Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 7
Synthesis, Spectral, Theoretical, Antimicrobial Evaluation, and Molecular Docking Studies of Some Novel Ethyl 5-(1-Methyl-1h-Tetrazol-5-Ylthio)-4-Oxo-2,6-Substituted Diphenylpiperidine-3-Carboxylate Derivatives
Srikanth R1,2, Venkatesan CS2, Sugumar P3, Sivarajan A1 and Ponnuswamy MN3*
1Department of Chemistry, Government Arts College, Tiruchirappali – 620 022, India
2Research and Development, Gland Pharma Ltd., Hyderabad 500 049, India
3Centre of Advanced Study in Crystallography & Biophysics, University of Madras, Guindy Campus, Chennai-600 025, India
- *Corresponding Author:
- Ponnuswamy MN
Centre of Advanced Study in Crystallography & Biophysics
University of Madras
Guindy Campus, Chennai-600 025, India
Abstract
A new series of heterocyclic compounds containing both N-methyl thiotetrazole (NMTT) and piperidine nuclei together, namely ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-caboxylate derivatives, were synthesized by cyclocondensation of ethyl 4-(1-methyl-1H-tetrazol-5-ylthio)-3-oxobutanoate with ammonium acetate and substituted aromatic aldehydes. The synthesized N-methylthio tetrazole substituted piperidine-4-one derivatives were evaluated for their antimicrobial activity using disc diffusion method. The structures of the synthesized compounds were characterized by Infrared Radiation (IR), Proton Nuclear Magnetic Resonance (1H-NMR), Carbon-13 (C13) Nuclear Magnetic Resonance (13C-NMR), 2D-NMR and mass spectral data. The antimicrobial activity reveals that several compounds exhibit good antibacterial activity when compared with the reference drug. Molecular docking studies were performed against malarial activities.
Keywords
Piperidone, Cyclo condensation, Antimicrobial activity, Molecular docking.
Introduction
A wide variety of heterocyclic systems have been explored for the development of pharmaceutically important activities. Heterocycles form by far the largest of the classical divisions of organic chemistry [1]. Generally N-based heterocycles have been the object of considerable focus since N-atom containing heterocycles are the structural components of many bioactive natural products such as vitamins, hormones, antibiotics, alkaloids, glycosides, and other compounds which play significant roles in human and animal health [2]. Many natural drugs such as quinine, papaverine, emetine, theophylline, atropine, codeine, morphine, and reserpine are N-containing heterocycles [3]. Therefore, N-containing heterocycles are especially considered as “privileged” structures for the synthesis and development of new drugs [4,5]. Tetrazoles and their derivatives have been reported as antibacterial [6], antiviral [7], herbicidal [8], anti-inflammatory [9], antitumor [10], analgesic [11], and antiproliferative [12] agents. Especially N-Methylthiotetrazoles (NMTT) containing side chains are present in many of the bioactive heterocyclic compounds that are of wide interest owing to their diverse biological, pharmaceutical and clinical applications.
Antibiotics that contain a 1-N-methyl-5-thiotetrazole (MTT) side group have been associated with hypoprothrombinemia [13]. Cefmetazole is a cephalosporin with a broad spectrum of antimicrobial activity. The structure of cefmetazole is similar to those of moxalactam, cefoperazone, cefotetan, and cefamandole in that all of these agents contain an N-methylthiotetrazole (NMTT) side chain. This side chain has been reported to dissociate from its parent molecule and has been implicated in the development of disulfiram-like reactions and changes in prothrombin time (1,7,9,11,12). It has been postulated that NMTT side chain which is cleaved from the cephalosporin is responsible for inhibition of vitamin K metabolism and subsequent development of antibiotic-associated hypoprothrombinemia (7,8,11,12,15).
In this context, a new series of novel heterocyclic compounds containing both N-methyl thiotetrazole and piperidine nuclei together, namely ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-caboxylate derivatives (1–9), were synthesized by the treatment of the ethyl 4-(1-methyl-1H-tetrazol-5-ylthio)-3-oxobutanoate with substituted aldehydes and ammonium acetate at room temperature. The ambition of the study is to provide an overview of pharmacological activity associated with the presence or influence of tetrazole containing moiety in the piperidine nuclei.
Herein, we wish to report the synthesis, characterization, theoretical, antimicrobial evaluation of the series of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-caboxylate derivatives. The structural confirmation was carried out using Fourier Transform Infrared (FTIR), Proton Nuclear Magnetic Resonance (1H-NMR), Carbon-13 (C13) Nuclear Magnetic Resonance (13C-NMR), 2D-NMR (1H-1H COSY, 1H-13C correlation spectroscopy) and theoretical calculations. Molecular docking studies were also performed on ethyl 5-(1-methyl-1Htetrazol- 5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-caboxylate derivatives with the targeted GTP binding protein.
Materials and Methods
Experimental procedure
General
The chemicals, benzaldehyde, 3,4,5-trimethoxy benzaldehyde, 3,5-dimethyl benzaldehyde, 3,5-dichloro benzaldehyde and 4- bromobenzaldehyde were purchased from Sigma Aldrich and used without further purification. Piperanol, 3-flurobenzaldehyde, 4- flurobenzaldehyde and 2-bromo-3-fluoro benzaldehyde were purchased from M/s SD Fine Chemicals (Mumbai, India) with the highest grade of purity. Silica gel (60–120 mesh) for column chromatography was purchased from M/s Acme Synthetic Chemicals (Mumbai, India) and pre-coated TLC plates (Silica gel 60F254) from Merck (Darmstadt, Germany). Melting points were determined in open capillary tubes and are uncorrected. IR spectra were taken as KBr pellets for solids on a Shimadzu IR Prestige-21 model. 1H-NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in CDCl3 solutions with TMS as an internal standard on a Bruker Avance instrument. Mass spectra were recorded on an Agilent Technologies 6310 Ion trap LC/MS.
Synthesis of Ethyl 5-(1-methyl-1H-tetrazol-5ylthio)-6-(benzo[d][1,3]dioxol-5-yl)-2-(benzo[d][1,3]dioxol-6-yl)-4-oxopiperidine-3-carboxylate, 2
To a solution of ammonia (0.38 ml, 25%, 2.12 mmol) and (L)-proline (73 mg, 0.636 mmol) in ethanol (3 ml), freshly distilled piperonal (638 mg, 4.24 mmol) and tetrazole ketone (500 mg, 2.12 mmol) were added. Then the resultant reaction mixture was stirred at room temperature until the mixture became a viscous liquid. To this ether (20 ml) was added and treated with aqueous hydrochloric acid [15 ml; 1: 1 (v/v)]. The hydrochloride salt of the crude product was filtered and washed with ether. The base was obtained from an alcoholic solution of the hydrochloride by adding a slight excess of aqueous ammonia and diluted with water at 0ºC. The resulting product was recrystallized from 3: 7 methanol-ethyl acetate mixtures (Yield: 65%). The compounds 1-9 were obtained using the same procedure. The present study reports the preparation, characterization and pharmacological evaluation of bis heterocycles containing piperidine moiety in combination with N-methylthiotetrazole. The schematic diagram of the synthesis is shown in Scheme 1.
