Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 5
Synthesis, Structural Characterization, Antimicrobial Activity of Complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) With 7-((2-Aminoethyl)Amino)-5-Bromo-6-Hydroxy-1-Methylquinolin-1- Ium-3-Sulfonate
Mai Phuong Chi, Nguyen Thi Quy and Le Thi Hong Hai*Le Thi Hong Hai, Chemistry Faculty, Hanoi National University of Education, Hanoi, Vietnam, Email: hailth@hnue.edu.vn
Received: 26-Apr-2021 Accepted Date: Jul 15, 2021 ; Published: 22-Jul-2021
Abstract
In this research, a new quinoline derivative, 7-((2-aminoethyl)amino)-5-bromo-6-hydroxy-1- methylquinolin-1-ium-3-sulfonate (QEt) as well as six complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) with this ligand [M(QEt-1H)2].nH2O (M: Co, Cu, Zn, Cd, Hg), [Ni(QEt-1H)Cl].2H2O were synthesized. The molecular formulas and structures of the complexes were determined using measurement as IR, EDX, ESI-MS, 1H NMR spectra and thermal analysis. The bioactivity tests shown that the ligand and CuQEt complex have limiting effects to microorganisms, complexes CoQEt, ZnQEt, CdQEt and HgQEt exhibit antibacterial activities with low IC50 values, from 0.5 to 56.0 μg/mL and be most effective to Bacillus subtilis. Especially HgQEt inhibits effectively to almost types of bacteria.
Keywords
Quinolinesulfonate, Transition-Metal Complexes, Antimicrobial Activity
Introduction
Quinoline is one of the popular heterocyclic compounds that have been utilized in variety fields like energy industry [1], environmental analysis [2], and especially applied pharmaceutical industry [3]. These compounds have attracted the large attention of scientists due to the potential biological properties and show excellent antifungal, antivirus and antibsacterial activities [4-6]. In addition, they have been used in the therapies of malaria disease, cancers, Alzheimer, Parkinson etc [7-9].
The complexes of quinoline derivatives with metal ions such as Co(II), Ni(II), Cu(II), Cd(II), Zn(II), Hg(II) have been reported to exhibit considerable antimicrobial activities, some complexes are better inhibitors than the ligands [10,11]. For instance, other complexes between (quinolin-2- yl)benzenediamine and Co(II), Cu(II) show the greater antibacterial activity than standard agents, ciprofloxacin, as well as the excellent antifungal ability compared to fluconazole [12]. Besides, quinoline complexes show the low toxicity to human, high cytotoxicity that have potential application in future [13].
Differently from quinoline compounds completely synthesized by artificial chemicals, a series of quinoline derivatives have been synthesized from Eugenol extracted from certain essential oils of plants such as tulsi (Ocimum sanctum L.) or clove (Syzygiumaromaticum) [14]. From key compound, 7-carboxymethoxy-6-hydroxyquinolin-1-ium-3-sulfonate (Q), many new derivatives were synthesized and studied about fluorescent property, structure and bioactivity of their complexes with transition metal ions [14,15]. Here in, we report the synthesis, structure and antimicrobial activity of complexes of a new quinoline derivative - 1-methyl-5-bromo-6-hydroxy-7-ethylendiamin-3- sulfoquinoline (QEt) with transition metal ions such as Co(II), Ni(II), Cu(II), Cd(II), Zn(II), Hg(II). In this ligand, there are aromatic rings, hydroxyl group (-OH), amine group (NH, NH2) that can coordinate with metal ions to form new complexes showing the potential antimicrobial activity.
Materials and Methods
All commercially available reagents and chemicals were of analytical grade purity and used without purification. The molecular formulas and structures of the complexes were determined by spectrometric methods. IR spectra were recorded from KBr pellets by an IMPACK-410 NICOLET IR spectrometer. 1H NMR spectra were recorded by a Bruker XL-500 MHz spectrometer in DMSO. Absorbance spectra were measured by UV-Vis Cary 60 spectrometer in distilled water. Mass spectra (ESI-MS) were measured by LC-MSD-Trap-SL spectrometer in DMSO or CHCl3. Thermal analysis was made on a DTG-60H detector in Argon with heating rate of 10°C min-1 in the range of temperature from 25 to 800°C. Energy-dispersive X-ray spectra (EDX) were recorded by FE-SEM instrument.
