Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 5
Tamarind Juice Catalyzed Green and Efficient Synthesis of Biscoumarin Derivatives in Aqueous Media
Amit S Waghmare1*, Ramrao A Mane2, Vinod T Kamble3 and Bhaskar S Dawane42Department of Chemistry, Marathwada University, Maharashtra, India
3Department of Chemistry, Government Institute of Science, Maharashtra, India
4Department of Chemical Sciences, SRTM University, Maharashtra, India
Amit S Waghmare, Department of Chemistry, Commerce and Science College, Maharashtra, India, Email: asw6807@gmail.com
Received: 24-May-2021, Manuscript No. DPC-23-32120; Editor assigned: 27-May-2021, Pre QC No. DPC-23-32120 (PQ); Reviewed: 10-Jun-2021, QC No. DPC-23-32120; Revised: 04-Aug-2023, Manuscript No. DPC-23-32120 (R); Published: 01-Sep-2023, DOI: 10.4172/0975-413X.15.5.1-4
Abstract
A green and efficient synthesis of biscoumarin derivatives is achieved in aqueous media catalyzed by tamarind juice as a natural catalyst. This Knoevenageal condensation is achieved using 4-hydroxycoumarin and different aromatic aldehyde in aqueous media at reflux condition. The present protocol avoids the use of hazardous reagents and toxic solvents. One-pot condensation, shorter reaction times, high yields, operational simplicity, simple workup procedure are some of the additional features of this protocol.
Keywords
Tamarind juice; Natural catalyst; Aqueous media; Biscoumarin; Green synthesis
Introduction
In the last couple of decades more attention is given for the development of green and eco-friendly processes as it does not involve the use of hazardous reagent, expensive catalyst and toxic solvents such as benzene, toluene, methanol, etc. Many of the organic transformations are carried out in aqueous media as it is inexpensive, easily available, non hazardous and environmentally benign solvent. Due to this reason water has get an attention and many of organic transformations are carried out in aqueous media [1]. Development of environmentally benign protocol for the organic transformations remains a challenging task for the organic chemists. In order to develop such type of protocols there is need to improve catalysis techniques. Now a day’s fruit juices, plant roots, plant tubers, vegetable extracts are explored for their catalytic activities in many organic reactions. The aqueous extract of naturally occurring fruit juice is a biocatalyst and also shows the applications in organic transformations. This fruit juice has got a considerable interest in different organic transformations as it is easily available, non toxic, inexpensive, safer and environmentally benign [2]. In the last decade different fruit juices are applied in organic synthesis as a homogeneous catalyst for the production of various derivatives. Fruit juices such as lemon, pineapple, coconut and tamarind are used as catalyst by various researchers. By using these juices different organic transformations are carried out such as Biginelli reaction, Knoevenagel-Michael condensation and in the preparation of benzimidazoles, benzothiazole and benzoxazoles, furanones and pyrrolones, etc [3].
Tamrindus indica is belonged to Leguminosea family. It is native to tropical Africa such as Sudan and Ethiopia. Now, it is widely distributed to other tropical climate areas such as Thailand and Indonesia [4]. There are two different types of tamarind which are acidic and sweet fruit. The sweet fruit contains more glucose than acidic fruit. Tamarind fruit pulp power shows various substances which are analyzed by high performance liquid chromatography technique. Organic acids such as tartaric acid, lactic acid, citric acid and maleic acid are found in tamarind fruit pulp. An aqueous extract of tamarind fruit juice is acidic having pH 3 and acidity percentage is 50% and hence it will be worked as an acid catalyst in acid catalyzed reactions [5].
Coumarins are naturally occurring substances observed in various species of plants, fungi and microorganisms. They are subdivided in different classes on their chemical diversity such as isocoumarins, furanocoumarins, pyranocoumarins, biscoumarins and other coumarins. Coumarin derivatives shows various biological activities such as antioxidant, anticancer, antibacterial, antifungal, antiviral, anti-inflammatory, anticonvulsant, anticoagulant and antidiabetic. Beside this they are also act as carbonic anhydrase inhibitors and use in Alzheimer’s disease [6].