Results and Discussion
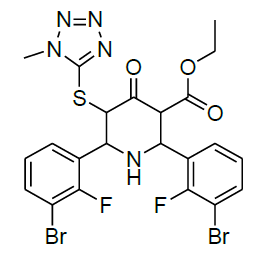
The present study describes synthesis and structural characterization of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio) substituted derivatives of piperdin-4-ones (Table 1). The ethyl 5-(1-methyl-1H-tetrazol-5-ylthio) substituted derivatives 1-9 were synthesized by the one pot condensation of ethyl 4-(1-methyl-1H-tetrazol-5-ylthio)-3-oxobutanoate with ammonium acetate and substituted aromatic aldehydes. The formation of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio) substituted derivatives of piperdin-4-ones was confirmed by IR, NMR and Mass spectral data. The results are presented in the experimental section.
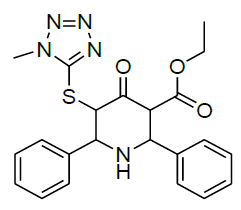 |
 |
| Compound 1 | Compound 2 |
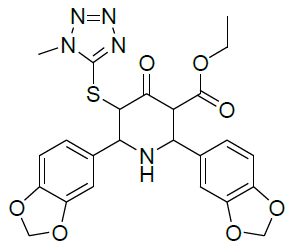
 |
 |
| Compound 3 | Compound 4 |
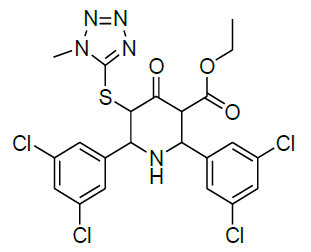 |
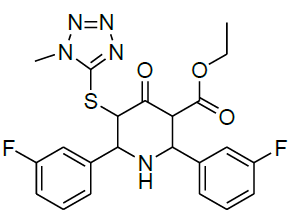 |
| Compound 5 | Compound 6 |
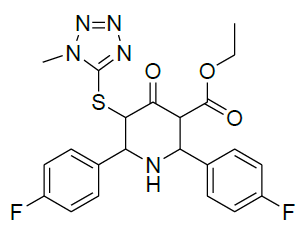 |
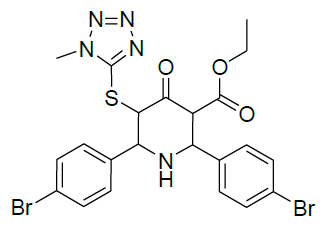 |
| Compound 7 | Compound 8 |
 |
|
| Compound 9 | |
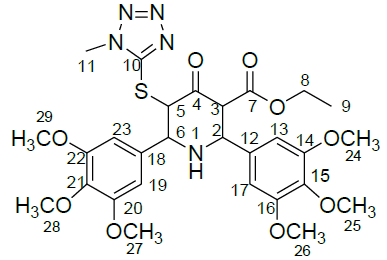
Table 1: Structure of compounds 1-9; the numbering assignment is shown in Compound 3
FTIR spectral analysis
The IR spectral analysis of compounds 1-7 were carried out to investigate the presence of functional groups and their vibration modes. The IR spectrum was recorded in the range 400-4000 cm-1 at room temperature using Shimadzu IR Prestige-21 model spectrophotometer. The IR spectrum of all compounds showed a characteristic signal at around 1665-1720 and 1730-1750 cm-1 attributing for keto and ester functionality, respectively. Besides, a band around 3065-3030 cm-1 indicates the aromatic C-H stretching vibration. In addition, a band observed in the region 3330-3410 cm-1 is due to NH group of the piperidine ring. Apart from these, other signals due to C-H and C=N functionality confirmed the structure of the present compounds.