Synthesis of 7-((2-aminoethyl)amino)-5-bromo-6-hydroxy-1-methyl quinolin-1-ium-3-sulfonate (QEt)
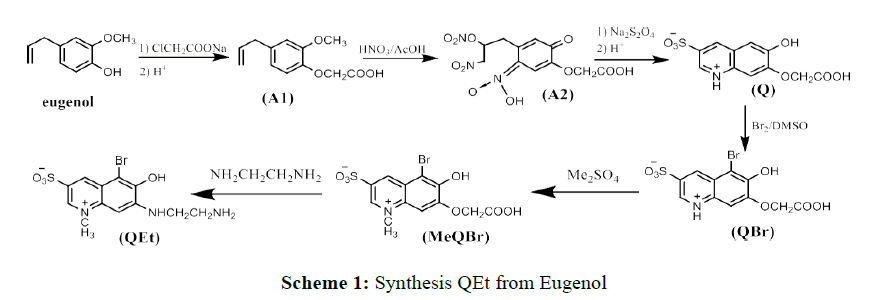
7-((2-aminoethyl)amino)-5-bromo-6-hydroxy-1-methyl quinolin-1-ium-3-sulfonate (QEt) was synthesized from eugenol, the main constituent of Ocimum sanctum L. oil, after 6 steps displayed in Scheme 1. The process to synthesize MeQBr from eugenol have been described in [15], in here the detail of the last step from MeQBr to QEt was presented.
5-bromo-7-(carboxymethoxy)-6-hydroxy-1-methylquinolin-1-ium-3-sulfonate (MeQBr) (0,392 g, 1 mmol) and ethylenediamine (3.0 mL, 0.045 mol) were solved in 10 mL water then refluxed for 3 hours at 80-90°C. After cooling by cold water, a large amount of appreciate appeared. The resulting solid was washed with cold water and acetone before being recrystallized in HCl 1M. 7-((2-aminoethyl)amino)-5- bromo-6-hydroxy-1-methylquinolin-1-ium-3-sulfonate (QEt) was afforded as needle-shaped yellow crystals. Yield of synthesis process was about 60%. IR (KBr, cm-1): 3436, 3257 (νOH,H), 3086 (νCH), 1608, 1544 (νC=C,C=N), 1199 (νC−C,C−O,C−N). 1H NMR (500 MHz, δ, ppm): 9.13s (1H, Ar-H), 8.78s (1H, Ar-H), 8.19s (3H, NH +3), 7.85t (1H, J=6,5, N-H), 7.01s (1H, Ar-H), 4.44 s (3H, N-CH3 ), 3.79m (2H,CH2), 3.11m (2H, CH2).
Synthesis of complex CoQEt
To an aqueous solution (20 mL) of QEt(0.09 g, 0.2 mmol) was added solution of C°Cl2.6H2O (0.029 g,0.12mmol)(CoQEt). The mixture was added drop wise the solution of NH3(4 mL,0.5M),stirring at room temperature for 3 hours. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The obtained solid was dark red, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 75%. IR (KBr, cm-1): 3355, 3252, 3177 (νOH, NH ), 1586, 1535 (νC=C, C=N ), 1327 (νSO3 ), 445, 524, 575 (νM-O, M-N ). ESI-MS (m/z): + MS, 807.06, {Co(QEt1H)2 + H+ }+ . Selected EDX (Energy dispersive X-ray spectra) (atomic %), S: Br: Co = 4.82: 4.49: 2.25 (Found.) ≈ 2: 2:1 (Calc.).
Synthesis of complex NiQEt
To an aqueous solution (20 mL) of QEt (0.09 g, 0.2 mmol) was added solution of NiCl2.6H2O (0.029 g, 0.12 mmol) (NiQEt). The mixture was added drop wise the solution of NH3 (4mL, 0.5 M), stirring at room temperature for 3 hours. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The resulting solid was dark red, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 80%. IR (KBr, cm-1): 3319, 3250, 3140 (νOH, NH ), 1585, 1553 (νC=C, C=N ), 1332 (νSO3 ), 437, 534, 577 (νM-O, M-N ). 1H NMR (500 MHz, δ, ppm): 8.54s (1H, Ar-H), 8.41s (1H, Ar-H), 7.86 (2H, NH2), 7.66s (1H, N-H), 6.62 (1H, Ar-H), 4.29 (3H, N-CH3). ESI-MS (m/z): -MS, 433.9, {Ni (QEt-1H)-2H+} - . Selected EDX spectra (atomic %), S: Br: Ni = 1.02: 1.05: 1.49 (Found.) ≈ 1: 1:1 (Calc.).