Bridge substituted dimers of 4-hydroxycoumarin known as biscoumarins have attracted a great importance in the last decade and act as potential anticoagulant and antiviral agent. Biscoumarins are applied as urease inhibitors and also used in the prevention and treatment of thrombosis [7].
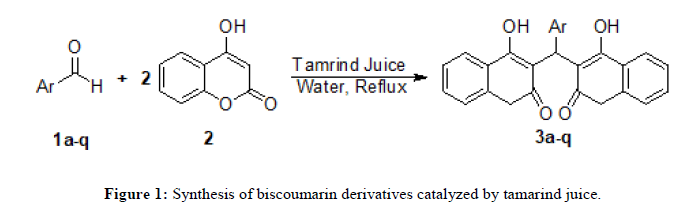
Biscoumarins are generally synthesized by Knoevenagel-Michael reaction from 4-hydroxy coumarin and different aldehyde. In the literature various methods are reported for the synthesis of biscoumarin derivatives such as use of microwave irradiation, ultrasonication, solvent free conditions, ionic liquid and iodine. Some methods are observed which includes different acid, base, inorganic salt and nanoparticles. These methods have their own advantages along with one of the limitations such as low yield, longer reaction time, harsh reaction condition tedious workup procedure use of hazardous solvents etc. In order to minimize these drawbacks there is need to develop an eco-friendly method for the synthesis of biscoumarin derivatives. In continuation of our research work for the synthesis of bioactive compounds using ecofriendly methods here, we report an efficient and green synthesis of biscoumarin derivatives catalyzed by tamarind juice in aqueous media (Figure 1) [8].
Materials and Methods
Experimental
General: All chemicals were purchased from sd fine and qualigens and used without further purification. All yields were referred to isolate products after purification. Melting points were determined by open capillary method and are uncorrected. IR spectra were recorded on KBr discs on a FT IR Jasco -4100 type A and the values are expressed as νmax cm-1. Nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on a Brucker avance II 400 NMR spectrophotometer using TMS as an internal standard. The chemical shifts values are reported in parts per million (δ), coupling constants (J values) are reported in Hertz (Hz) [9]. The progress of the reaction was monitored by TLC using silica gel-G (Merck). All products are known compounds and were characterized by comparison of their spectral and physical data with literature values [10].
Procedure for extraction of tamarind juice: The upper shell and inner grain of unripe tamarind fruit were removed with the help of a knife. The hard green 10 g pulp was boiled in 50 ml water, cooled and it was centrifuged. The clear portion of the aqueous extract (pH 3) of tamarind fruit was used as catalyst for the reaction [11].
General procedure for the synthesis of biscoumarin derivatives: A mixture of aromatic aldehydes (1 mmol), 4-hydroxycoumarin (2 mmol) and tamarind juice-water (5 ml, 4:1, pH 3) was taken in a 50 ml round bottom flask and refluxed for appropriate time as mentioned in Table 2. After completion of reaction (monitored by TLC), the reaction mixture was diluted with cold water. The solid product obtained was collected by simple filtration, washed with water and dried. The crude product obtained was purified by re-crystallization with ethanol to afford pure product [12].
Spectral data of some compounds
3, 3’-Benzylidene-bis-(4-hydroxycoumarin) (3a): IR (KBr) νmax: 2361, 1675, 1609, 1566, 1494, 1352, 1110 cm-1; 1H NMR (CDCl3, 400 MHz): δ 6.11 (s, 1H, CH), 7.14-8.06 (m, 13H, 13 × CH), 11.31 (s, 1H, OH), 11.53 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100MHz): δ 36.05, 103.75, 105.47, 116.51, 117.92, 124.24, 124.76, 126.34, 126.73, 128.50, 132.75, 135.10, 151.20, 164.45, 165.67, 166.77, 169.10 ppm [13].
3, 3’-(4-Nitrobenzylidene)-bis-(4-hydroxycoumarin) (3b): FT-IR (KBr) νmax: 2604, 1660, 1599, 1566, 1520, 1347 cm-1; 1H NMR (CDCl3, 400 MHz): δ 6.13 (s, 1H, CH), 7.26-8.15 (m, 12H, 12 × CH), 11.52 (s, 1H, OH), 11.58 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100MHz): δ 36.41, 103.15, 104.63, 116.09, 116.67, 123.74, 124.37, 125.10, 127.48, 133.27, 143.30, 146.69, 152.44, 164.69, 166.31, 166.87, 168.97 ppm [14].