1H and 13C-NMR analysis
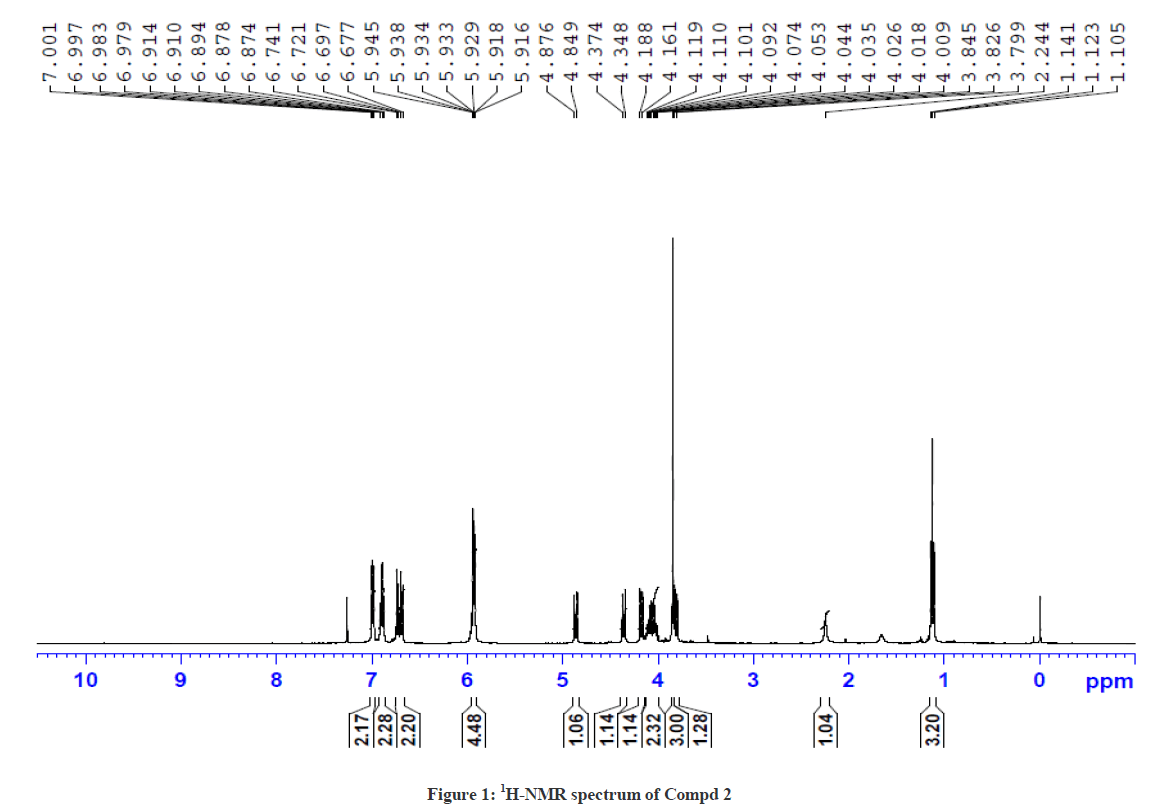
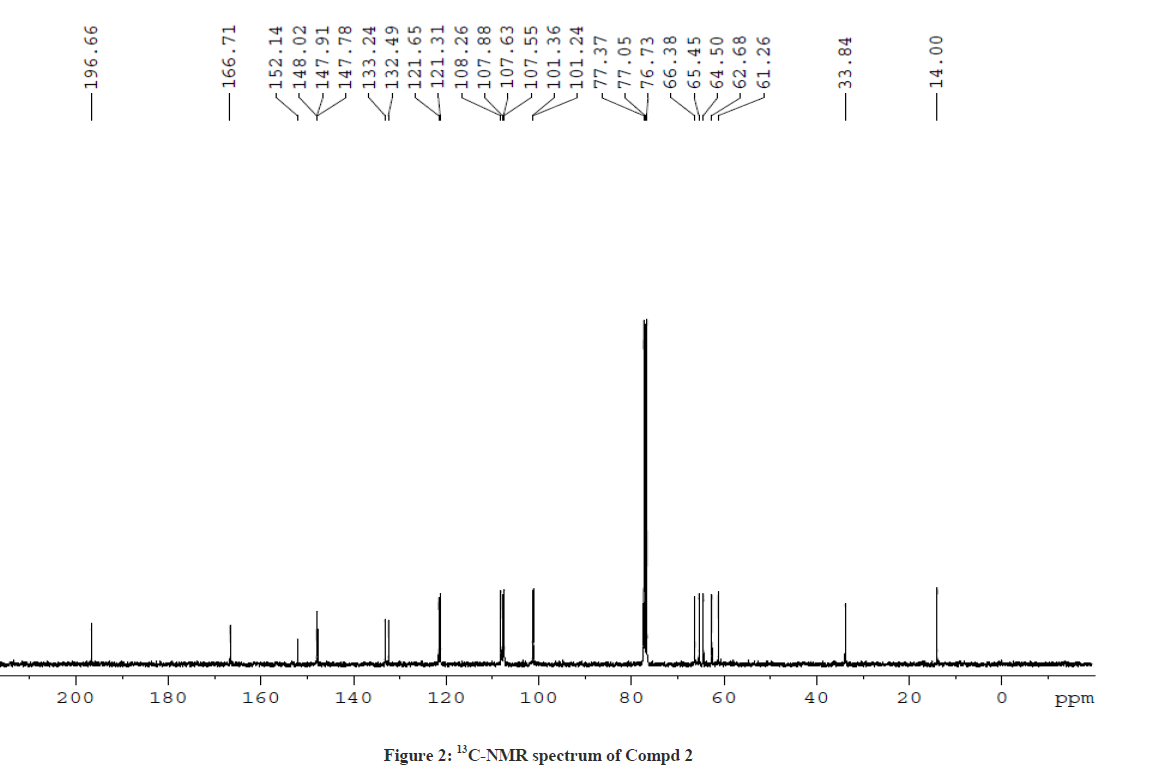
The 1H-NMR spectrum of Compound 2 showed a triplet at δ 1.12 ppm and a multiplet at δ 4.11 ppm corresponding to ethyl side chain (ester) of piperidin-4-one. In addition, the doublets at δ 4.86 ppm (J =10.8 Hz) and δ 4.17 ppm (J = 10.8 Hz) correspond to the protons at C-5 and C-6 positions, respectively. The deshielding effect of C-5 proton is due to the attachment of hetero sulphur atom. The singlet at δ 3.85 ppm corresponds to N-methyl proton of the thiotetrazole side chain. The aromatic and the piperanol methylene protons at δ 5.93 ppm were also observed. The 13C-NMR spectrum of Compound 2 showed a signal at δ 196.7 ppm and δ 166.71 ppm corresponding to the keto group in the piperidin-4-one ring and the ester carbonyl function of the side chain, respectively. The signals observed at δ 61.3 ppm and δ 101.3 ppm were attributed for the methylene protons of the ester side chain and the piperanol ring, respectively. Besides, the other signals due to phenyl ring carbons were also observed. This was further confirmed by DEPT-135 NMR and Mass spectral studies. The 1H-NMR and 13C-NMR spectra of Compound 2 are shown in Figures 1 and 2, respectively.
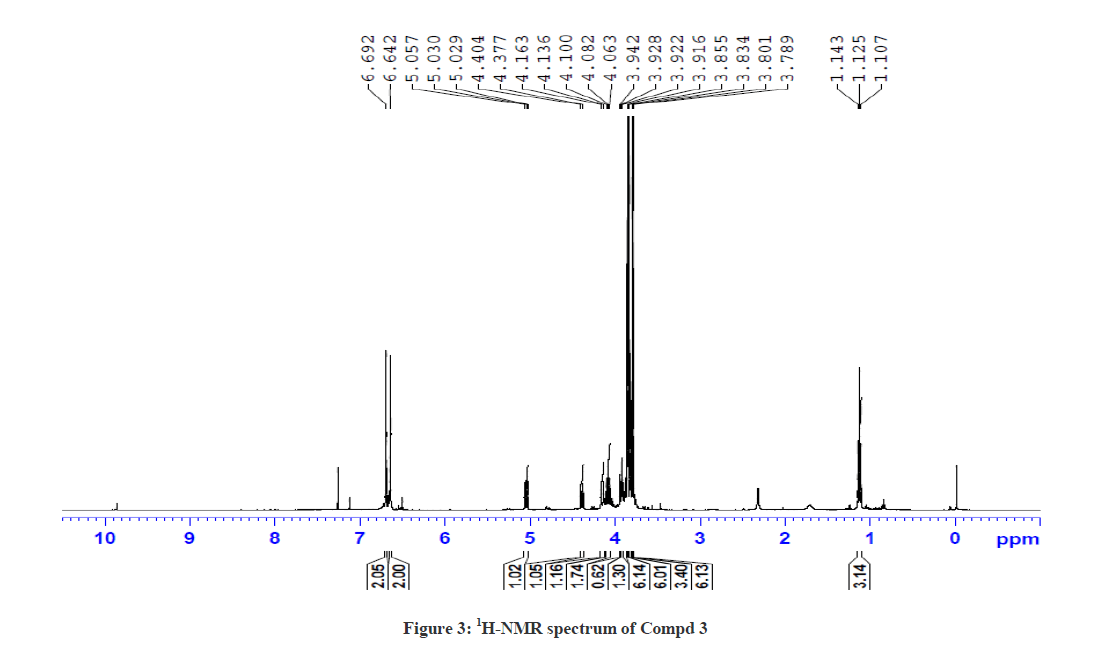
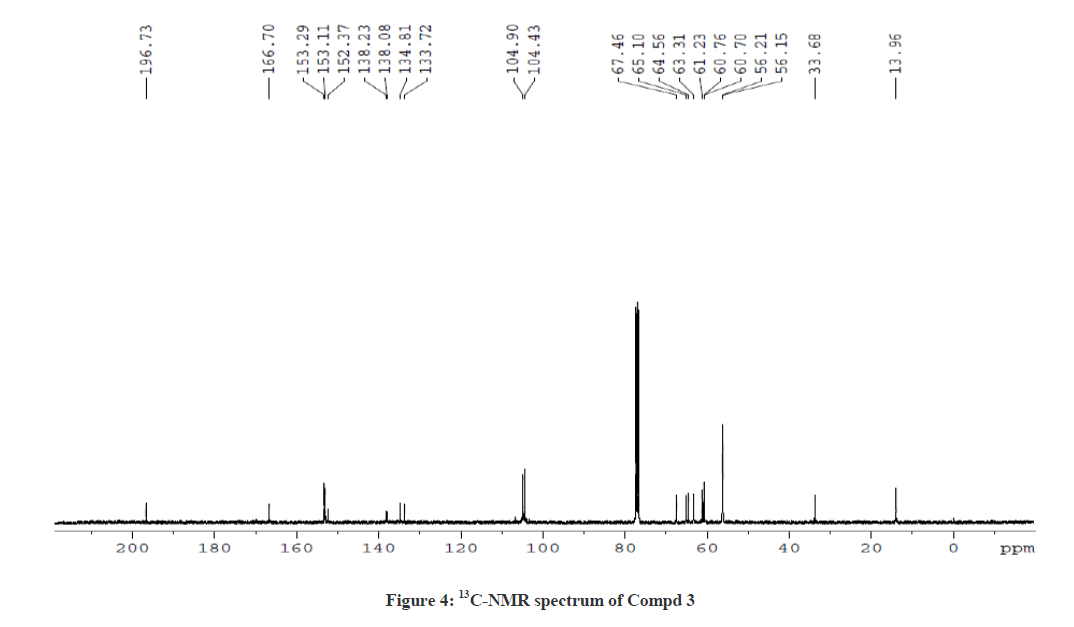
The 1H-NMR spectrum of Compound 3 showed a triplet at δ 1.12 ppm and a multiplet at δ 4.08 ppm corresponding to ethyl side chain (ester) of piperidin-4-one ring. In addition, the doublets at δ 5.04 ppm (J =10.8 Hz) and δ 4.39 ppm (J = 10.8 Hz) correspond to the protons at C-5 and C-2 positions, respectively. The downfield shift of C-5 proton in Compound 3 also shows a similarity with Compound 