Synthesis of complex CuQEt
QEt (0.090 g, 0.20 mmol) was dissolved in acetate buffer solution (20 mL, 50°C). To the solution was added salt of Cu (CH3COO)2.2H2O (0.026 g, 0.12 mmol) and stirred for 3h at room temperature. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The resulting solid was green, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 70%. IR (KBr, cm-1): 3574, 3337, 3257 (ν OH, NH), 1586 (νC=C, C=N), 1336 (νSO3), 540, 557 (νMO, M-N). ESI-MS (m/z): -MS, 910.6, {Cu (QEt-1H)2 + Br- + H2O}-
.
Synthesis of complex ZnQEt
To an aqueous solution (20mL) of QEt (0.09 g, 0.2 mmol) was added solution of ZnCl2.6H2O (0.029 g, 0.12 mmol) (ZnQEt). The mixture was added drop wise the solution of NH3 (4mL, 0.5 M), stirring at room temperature for 3 hours. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The resulting solid was yellow, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 75%. IR (KBr, cm-1): 3680-3100 (νOH, NH), 1594, 1548, 1518 (νC=C, C=N), 1320 (νSO3), 451, 525 (νM-O, M-N). 1H NMR (500 MHz, δ, ppm): 8.41s (1H, Ar-H), 8.32s (1H, Ar-H), 7.88 (2H, NH2), 7.68s (1H, N-H), 6.57 (1H, Ar-H), 4.27s (3H, N-CH3), 3.97 (2H, CH2), 3.67 (2H, CH2). Selected EDX spectra (atomic %), S: Br: Zn = 3.30 : 3.22 : 1.30 (Found.) ≈ 2 : 2 :1 (Calc.).
Synthesis of complex CdQEt
To a water solution (20mL) of QEt (0.09 g, 0.2 mmol) was added solution of CdCl2.2,5H2O (0.027 g, 0.012 mmol) (CdQEt). The mixture was added drop wise the solution of NH3 (4mL, 0.5 M), stirring at room temperature for 3 hours. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The resulting solid was yellow, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 70%. IR (KBr, cm-1): 3600÷3100 (νOH, NH ), 1591 (νC=C, C=N ), 1316 (νSO3 ), 464 (νM-O, M-N ). 1H NMR (500 MHz, δ, ppm): 8.41s (1H, Ar-H), 8.32s (1H, ArH), 7.88 (2H, NH2), 7.68s (1H, N-H), 6.57 (1H, Ar-H), 4.26s (3H, N-CH3), 3.67m (2H, CH2), 3.19m (2H, CH2).
Synthesis of complex HgQEt.
To an aqueous solution (20mL) of QEt (0.09 g, 0.2 mmol) was added a solution of Hg(CH3COO)2.H2O (0.031 g, 0.12 mmol). The mixture was added drop wise the solution of NH3 (4mL, 0.5 M), stirring at room temperature for 3 hours. Appeared precipitate was filtered, washed by cold water and ethanol and dried at 50°C. The resulting solid was orange, soluble in DMSO and slightly soluble in acetone, ethanol and methanol. Yield of synthesis process was about 60%. IR (KBr, cm-1): 3600-3100 (νOH, NH ), 1591, 1547 (νC=C, C=N ), 1318 (νSO3 ), 559 (νM-O, M-N ). 1H NMR (500 MHz, δ, ppm): 8.40s (1H, Ar-H), 8.33s (1H, Ar-H), 7.66d (N-H), 6.57 (1H, Ar-H), 4.26s (3H, N-CH3), 3.66 (2H, CH2), 3.13 (2H, CH2). EDX spectra (atomic %): C, 51.39; O, 24.97; S, 2.13; Br, 1.89; N, 17.73; Hg, 1.42. Selected EDX spectra (atomic %), S: Br: Hg = 2.13:1.89: 1.42 (Found.) ≈ 2:2:1 (Calc.).
Antimicrobial activity
Antimicrobial activity of QEt and the complexes were tested by Broth micro dilution method [16-18] for 7 types of microorganisms: Gram (+) (Staphylococcus aureus, Bacillus subtilis, Lactobacillus 5 fermentum), Gram (-) (Salmonella enterica, Pseudomonas aeruginosa, Pseudomonas aeruginosa) and Candida albicans fungus. The microorganisms are stored at -80°C. Before the experiment, the bacteria and fungus were activated in germination medium and reached the concentration of 5x105 CFU/mL to bacteria and 1x103 CFU/mL to fungus. The testing mixture was incubated for 16-24 hours at 37°C. The IC50 value is determined via the percentage of inhibition and by the Raw data computation program.
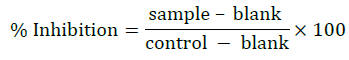
(sample - serial diluted test compound incubated for 18 h, control – stock solution with pure organic solvent, blank – control with ungerminated conidia suspension)
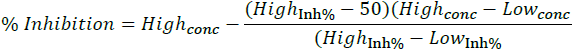
(Highconc/Lowconc: maximum/minimum value of the concentration, Higinh% / Lowinh%: the maximum/minimum value of the inhibition percentage).
Reference substances: Ampicillin antibiotics for Gram (+) bacterial strains with MIC values between 0.004-1.2 μg/mL, Cefotaxime antibiotics for Gram (-) bacterial strains with MIC values between 0.07- 19.23 μg/mL, Nystatin antifungal resistance for fungal strains with MIC value between 2.8-5.0 μg/mL.
Results and Discussions
Electrospray Ionization Mass Spectra (ESI-MS)
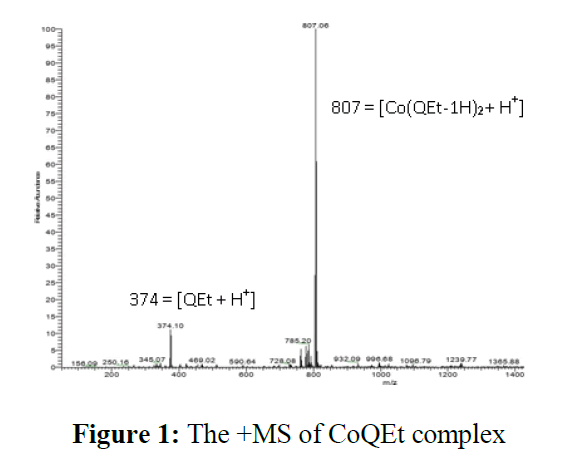
The obtained products were characterized by ESI-MS method and the ESI-MS spectrum of NiQEt complex is shown in Figure 1.
In the mass spectrum of CoQEt, thereisa 100% peak atm/z=807.06 in positive-ion mode corresponded to the species of{Co(QEt-1H)2+H+}to show the1:2 ratio of metal:ligand. Similarly, in mass spectrum of CuQEt, the 100% signal at m/z = 910.6 was assigned to {Cu(QEt-1H)2 + Br-+ H2O}-, shows that the ratio between metal-ligand is 1:2. Besides, the mass spectrum of NiQEt shows the present of one intense peak at 433.9 attributed to [Ni(QEt-1H)]+ion, confirming the metal–ligand radio of 1:1.
Infrared spectra (IR)
IR spectra of ligand and complexes display all of functional groups such as C=C,C=N bonds inaromatic rings, amine(-NH,NH2), phenol(-OH), sulfate(-SO3),that are assigned and showed in Table 1.
| Compound | IR absorption data (cm-1) | Thermal analysis | ||||
|---|---|---|---|---|---|---|
| u ,u ,u OH NH NH2 |
u u C=C C=N |
u SO3 |
u u M-O M-N |
%weight water (exp/calc) |
%weight oxide (exp/calc) |
|
| QEt | 3436, 3257 | 1608, 1544 | 1336 | - | - | - |
| CoQEt | 3355, 3252, 3177 |
1586, 1535 | 1327 | 575, 524, 445 |
9.27/10.01 | 10.97/9.56 |
| NiQEt | 3600÷3200 (broad) |
1585, 1553 | 1332 | 577, 534, 437 |
8.43/7.12 | 15.42/14.84 |
| CuQEt | 3574, 3337, 3257 |
1586 | 1336 | 540, 557 | 7.61/8.13 | 5.29/8.14 |
| ZnQEt | 3680-3000 (broad) |
1594, 1548 1518 |
1320 | 525, 451 | 5.78/6.20 | 10.60/9.32 |
| CdQEt | 3600-3090 (broad) |
1591,1552 | 1316 | 464 | 9.46/8.59 | 13.46/11.88 |
| HgQEt | 3600-3000 (broad) |
1591,1547 | 1318 | 559 | - | - |
In IR spectrum of QEt, there are absorption bands in 3400-3100 cm-1 area presented to the vibration of amine and hydroxyl groups. In addition, the intense absorptions at 1608 cm-1, 1544 cm-1 were assigned to the double bonds in aromatic rings and the vibration of -SO3- group was observed at 1336 cm-1 in the ligand IR spectrum [19]. In complex spectra, the broad signals at 3600–3100 cm-1 were caused by crystalized water, so the distinct peaks of -OH, NH groups did not be shown clearly in the IR spectra [20]. Besides, the C= C, C=N bond in aromatic rings, sulfate groups produce the IR absorption at 1590 cm-1, 1550 cm-1 and 1320 cm-1 areas, respectively [21]. These signals of similar vibrations of complexes slightly decrease in wavenumber compared to the ligand, which explained by the formation of coordinated bonds. Especially, the appearance of several M-O, M-N characteristic absorption at 400-600 cm-1 confirms the existence of complexes and the ligands coordinate with metal ion through -OH and -NH, -NH2 group [22].