3, 3’-(2-Nitrobenzylidene)-bis-(4-hydroxycoumarin) (3d): FT-IR (KBr) νmax: 2601, 1659, 1611, 1524, 1358, 1309 cm-1; 1H NMR (CDCl3, 400 MHz): δ 6.13 (s, 1H, CH), 7.28-8.15 (m, 12H, 12 × CH), 11.38 (s, 1H, OH), 11.57 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100MHz): δ 33.80, 103.66, 116.30, 116.46, 124.29, 124.57, 124.60, 127.95, 129.44, 131.10, 132.15, 132.80, 149.70, 152.35, 164.88, 166.48 ppm [14].
3, 3’-(4-Chlorobenzylidene)-bis-(4-hydroxycoumarin) (3e): FT-IR (KBr) νmax: 2609 , 1672, 1564, 1493, 1307 cm-1; 1H NMR (CDCl3, 400 MHz): δ 6.00 (s, 1H, CH), 7.09-8.10 (m, 12H, 12 × CH), 11.10 (s, 1H, OH), 11.56 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100MHz): δ 35.19, 108.98, 115.90, 116.59, 123.70, 123.90, 127.90, 128.20, 130.80, 132.20, 136.89, 151.89, 164.16, 165.20 ppm [15].
3, 3’-(4-Methoxybenzylidene)-bis-(4-hydroxycoumarin) (3j): FT-IR (KBr) νmax: 2626, 1671, 1564, 1510, 1351, 1259 cm-1; 1H NMR (CDCl3, 400 MHz): δ 3.79 (s, 6H, 2 × OCH3), 6.04 (s, 1H, CH), 7.11-8.05 (m, 12H, 12 × CH), 11.29 (s, 1H, OH), 11.50 (s, 1H, OH) ppm; 13C NMR (CDCl3, 100MHz): δ 26.90, 28.20, 31.48, 34.58, 49.97, 127.80, 128.40, 129.30, 131.30, 141.67, 158.07, 161.50, 186.70, 195.30 ppm.
3, 3’-(3,4,5-Trimethoxybenzylidene)-bis-(4-hydroxycoumarin) (3l): FT-IR (KBr) νmax: 2609, 1660, 1564, 1510, 1454, 1348, 1129 cm-1; 1H NMR (CDCl3, 400 MHz): δ 3.60 (s, 6H, 2 × OCH3), 3.70 (s, 3H, 1 × OCH3), 6.00 (s, 1H, CH), 6.35 (s, 2H, 2 × CH), 7.22-7.95 (m, 8H, 8 × CH), 11.38 (s, 2H, 2 × OH) ppm; 13C NMR (CDCl3, 100 MHz): δ 35.82, 55.90, 60.48, 103.84, 104.40, 116.20, 116.28, 123.89, 124.58, 130.87, 132.60, 136.65, 151.98, 152.94, 164.70, 167.39 ppm.
3, 3’-(4-Hydoxy-3-methoxybenzylidene)-bis-(4-hydroxycoumarin) (3m): FT-IR (KBr) νmax: 2614, 1570, 1516, 1452, 1345, 1274 cm-1; 1H NMR (CDCl3, 400 MHz): δ 3.74 (s, 3H, OCH3), 5.60 (s, 1H, CH), 6.06 (s, 1H, OH) 6.67-8.05 (m, 11H, 11 × CH), 11.31(s, 1H, OH), 11.51(s, 1H, OH) ppm; 13C NMR (CDCl3, 100 MHz): δ 35.80, 56.60, 103.45, 113.58, 115.79, 115.90, 118.71, 123.38, 123.89, 131.56, 132.18, 136.44, 140.90, 149.18, 152.36, 164.37, 166.46 ppm
Results and Discussion
In the optimization of amount of tamarind juice for the synthesis of biscoumarin derivatives initially benzaldehyde is selected as a probe aldehyde. When reaction is carried out in water without tamarind juice, it does not proceed to completion even after refluxing for two hours. We the amount of tamarind juice increased slowly from 1 ml to 5 ml and decreasing amount of water from 5 ml to without water, it is observed that amount of yield increases slowly and time for completion of reaction decreases up to 20 minutes. From the optimization it is observe that 4 ml of tamarind juice and 1 ml of water is sufficient for completion of reaction in the forward direction. Good yield obtained within short reaction time at this condition (Table 1). Further increase in the amount of tamarind juice does not show significant effect on the yield and reaction time.
| Entry | Amount of tamarind juice | Amount of water | Time (Min.) | Yield (%)b |
|---|---|---|---|---|
| 1 | 0 | 5 | 120 | --- |
| 2 | 1 | 4 | 60 | 48 |
| 3 | 2 | 3 | 40 | 60 |
| 4 | 3 | 2 | 30 | 75 |
| 5 | 4 | 1 | 20 | 90 |
| 6 | 5 | 0 | 20 | 90 |
Note: Reaction conditions: a4-hydroxycoumarin (2 mmol), benzaldehyde (1mmol), reflux, bIsolated yields
Table 1: Optimization of amount of tamarind juice for the synthesis of (3a)a.
From these results other aldehyde were also reacted with 4-hydroxycoumarin at reflux condition catalyzed by tamarind juice in water to obtain the derivatives of biscoumarin. Other aromatic aldehyde containing electron donating, withdrawing groups and heterolytic aromatic aldehyde were employed and reacted successfully at the optimized condition to give the corresponding biscoumarin derivative with good to excellent yield (Table 2).
| Entry | Aldehyde | Product | Time (Min.) | Yield (%)b | M.P. (°C) | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 1 | C6H5- | 3a | 20 | 85 | 218-220 | 226-228 |
| 2 | 4-NO2-C6H4- | 3b | 15 | 95 | 230-232 | 236-237 |
| 3 | 3-NO2-C6H4- | 3c | 15 | 86 | 208-210 | 234-236 |
| 4 | 2-NO2-C6H4- | 3d | 10 | 80 | 204-206 | 202 |
| 5 | 4-Cl-C6H4- | 3e | 15 | 85 | 256-258 | 258-260 |
| 6 | 3-Cl-C6H4- | 3f | 20 | 94 | 218-220 | 215 |
| 7 | 2-Cl-C6H4- | 3g | 20 | 90 | 218-220 | 224-226 |
| 8 | 4-OH-C6H4- | 3h | 20 | 82 | 218-220 | 220-224 |
| 9 | 3-OH-C6H4- | 3i | 15 | 93 | 212-214 | 210.5 |
| 10 | 4-OMe-C6H4- | 3j | 20 | 91 | 238-240 | 244-246 |
| 11 | 3,4-(MeO)2C6H3- | 3k | 15 | 95 | 260-262 | 264-266 |
| 12 | 3,4,5-(OMe)3-C6H2- | 3l | 15 | 94 | 244-246 | 240-242 |
| 13 | 4-OH-3-MeO-C6H3- | 3m | 20 | 94 | 208-210 | 199.6 |
| 14 | 4-CH3-C6H4- | 3n | 20 | 90 | 200-202 | 270-271 |
| 15 | 4-Br-C6H4- | 3o | 15 | 95 | 262-264 | 269-270 |
| 16 | 4-N(Me)2-C6H4- | 3p | 15 | 90 | 220-222 | 210-215 |
| 17 | 2-Thiophenyl | 3q | 20 | 95 | 210-212 | 210 |
Note: Reaction conditions: a4-hydroxycoumarin (2 mmol), aldehyde (1 mmol), tamarind juice-water (5 ml, 4:1), reflux, bisolated yield
Table 2: Tamarind juice catalyzed synthesis of biscoumarin deriveativesa.