2, which further ascertain the attachment of proton to the hetero sulphur atom. The singlet at δ 3.80 ppm corresponds to the N-methyl proton of the thiotetrazole side chain. The aromatic protons and the O-methoxy protons at δ 3.78 ppm, δ 3.83 ppm and δ 3.86 ppm were also observed. The 13C-NMR spectrum of Compound 3 showed a signal at δ 196.7 ppm and δ 166.71 ppm corresponding to the keto group in the piperidin-4-one ring and the ester carbonyl function of the side chains, respectively. The signal observed at δ 61.24 ppm was attributed for the methylene protons of the ester side chain. Besides, the other signals were also observed due to phenyl ring carbons. This was further confirmed by DEPT NMR and Mass spectral analysis. The 1H-NMR and 13C-NMR spectra of Compound 3 are also shown in Figures 3 and 4, respectively.
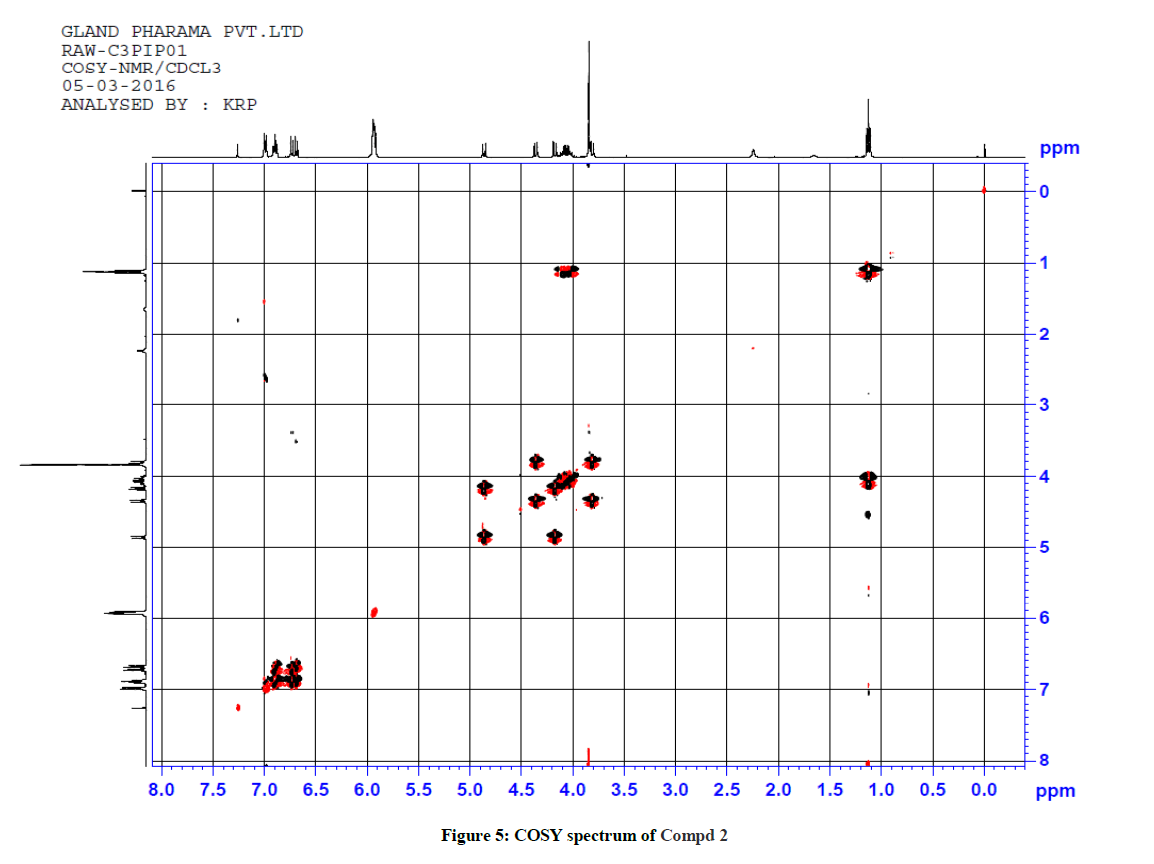
For ethyl 5-(1-methyl-1H-tetrazol-5ylthio)-6-(benzo[d][1,3]dioxol-5-yl)-2-(benzo[d][1,3]dioxol-6-yl)-4-oxopiperidine-3-carboxylate 2, 1H-1H COSY and 1H-13C HSQC spectra were recorded to confirm the assignments. The COSY spectrum reveals that the upfield signal at δ 1.12 ppm is unambiguously assigned at side chain ethyl hydrogen H(9) and it shows a cross peak with the multiplet at δ 4.11 ppm. Therefore, the signal at δ 4.11 ppm is obviously due to H(8) only. The doublet signals at δ 3.81 ppm H(3) shows cross peak with δ 4.36 ppm for H(2), indicating that both are adjacent to each other. Similarly, the doublet signals at δ 4.86 ppm H(5) correlates with a signal at δ 4.17 ppm H(6), which also indicate the positions adjacent to each other. The signal at 4.86ppm corresponding to H(5) was unambiguously assigned based on the evidence of connectivity with the hetero sulphur atom and HMBC correlation experiments. Besides, other correlations also confirmed the structure of the compound.