Thermal analysis
By studying the thermal decomposition process of the compound, the number of crystalline and coordinated waters were determined, the data are presented in Table 1 and the thermal analysis curve of NiQEt complex is shown in Figure 2. In the DTA curve of this complex, there is one endothermal peak at about 95°C, combining with the first mass loss occurring from 50°C to 200°C in the TGA curve and the minimum peak in DrTGA curve at 92°C, corresponding to the release of two crystalline water molecules in [Ni(QEt-1H)Cl].2H2O. The mass loss of 8,43% is in good agreement with the calculated result of 7,12%. In the next stages, two exothermic peaks at 317°C and 481°C in the DTA curve correspond to decomposition reactions and oxidation processes of the complex to finally form nickel(II) oxide , it is acceptably stable at 800°C. After 800°C, the complex completely decomposes and the experimental percentage mass of the final residue (NiO)is15,42%,the data is consistent with the calculated percentage (14,84%). The results of the thermal analysis confirms the composition of the obtained compound that it is [Ni(QEt-1H)Cl].2H2O. Similarly, the results of thermal analysis (Table 1) further ascertain that the molecule composition of complexes is suitable with the predicted structures, the metal-ligand ratio in CoQEt, CuQEt, ZnQEt, CdQEt complexes are 1:2, and this ratio in NiQEt complex is1:1.
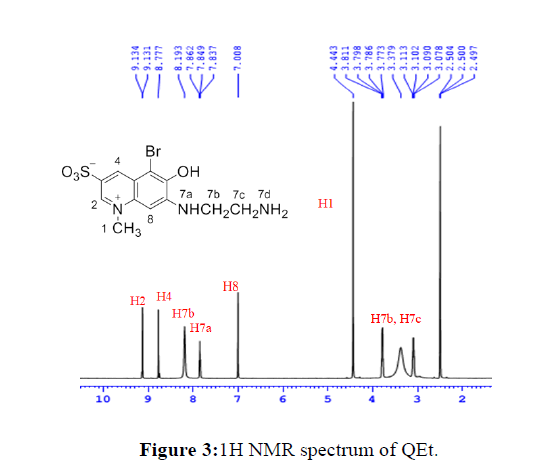
Proton nuclear magnetic resonance spectra (1H NMR)
All spectral data were assigned to protons of the compounds and presented in Table 2, the 1H NMR spectra of QEt and HgQEt complexes are showed in Figure 3. In the spectrum of the ligand, the resonance signals at 8.19, 7.89 ppm of amine protons are evident that -OCH2COOH of MeQBr was replaced by -NH(CH2)2NH2. The directly nucleophile substitution occurring easily were explained as the strong electron withdrawing group – imine (N+-CH3) and sulfate (-SO3-) in the quinoline ring that increasing positive charge in benzene ring. The aromatic signals at 9.13, 8.78 and 7.01 ppm were assigned to three protons - H2, H4, H6 in quinoline ring. In up field area, the signals were resolved into the protons bonding with three saturated carbon, which is consistent with the relative intensities of the signals.
