Research - Der Pharma Chemica ( 2023) Volume 15, Issue 3
TiI4 mediated Prins-type cyclization of homoallylic alcohols and epoxides: An efficient method for the synthesis of 4-Iodotetrahydropyran derivatives
Tirukovela Manjula1,2 and Jeyanthi Arasan1*2Department of Chemistry, Government Degree College, Luxettipet-504231, Telangana, India
Jeyanthi Arasan, Department of Chemistry, Government Degree College, Luxettipet-504231, Telangana, India, Email: jayanthi.arasan@gmail.com
Received: 01-May-2023, Manuscript No. dpc-23-102396; Accepted Date: May 29, 2023 ; Editor assigned: 05-May-2023, Pre QC No. dpc-23-102396; Reviewed: 19-May-2023, QC No. dpc-23-102396; Revised: 26-May-2023, Manuscript No. dpc-23-102396; Published: 31-May-2023, DOI: 10.4172/0975-413X.15.3.80-83
Abstract
The cyclization reaction between homoallylic alcohols and epoxides using Titanium (IV) Iodide generates 4-Iodo tetrahydropyran derivativeswith good yields under mild reaction conditions.
Keywords
Tetrahydropyran; Epoxide; Homoallylic alcohol; Iodo; Titanium (IV) Iodide
INTRODUCTION
Oxygen- and nitrogen-containing heterocyclic systems such as pyran and piperidine systems occur in many natural products and are important intermediates for the synthesis of complex molecules [1-3]. In particular, these ring systems with labile groups such as halogen and hydroxyl prove useful for further functionalization [4-6]. In particular, iodine substitution on pyran and piperidine systems proves useful due to their labile functionality [7-9]. The Prins reaction, which involves acid-catalyzed aldehyde or epoxide-olefin condensation, is one of the most common and simplest methods for the synthesis of pyran and pyperidine systems [10-13]. Many synthetic methods are available in the literature for the synthesis of iodine-substituted tetrahydropyrans, but their application is limited due to low yield, prolonged reaction time, and high reagent loading [14-19].
Because of their wide application, the development of improved methods that minimize reaction time and reagent loading is highly desirable. In this regard, titanium (IV) iodide proves to be more effective for the synthesis of tetrahydropran due to its low catalyst loading and shorter reaction time with good yield. In the literature, titanium (IV) iodide has been widely used for carbon-carbon bonding reactions such as Diels-Alder reactions, Mukaiyama Aldol reactions, and carbonyl-ene reactions [20-22]. In this report, we would like to describe the synthesis of iodo-tetrahydropyrans by the Prins-type cyclization reaction between homoallylic alcohols and epoxides using titanium (IV) iodide.
EXPERIMENTAL
Material and Methods (2)
The chemicals used for these reactions were purchased from Sigma-Aldrich and used as received. All epoxides were prepared from the relevant olefins according to the methods described in the literature. For TLC, silica gel 60 F254 coated on aluminium sheets was used and purchased from Merck. Silica gel with 60-120 meshes was used for column chromatography. 1HNMR and 13CNMR spectra were recorded on a Bruker 300-MHz NMR spectrometer using trimethylsilane as an internal standard on a δ-scale of parts per million. Mass spectra were recorded using a Waters G2- XS QTof mass spectrometer. IR Spectra were recorded using the Nexus 670 Thermo Nicolet Fourier transform infrared spectrometer.