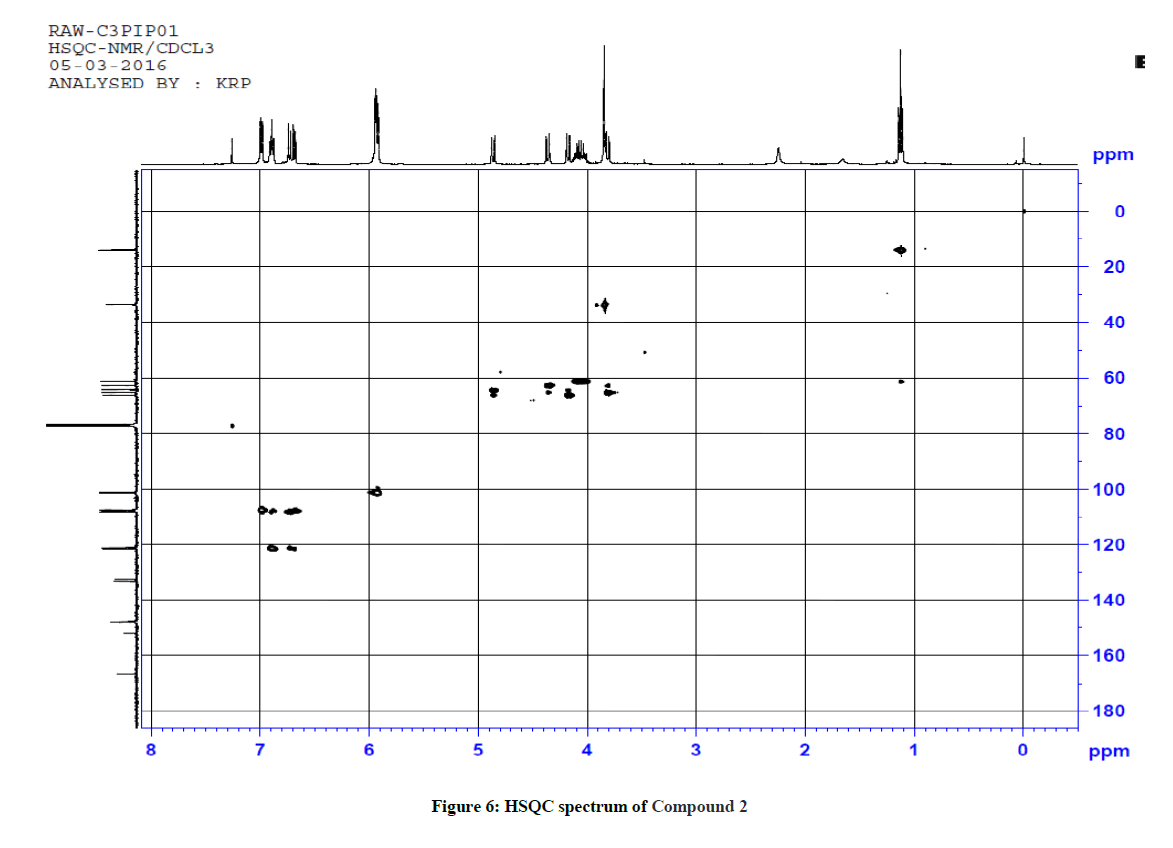
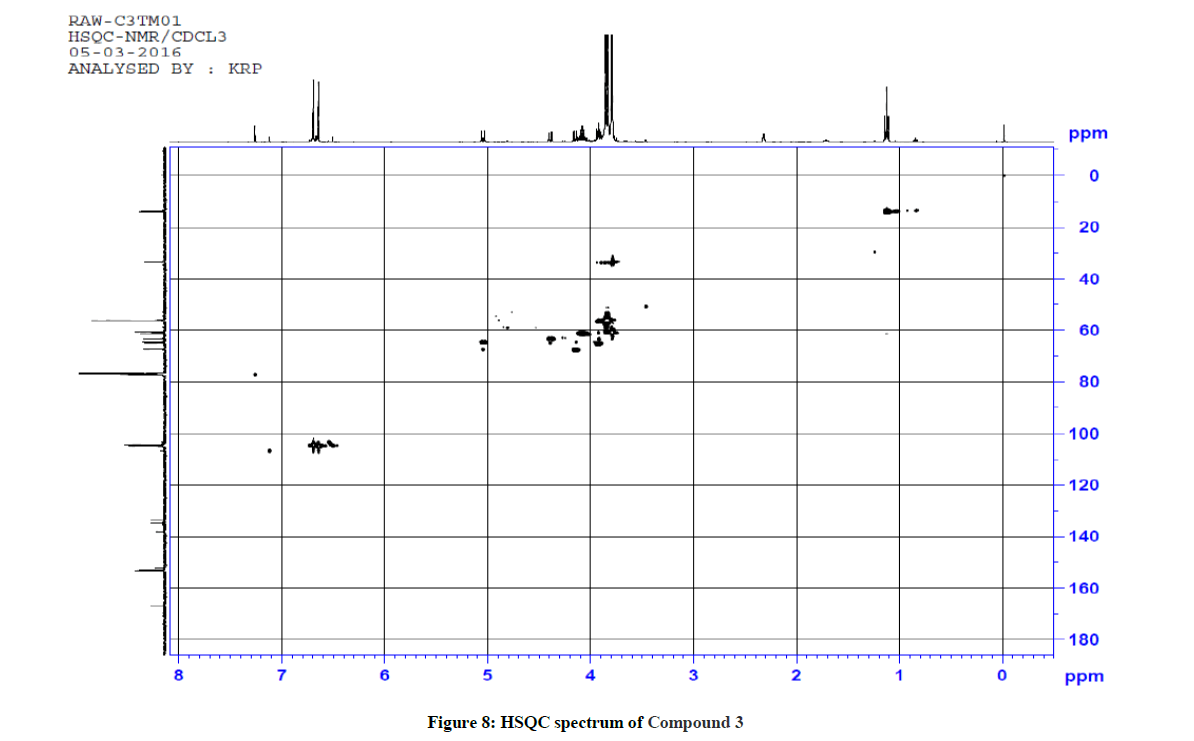
1H-13C HSQC shows a cross peak at δ 1.12 ppm and 14.0 ppm indicates the –CH3 group of the ester side chain. Similarly H(3), H(5), H(6), H(2), H(24) and H(25) are correlated with the corresponding carbons, further confirming the structure of the compound. The observed correlations in HSQC and HOMOCOSY spectra are given in Table 2. The COSY and HSQC spectra of compound 2 are shown in Figures 5 and 6, respectively.