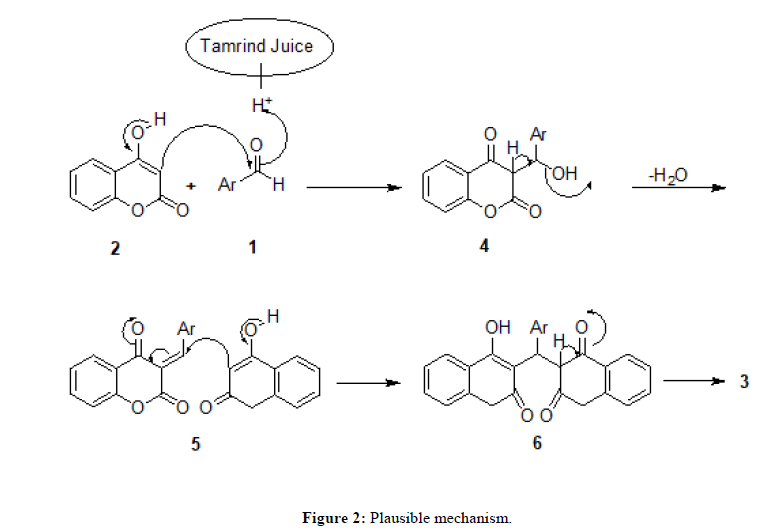
Initially, activation of 4-hydroxycoumarin (2) and benzaldehyde (1) was carried out by tamarind juice to give (4). After the condensation between first molecule of 4-hydroxycoumarin and aldehyde a double bond is formed with the elimination of water molecule (5). Michael addition takes place between second molecule of biscoumarin and (5) to give (6) in the next step. In the last step keto enol tautomerism takes place to give the final product (3). In the progress of reaction the tamarind juice performs important role of activation for the substrate to proceed in the forward direction within short duration with good efficiency and high selectivity (Figure 2).
Conclusion
In conclusion we have developed simple, efficient and environmentally benign protocol for the synthesis of biscoumarin derivatives by condensation of 4-hydroxycoumarin and different aromatic aldehydes catalyzed by tamarind juice in aqueous media at reflux condition. Present protocol shows various applications such as shorter reaction time, good to excellent yields, simple workup procedure.
Acknowledgement
Author is highly thankful to Prof. S. S. Gholap, principal, arts, commerce and science college, Satral, for the support of this work and for providing all necessary facilities. Author is also thankful to SAIF, Panjab university Chandigarh for providing spectral facilities.
References
- Anastas PT, Kirchhoff MM. Acc Chem Res. 2002, 35(9): p. 686-694.
[Crossref] [Google Scholar] [PubMed]
- Ahmad RNA, Kan SY, Hamzah AS, et al. Environ Chem Lett. 2021, 19: p. 3359-3380.
- Comasseto JV, Omori AT, Porto AL, et al. Tetrahedron Lett. 2004, 45(3): p. 473-476.
- Mironowicz A. Phytochem. 1998, 47(8): p. 1531-1534.
- Bedi P, Behera AK, Alanazi AK, et al. J Chem Sci. 2023, 135(2): p. 34.
- Fiorito S, Taddeo VA, Genovese S, et al. Tetrahedron Lett. 2016, 57(43): p.4795-4798.
- Pal R. Open J Org Chem, 2013; 1(4): p. 47-56.
- Patil S, Jadhav SD, Mane SY. Int J Organ Chem. 2011, 1(3): p. 125.
- Fonseca AM, Monte FJ, Maria da Conceicao F, et al. J Mole Cataly B: Enzym. 2009, 57(1-4): p. 78-82.
- Vekariya RH, Patel KD, Patel HD. Res Chem Inter. 2016, 42: p. 7559-7579.
- Patil MA, Ubale PA, Karhale SS, et al. Der Chemica Sinica. 2017, 8(1): p.198-205.
- Gulati S, Singh R, Sindhu J, et al. Organ Prep Proced Int. 2020, 52(5): p. 381-395.
- Tril U, Fernandez-Lopez J, Alvarez JA, et al. Ind Crop Prod. 2014, 55: p. 155-162.
- Borges F, Roleira F, Milhazes N, et al. Curr Med Chem. 2005, 12(8): p. 887-916.
[Crossref] [Google Scholar] [PubMed]
- Hoult JR, Paya M. Gener Pharmacol. 1996, 27(4): p. 713-722.