Representative Procedure (2.1)
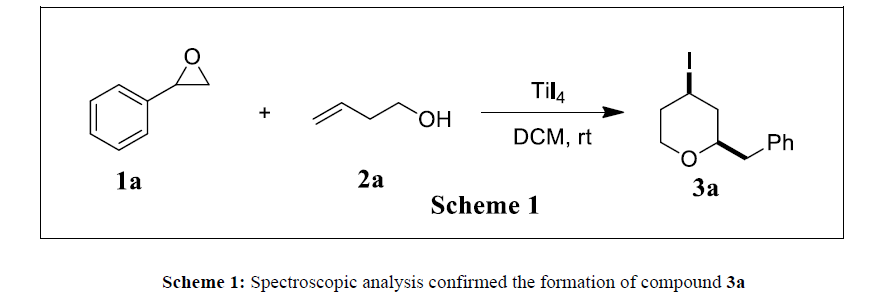
To a magnetically stirred mixture of styrene oxide (200 mg, 1.66 mmol) and 3-buten-1-ol (96 mg, 1.33mmol) in anhydrous methylene dichloride (10 mL) under nitrogen atmosphere was added titanium (IV) iodide (230 mg, 0.4 mmol) and stirred for 15 minutes at room temperature (25-30oC). TLC monitoring after 15 minutes indicated the completion of the reaction. The reaction mixture is then poured onto crushed ice, extracted with dichloromethane and concentrated. The reaction mixture was purified by silica gel column chromatography and the product was eluted with an ethyl acetate-hexane mixture (2:8) as the mobile phase, yielding 290 mg of compound 3a as light yellow oil.
RESULTS AND DISCUSSION
The applicability of this method was first investigated by stirring a mixture of 3-buten-1-ol and styrene oxide with Titanium (IV) Iodide in anhydrous dichloromethane under a nitrogen atmosphere at ambient temperature (25-30oC). TLC was monitored on the progress of reaction, and after 15 minutes the disappearance of the starting materials was observed. Then the reaction mixture was poured into ice, extracted with DCM and concentrated. The product was isolated by column chromatography over silica gel and eluted with ethyl acetate and hexane as mobile phase. After purification, the product was analyzed by NMR (1H &13C), IR and mass spectroscopy. All spectroscopic analysis confirmed the formation of compound 3a, and the data obtained are comparable to literature reports [16-19] (Scheme 1).
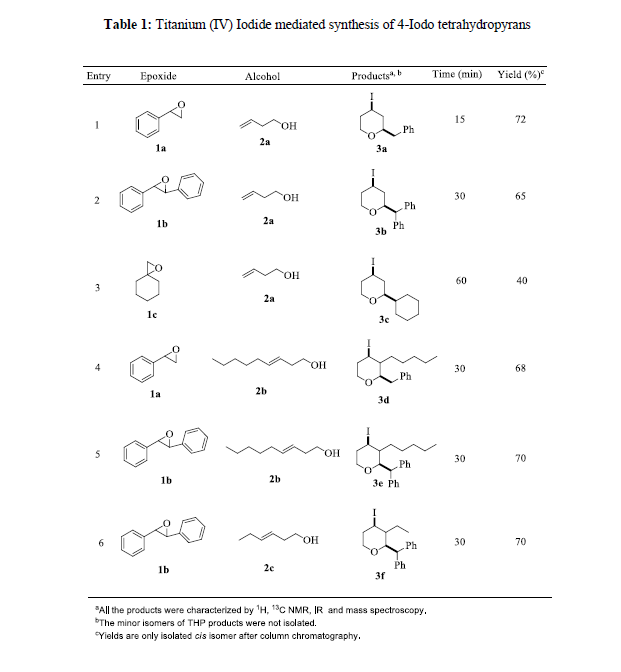
Based on the above results, the feasibility of this method was extended to various combinations of epoxides and homoallyl alcohols. The reaction with all combinations gave corresponding tetrahydropyrans derivatives. This demonstrates the wide applicability of this method for the synthesis of a variety of Iodo-tetrahydropyrans using epoxides as starting materials (Table 1).
It is observed that steric hindrance plays an important role in the reaction time and product yield. For example, the reaction of substituted epoxides with substituted homoallyl alcohols gave lower yield with longer reaction time compared to unsubstituted epoxides and homoallyl alcohols. The reaction of methylene cyclohexane oxide with 3-buten-1-ol took longer with lower yield than aryl epoxides. This could be due to the tertiary and hindered formation of cyclohexyl carbonium ions. The reaction is stereo-selective and the formation of a cis-trans isomer mixture was observed. The major product was cisstereo chemistry and the trans minor isomer was not isolated. The formation of the cis-isomer was confirmed by 1HNMR spectroscopy and compared with literature data. The important formation of cis-stereoisomer is likely due to thermodynamic stability.