| 1H-NMR signal | Correlation in HSQC | Correlation in HOMOCOSY |
|---|---|---|
| 1.12 (H(9)) | 14.00 (C(9)) | 4.11 (H(8)) |
| 3.81 (H(3)) | 65.45 (C(3)) | 4.36 (H(2)) |
| 4.11 (H(8)) | 61.26 (C(8)) | 1.12 (H(9)) |
| 4.17 (H(6)) | 66.38 (C(6)) | 4.86 (H(5)) |
| 4.36 (H(2)) | 62.68 (C(2)) | 3.81 (H(3)) |
| 4.86 (H(5)) | 64.50 (C(5)) | 4.17 (H(6)) |
| 3.84 (H(11)) | 33.84 (C(11)) | - |
| 5.92 (H(24,25)) | 101.24, 101.36, (C(24,25) | - |
| 6.71 (H(14,22)) | 107.88, 108.26 (C(14,22)) | 6.89 (H(23,13)) |
| 6.89 (H(23,13)) | 121.31, 121.65 (C(23,13)) | 6.71 (H(14,22)) |
| 6.99(H(17, 19)) | 107.55, 108.63 (C17, 19) | - |
Table 2: Correlation in HSQC and HOMOCOSY of Compd 2 (δ, ppm)
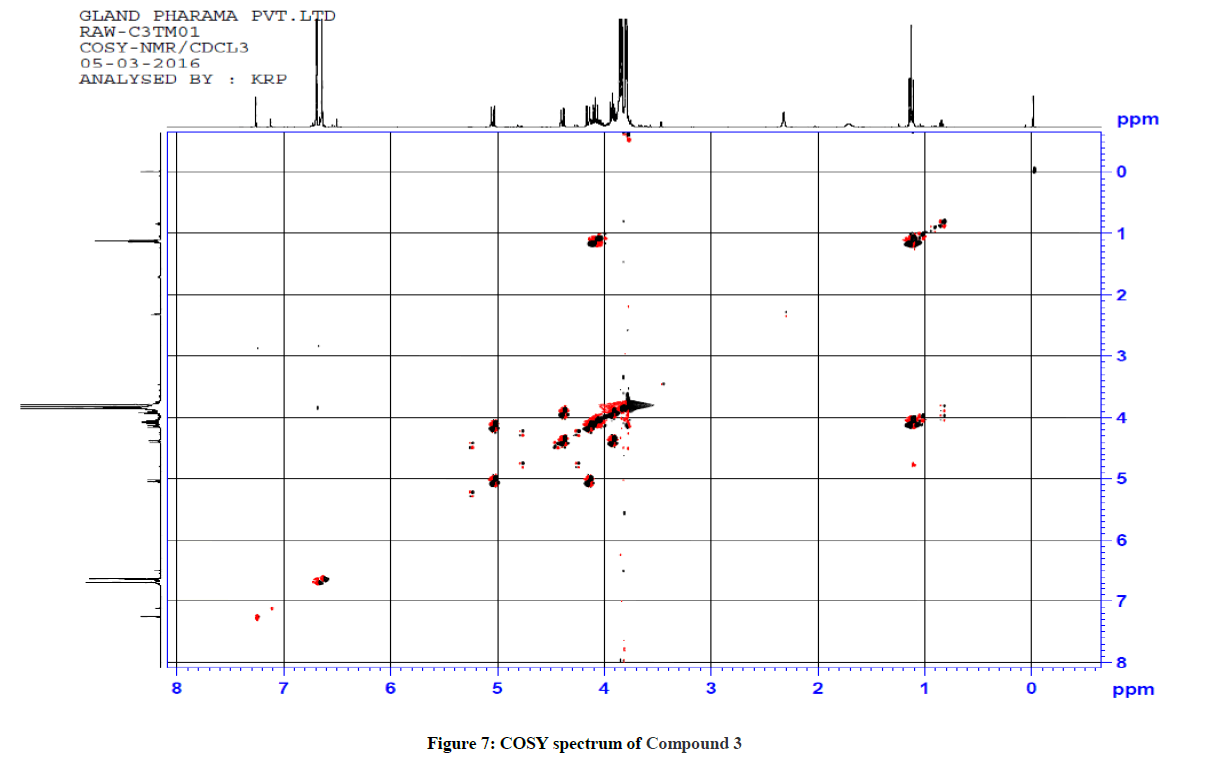
Similarly for compound 3 (Ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(3,4,5-trimethoxyphenyl)-4-oxopiperidine-3-carboxylate), 1H-1H COSY and 1H-13C HSQC spectra were recorded to reconfirm the assignments in the presence of other substituents. The observed correlations in HSQC and HOMOCOSY spectra of compound 3 are given in Table 3. The COSY and HSQC spectra of compound 3 are shown in Figures 7 and 8, respectively. The IR spectral and analytical data of compounds 1-7 are presented in Tables 4 and 5, respectively.