| Chemical shift, δ (ppm) | |||||
|---|---|---|---|---|---|
| N-CH3 | H2, H4 | NH | NH2 | H8 | |
| QEt | 4,44 s | 9,13s, 8,78 s | 7,85t | 8,19s | 7,01s |
| NiQEt | 4.29 | 8,54s, 8,41s | 7.66s | 7.86 | 6.62 |
| ZnQEt | 4.26s | 8.41s, 8.32s | 7.68s | 7.88 | 6.57 |
| CdQEt | 4.26s | 8.40s | 7.66s | - | 6.57 |
| HgQEt | 4.26s | 8.40s, 8.32s | 7.61 | 7. 82 | 6.56 |
In the 1H NMR spectra of complexes, the proton resonances have the same pattern with the ligand, but the chemical shifts change slightly (Table 3). The decrease in chemical shift of almost protonswas explained as the impact of bonding between the metal center and ligands through -OH phenol and -NH amine.
| IC50 Values (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (-) | Fungus | |||||
| Staphylococ cusaureus |
Bacillus subtilis |
Lactobacillu sfermentum |
Salmonella enterica |
Escherichia coli |
Pseudomona saeruginosa |
Candida albican |
|
| QEt | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| CuQEt | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| NiQEt | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| CoQEt | >128 | 0.69 ± 0.02 | >128 | >128 | >128 | >128 | >128 |
| ZnQEt | >128 | 0.5 ± 0.03 | >128 | >128 | >128 | >128 | >128 |
| CdQEt | >256 | 21,94 ± 2,59 | 1,87 ± 0,13 | >256 | >256 | >256 | 34,21 ± 1,90 |
| HgQEt | 15.5 ± 0.35 | 0.69 ± 0.02 | 6.00 ± 0.17 | 56.0 ± 1.05 | 18.45 ± 0.33 | >128 | 8.0 ± 0.19 |
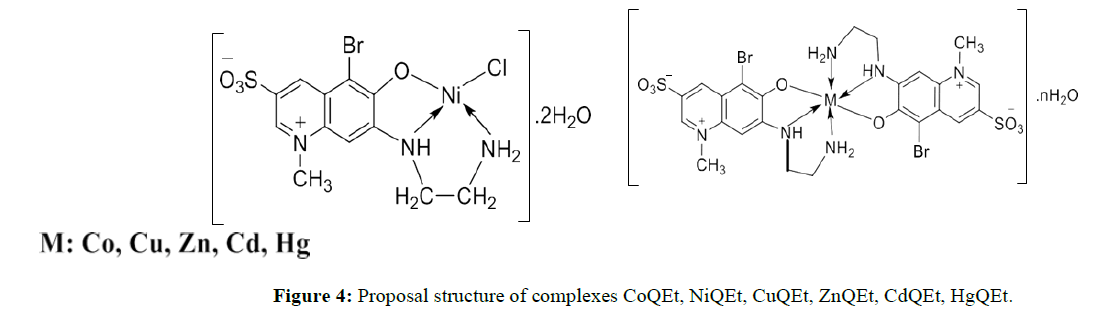
The strong decrease of chemical shift from 8.19 ppm (equivalent to –NH3+ group) to about 7.90 ppm and from 7.85 ppm (-NH, triplet) to about 7.70 ppm (singlet) are evident that QEt coordinates with metal ion through -NH and -NH2. Additionally, the 1H NMR spectra of ZnQEt, CdQEt and HgQEt appearing only one set of signals indicates that two ligands in the complexes are similar and symmetry. In 1H NMR spectrum of NiQEt complex, only one set of signals appears clearly and based on the dark red color of the compound, which shows that NiQEt complex is diamagnetic so that confirms the Ni(II) complex is square planar (Figure 4).
The combination of IR, 1H NMR, EDX, ESI-MS spectra and thermal analysis, the structure of complexes were proposed: CoQEt, ZnQEt, CdQEt, CuQEt, HgQEt have same structure with ratio of metal : ligand is 1:2 while NiQEt complex is square planar with the ratio 1:1.
Antimicrobial activity
The ligand and six complexes were tested the antimicrobial activity to both Gram positive and Gram negative strains like Staphylococcus aureus, Bacillus subtilis, Lactobacillus fermentum, Salmonella enterica, Escherichia coli, Pseudomonas aeruginosa and Candida albicans fungus. The results show that the ligand, NiQEt and CuQEt have no effect on the microorganisms. In contrast, the CoQEt, ZnQEt complexes show a great inhibition to Gram (+) Bacillus subtilis (IC50=0.69 and 0.50 μg/mL) while the complex of CdQEt has ability to inhibit Gram (+) Bacillus subtilis, Candida albican fungus and Gram (+) Lactobacillus fermentum with the moderate to low IC50 values are 21,94, 34,21 and 1,87 μg/mL respectively. Especially, the complex HgQEt exhibits the excellent activities against six types of microorganism, (IC50=0.69 ÷ 56.0 μg/mL), most effectively inhibits Gram (+) Bacillus subtilis with the value of IC50 is 0.69 μg/mL (Table 3).