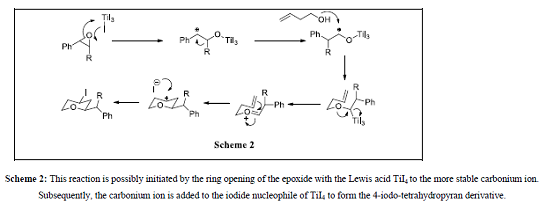
The expected mechanism of this reaction is possibly initiated by the ring opening of the epoxide with the Lewis acid TiI4 to the more stable carbonium ion. Subsequently, the carbonium ion is added to the iodide nucleophile of TiI4 to form the 4-iodo-tetrahydropyran derivative (Scheme 2)
CONCLUSION
Briefly, we have invented an improved and simple method for the synthesis of 4-iodo-tetrahydropyrans by the cyclization reaction between homoallylic alcohols and epoxides with Lewis acid Titanium (IV) Iodide. The mild reaction conditions, low reagent usage and improved yield make this method more useful than the existing methods for the synthesis of iodotetrahydropyran derivatives.
ACKNOWLEDGEMENT
The Authors thank the Department of Chemistry, Satavahana University for the cooperation and support.
CONFLICT OF INTEREST
The Authors declare no conflict of interest.
REFERENCES
- Sudhakar A, Chandrasekhar B, Saumya R, et al., J Org Chem. 2012, 77: p. 9840.
- Françoise C, Renaud DM, Guy S, et al., Organic Lett. 2002, 4: p. 1723.
- Fettes A, Carreira EM. J Org Chem. 2003, 68: p. 9274.
- Setsuko F, Yoshiteru T, Tadahito N, et al., European Patent 2004.
- Michael B, Plake BH, Xiaoqi C, et al., WIPO Patent 2013.
- Marcello A, Riccardo B, Maria CC, et al., J Med Chem. 2005, 48: p. 4312.
- Philipp MH, Mariar V, Paolo L, et al., ACS Catalysis.2015, 5: p. 4300.
- Christopher JC, Rylan JL, Gregory CF. J Am Chem Soc. 2013, 135: p. 10946.
- Chi WC, Peng R, Xile H. Org Lett. 2014, 16: p. 2566.
- Reyes E, Prieto L, Uria U, et al., ACS Omega. 2022, 7: p. 31621.
- Yadav JS, Reddy BVS, Reddy MS, et al., Eur J Org Chem. 2003, 9: p. 1779.
- Li J, Li CJ. Tetrahedron Lett. 2001, 42: p. 793.
- Yadav JS, Rajasekhar K, Murty MSR. Tetrahedron Lett. 2005, 46: p. 2311.
- Sabitha G, Reddy KB, Bhikshapathi M, et al., Tetrahedron Lett. 2006, 47: p. 2807.
- Yadav JS, Reddy BVS, Krishna VH, et al., Can J Chem. 2007, 85: p. 412.
- Yadav JS, Reddy BVS, Kumar GGKSN, et al., Chemistry Lett. 2007, 36: p. 426.
- Träff AM, Janjetovic M, Linda T, et al., Chem Int Ed. 2013, 52: p. 12073.
- Chalopin T, Jebali K, Nourry CG, et al., Tetrahedron. 2016, 72: p. 318.
- Anil SK, Somasekhar B, Kiran I, et al., Chemistry Lett. 2011, 40: p. 1176.
- Haneishi T, Hachiya I, Shimizu M. Arab J Sci Eng. 2014, 39: p. 6599.
- Hachiya I, Nagoshi S, Shimizu M. ACS Omega. 2019, 4: p. 10463.
- Hayakawa R, Shimizu M. Chemistry Lett. 2000, 29: p. 724.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref