
| Gentamycin | 0.5 | 0.72 | 1 | 1.3 | - |
| Flucanozole | - | - | - | - | 0.75 |
Table 4: Invitro anti-microbial activities of synthesized compounds.
Conclusion
Using trimethyl phosphate, benzothiazepine derivatives have been synthesized easily. The transformation can be carried out at room temperature and the reaction condition is straightforward. When the newly created compounds (4a-g) were tested for their in vitro antimicrobial activity, 4a, 4b, 4d, 4f, and 4g had great antifungal activity and, when compared to other compounds, 4a and 4d demonstrated excellent antibacterial activity. Both antioxidant and antimicrobial activities were demonstrated by the entire molecule.
References
- El-Bayouki KA. J of Sulfur Chem. 2011; 32(6): p. 623-690.
- Saha D, Jain G, Sharma A. RSC Adv. 2015; 5(86): p. 70619-70639.
- Zamania F, Doustkhahb E. Benzodiazepine Based Drug Dis. 2022; 15: p.1-10.
- Kamble RR, Sudha BS. Phosphorus Sulfur Relat Elem. 2008; 183(7): p.1691-1695.
- Terada T, Fujimoto K, Nomura M, et al. J Med Chem. 1993; 36(12): p. 1689-1699.
- Bariwal JB, Upadhyay KD, Manvar AT, et al. Eur J Med Chem. 2008; 43(11): p. 2279-2290.
- Mali JR, Jawale DV, Londhe BS, Mane RA. Green Chem Lett Rev. 2010; 3(3): p. 209-212.
- Katritzky AR, Rachwal S. Chemical Rev. 2011; 111(11): p. 7063-7120.
- Chua KE, Stinson SF, Phillips LR. et al. J Med Chem. 1999; 42: p. 4172.
- Moreau S, Coudert P, Rubat C, et al P. J Med Chem. 1994; 37(14): p. 2153-2160.
- Zhang XH, Chen BJ, Lin XQ, et al. Chem Mater. 2001; 13: p. 1565-1569.
- Foldesi T, Volk B, Milen M. Curr Org Synth. 2018: p. 729-754.