Conclusion
In this work, we have successfully synthesized an ewquinoline derivative, 1-methyl-5-bromo-6-hydroxy- 7-ethylendiamin-3-sulfoquinoline(QEt) as well as six complexes of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) with this ligand: [M(QEt-1H)2].nH2O (M is Co, Cu, Cd, Zn, Hg) and [Ni(QEt-1H)Cl]. 2H2O. Based on physical measurement such as IR, ESI-MS, 1HNMR, EDX, thermal analysis, the structure of six complexes was initiatively determined. In most complexes, the mole ratio between metal center:ligand is 1: 2, exceptionally, the ratio is 1:1 in NiQEt complex. The center metals coordinate with the ligand through -OH and -NH,NH2.
The bioactivity tests show that QEt, CuQEt, NiQEt have no bioactivity to microorganisms while CoQEt, ZnQEt show effective impact on Bacillus subtilis with IC50 value <1.0 μg/mL, CdQEt possesses the antimicrobial activity to Gram (+) Bacillus subtilis, Candida albican fungus and Gram (+) Lactobacillus fermentum with IC50 value is 1.87 ÷ 34.51 μg/mL, HgQEt shows further great antimicrobial activity that inhibits six types of microorganisms with IC50 = 0.6 ÷ 56 μg/mL.
References
- Li G, Wu J, Song J et al., J Power Sources, 2021. 48:p. 228857.
- Fu Z, Qin J, Wang Y et al., Dyes and pigments, 2021. 185: p. 108896.
- ]Weyesa A and Mulugeta E. RSC Advances, 2020. 10: p. 20784.
- Jianing S, Yuqin Z, Weidong Z et al, Bioorganic & Medicinal Chemistry, 2020.p. 115856.
- Hai-Gen F, Zhi-Wen L, Xin-Xin H et al., Molecules, 2019. 24: p. 548.
- Rashmi S, Parteek K and Anil K. JChemSci, 2018. 130(6).
- Nijssen S, Fluit A, van de Vijver D et al., J., Intensive Care Med, 2010. 36(3): p. 512-519.
- Li X, TjalkensR, ShresthaR et al., Chemical Biology & Drug Design, 2019. 94: p. 1711- 1720.
- DuarteY, FonsecaA, GutiérrezM et al., Medicinal Chemistry & Drug Discovery, 2019. 4: p. 551-558.
- ChaudharyNK, GuragainB, ChaudharySK et al., Bibechana, 2021. 18(1): p. 218- 230.
- AdelekeAA, ZamisaSJ, IslamS et al., Molecules, 2021. 26: p. 1205.
- VenugopalN, Krishnamurthy G, BhojyanaikHS et al., Inorganic and Nano-Metal Chemistry, 2020.p. 1-11.
- Ramachandran E, Gandin V, Bertani R et al., Molecules, 2020. 25(8): p. 1868.
- Nguyen HD, Le VC, Nguyen MT et al., Heterocycles, 2012. 85(3): p. 627 – 637.
- Le THH, Nguyen TNV, Ngo TC et al., J Fluor, 2020. 31(1):p. 195-208.
- Hadacek F andGreger H, Phytochemical Analysis, 2000. 90: p. 137-147.
- Cos P, Maes L, Sindambiwe JB et al., Trieste: Unido‑Ics.2006. p.19–28
- Cos P, Vlietinck AJ, Vanden BD., Maes L., JEthnopharma, 2006. 106(3): p. 290-302.
- Moosavinejad SM, Madhoushi M, Vakili M et al., Ciencia y Tecnología, 2019. 21(3): p. 381-392.
- Aljerf L and Nadra R., Int J Nanomanufacturing, 2019. 15(3): p. 269.
- Bartošová A, Blinová L, Sirotiak M et al., 2017. 25(40): p. 103-111.
- Reiss A, Samide A, Ciobanu G et al., J Chilean Chem Society, 2015. 60(3): p. 3074-3079.