| 1H-NMR signal | Correlation in HSQC | Correlation in HOMOCOSY |
|---|---|---|
| 1.12 (H(9)) | 13.96 (C(9)) | 4.08 (H(8)) |
| 3.93 (H(3)) | 65.10 (C(3)) | 4.39 (H(2)) |
| 4.08 (H(8)) | 61.24 (C(8)) | 1.12 (H(9)) |
| 4.10-4.16 (H(6)) | 67.46 (C6)) | 5.04 (H(5)) |
| 4.39 (H(2)) | 63.31 (C(2)) | 3.93 (H(3)) |
| 5.04 (H(5)) | 64.56 (C(5)) | 4.10-4.16 (H(6)) |
| 6.64 (H(13)) & H(17)) | 104.89 (C(13)) & (C(17)) | - |
| 6.69 ((H19)) & H(23)) | 104.43 (C(19)) & (C(23)) | - |
Table 3: Correlation in HSQC and HOMOCOSY of Compd 3 (δ, ppm)
| Compound No | Aromatic C-H str cm-1 | Aliphatic C-H str cm-1 | C=N str | C=O (keto) cm-1 | C=O (ester) cm-1 | N-H str cm-1 |
|---|---|---|---|---|---|---|
| 1 | 3061, 3028 | 2976, 2941, 2820 | 1595 | 1718 | 1749 | 3311 |
| 2 | 3065 | 2936 | 1653 | 1701 | 1740 | 3329 |
| 3 | 3014 | 2978, 2955, | 1657 | 1718 | 1749 | 3309 |
| 4 | 3003 | 2968, 2940, 2843 | 1627 | 1719 | 1749 | 3316 |
| 5 | 3070 | 2986, 2890 | 1630 | 1669 | 1730 | 3406, 3304 |
| 6 | 3061, 3038 | 2982, 2945, 2822 | 1657 | 1719 | 1750 | 3333 |
| 7 | 3061 | 2982, 2941, 2812 | 1649 | 1719 | 1748 | 3312 |
Table 4: Characteristic IR stretching frequencies (cm-1) of compounds 1–7
Ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-diphenylpiperidine-3-carboxylate (1)
1H-NMR (400 MHz, CDCl3) δ(ppm)=0.88 (t, 3H, 9-CH3), 3.90-3.96 & 3.99-4.05 (m, 2H, 8-CH2), 3.68 (s, 3H, 11H, N-Methyl proton), 4.65 (m, 2H, 3&6-CH), 4.93-4.95 (m, 2H, 2&5-CH), 7.18-7.39 (m, 10H, aromatic), 12.13 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ(ppm)=13.5 (C-9), 33.5(C-11, N-CH3), 55.0 (C-2), 58.4 (C-6), 59.1 (C-3), 59.9 (C-5), 60.7 (C-8), 126.9 (C-13 & C-17), 127.6 (C-14 & C-16), 128.0 (C-15), 128.1 (C-19 & C-23), 128.3 (C-20 & C-22), 128.7 (C-21), 137.4 (C-12), 137.9 (C-18), 153.4 (C-10), 170.7 (C-7, C=O, Ester), 197.9 (C-4, C=O, Keto) ppm; MS (ESI): calculated for C22H23N5O3S: 437.51; Observed Mass: 438.1 (M+H)+
Ethyl 5-(1-methyl-1H-tetrazol-5ylthio)-6-(benzo[d][1,3]dioxol-5-yl)-2-(benzo[d][1,3]dioxol-6-yl)-4-oxopiperidine-3-carboxylate (2)
1H-NMR (400 MHz, CDCl3) δ(ppm)=1.12 (t, 3H, 9-CH3), 2.2 (1H, NH), 3.81 (d, J = 10.8 Hz, 1H, 3-CH), 3.84 (s, 3H, 11-CH, N-Methyl proton), 4.01-4.12 (m, 2H, 8-CH2), 4.17 (d, J = 10.8 Hz,1H, 6-CH), 4.36 (d, J = 10.4 Hz, 1H, 2-CH), 4.86 (d, J = 10.8 Hz, 1H, 5-CH), 5.93 (m, 4H, 24 & 25-CH2, 6.68-6.74 (2d, 2H, J = 17.6 Hz & 8 Hz, 14&22-CH, aromatic), 6.87-6.91 (m, 2H, J = 8 Hz & 1.6 Hz, 13&23-CH, aromatic), 6.98-7.00 (d, 2H, J = 7.2 Hz & 1.6 Hz, 17&19-CH, aromatic); 13C-NMR (100 MHz, CDCl3) δ(ppm)=14.0 (C-9), 33.7 (C-11, N-CH3), 61.3 (C-8), 62.7(C-2), 64.5 (C-5), 65.45 (C-3), 66.4 (C-6), 101.2 & 101.4 (C-24 & C-25, CH2), 107.55 & 108.63 (C-17 & C-19, aromatic), 107.88 & 108.26 (C-14 & C-22, aromatic), 121.31 & 121.65 (C-13 & C-23, aromatic), 132.5 (C-12), 133.2 (C-18), 147.8 (C-15 & C-21), 147.9 (C-16 & C-20), 152.1 (C-10),166.7 (C-7, C=O, Ester), 196.7 (C-4, Keto), ppm; MS (ESI): calculated for C24H23N5O7S ; 525.53; Observed Mass: 526.3 (M+H)+
Ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(3,4,5-trimethoxyphenyl)-4-oxopiperidine-3-carboxylate (3)
1H NMR (400 MHz, CDCl3) δ(ppm)=1.13 (t, 3H, J = 7.2 Hz, 9-CH3), 3.79 (S, 6H, 24 & 26-OCH3), 3.80 (s, 3H, N-Methyl proton), 3.83 (s, 6H, 25 & 28-OCH3), 3.86 (s, 6H, 27 & 29-OCH3), 3.93 (d, 1H, 3-CH), 4.09 (q, 2H, 8-CH2), 4.15(d, 1H, 6-CH), 4.39 (d, 1H, J = 10.8 Hz, 2-CH), 5.04 (d, 1H, J = 10.8 Hz, 5-CH), 6.64 (s, 2H, 13 & 17-CH aromatic proton), 6.69 (s, 2H, 19 & 23-CH aromatic proton); 13C-NMR (100 MHz, CDCl3): δ 13.96 (C-9), 33.68 (N-CH3), 56.15 (C-27 & C-29), 56.21 (C-24 & C-26), 60.70 (C-25), 60.76 (C-28), 61.23 (C-8), 63.31 (C-2), 64.6 (C-5), 65.1 (C-3), 67.5 (C-6), 104.43 (C-19 & C-23 aromatic), 104.90 (C-13 & C-17 aromatic), 133.72 (C-12), 134.81 (C-18), 138.08 (C-15), 138.23 (C-21), 153.11 (C-14 & C-16), 153.29 (C-10, C-20 & C-22), 166.70 (C-7, C=O, Ester), 196.73 (C-4, C=O, Keto) ppm; MS (ESI): calculated for C28H35N5O9S, 617.7; Observed Mass: 618.4 (M+H)+
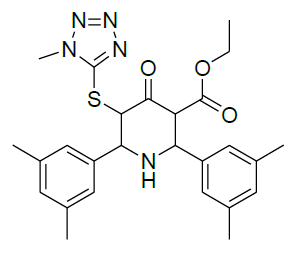
Ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(3,5-dimethylphenyl)-4-oxopiperidine-3-carboxylate (4)
1H-NMR (400 MHz, CDCl3) δ(ppm)=0.96 (t, 3H, J = 7.2 Hz, 9-CH3), 2.29 & 2.39 (s, 12H, 24, 25, 26 & 27-CH3), 3.98 (s, 3H, 11-CH, N-Methyl proton), 4.01-4.15 (m, 3H, 8-CH2 & 3-CH), 5.14 (s, 2H, 2&6-CH), 5.42 (bs, 1H, 5-CH), 7.02 & 7.05 (2s, 2H, 15&21-CH, aromatic proton), 7.16 (s, 2H, 13&17-CH aromatic proton), 7.35 (s, 2H, 19&23-CH aromatic proton); 13C-NMR (100 MHz, CDCl3) δ(ppm)=13.68 (C-9), 21.30 (C-24 & C-25), 21.35 (C-26 & C-27), 34.11 (C-11), 49.25 (C-2 & C-5), 55.35 (C-3), 55.43 (C-6), 61.71 (C-8), 126.98 (C-13 & C-17), 127.38 (C-15 & C-21), 131.53 (C-19 & C-23), 133.78 (C-20 & C-22), 138.72 (C-14 & C-16), 138.88 (C-12 & C-18), 149.85 (C-10), 165.26 (C-7, C=O, Ester), 169.70 (C-4, Keto) ppm; MS (ESI): calculated for C26H31N5O3S, 493.62; Observed Mass: 494.3 (M+H)+
Ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(3,5-dichlorophenyl)-4-oxopiperidine-3-carboxylate (5)
1H-NMR (400 MHz, CDCl3) δ(ppm)=0.90 (t, 3H, J = 7.2 Hz, 9-CH3), 3.75 (s, 3H, 11-CH3, N-Methyl proton), 3.86-3.95 (m, 3H, 8-CH2 & 3-CH), 4.90 (s, 1H, J = 1.2 Hz, 6-CH), 4.96 (bs, 1H, 2-CH), 5.61 (bs, 1H, 5-CH), 7.20-7.43 (m, 6H, 13, 15, 17, 19, 21 & 23-CH aromatic protons); 13C-NMR (100 MHz, CDCl3) δ(ppm)=13.77 (C-9), 33.47 (C-11), 51.87 (C-2), 54.76 (C-5), 55.67 (C-3 & C-6), 59.24 (C-8), 91.39 (C-10), 126.80 (C-13), 127.51 (C-15), 128.52 (C-17), 129.58 (C-23), 129.87 (C-19), 130.00 (C-21), 132.91 (C-20), 133.67 (C-16), 133.87 (C-14), 134.64 (C-22), 142.04 (C-12 & C-18), 155.21 (C-10), 157.18 (C-7, C=O, Ester), 168.13 (C-4, Keto) ppm; MS (ESI): calculated for C22H19Cl4N5O3S, 575.29; Observed Mass: 575.2 (M+H)+
Ethyl-5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(3-fluorophenyl)-4-oxopiperidine-3-carboxylate (6)
1H-NMR (400 MHz, CDCl3) δ(ppm)=0.93 (t, 3H, J = 7.2 Hz, 9-CH3), 3.78 (s, 3H, 11-CH, N-Methyl proton), 3.92-4.10 (m, 3H, 8-CH2 & 3-CH), 4.64 (s, 1H, 6-CH), 4.93-4.96 (s, 2H, 2-CH & 5-CH), 6.87-7.35 (m, 8H, 13, 15, 16 & 17-CH & 19, 20, 21 & 23-CH aromatic proton), 12.15 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ(ppm)=13.57 (C-9), 33.54 (C-11), 54.31 (C-2 & C-5), 57.91 (C-3), 58.68 (C-6), 60.93 (C-8), 114.46 (C-13), 114.67 (C-15), 114.94 (C-16), 115.88 (C-17), 122.99 (C-19), 123.35 (C-20), 123.87 (C-21), 129.84 (C-23), 140.43 (C-12 & C-18), 153.18 (C-10), 161.54 (C-14), 163.98 (C-22), 167.79 (C-7, C=O, Ester), 170.47 (C-4, keto) ppm; MS (ESI): calculated for C22H21F2N5O3S, 473.5; Observed Mass: 474.1 (M+H)+, 496 (M+23)+ Sodium adduct.
Ethyl-5-(1-methyl-1H-tetrazol-5-ylthio)-2,6-bis(4-fluorophenyl)-4-oxopiperidine-3-carboxylate (7)
1H-NMR (400 MHz, CDCl3) δ(ppm)=0.92 (t, 3H, J = 7.2 Hz, 9-CH3), 3.76 (s, 3H, 11-CH, N-Methyl proton), 3.99-4.10 (m, 3H, 8-CH2 & 3-CH), 4.61 (s, 1H, 6-CH), 4.92 (bs, 2H, 2-CH & 5-CH), 6.91-7.10 (m, 8H, 13, 14, 16 & 17-CH & 19, 20, 22 & 23-CH aromatic proton), 12.10 (s, 1H, NH); 13C-NMR (100 MHz, CDCl3) δ(ppm)=13.95 (C-9), 33.80 (C-11), 54.57 (C-2 & C-5), 57.70 (C-3), 58.53 (C-6), 64.08 (C-8), 114.96 (C-13), 115.18 (C-14), 128.73 (C-16), 128.81 (C-22), 129.59 (C-23), 129.68 (C-17), 129.77 (C-19), 133.71 (C-20), 139.55 (C-12 & C-18), 153.26 (C-10), 163.51 (C-15 & C-21), 167.74 (C-7, C=O, Ester), 170.47 (C-4, Keto) ppm; MS (ESI): calculated for C22H21F2N5O3S, 473.5; Observed Mass: 474.1 (M+H)+, 495.9 (M+23)+ Sodium adduct.
Where, R= Ph, Piperanol, 3,4,5-trimethoxy, 3,5-Dimethyl, 3,5-dichloro, 3-Fluoro, 4-Fluoro, p-Bromo, 2-Fluoro-3-Bromo-
Microbiological screening
Antibacterial activity by disc diffusion assay
The antimicrobial activity of the newly synthesized ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3- carboxylate derivatives were screened against the following species:
| Grams strain | Name of the organism | Code |
|---|---|---|
| Gram-negative | Klebsiella pneumoniae | (MTCC-3384) |
| Gram negative | Escherichia coli | (ATCC-25922) |
| Gram- positive bacterium | Bacillus subtilis | (ATCC-6051) |
| Gram-positive spherical bacteria | Staphylococcus aureus | (ATCC-9144) |
Standard: Streptomycin
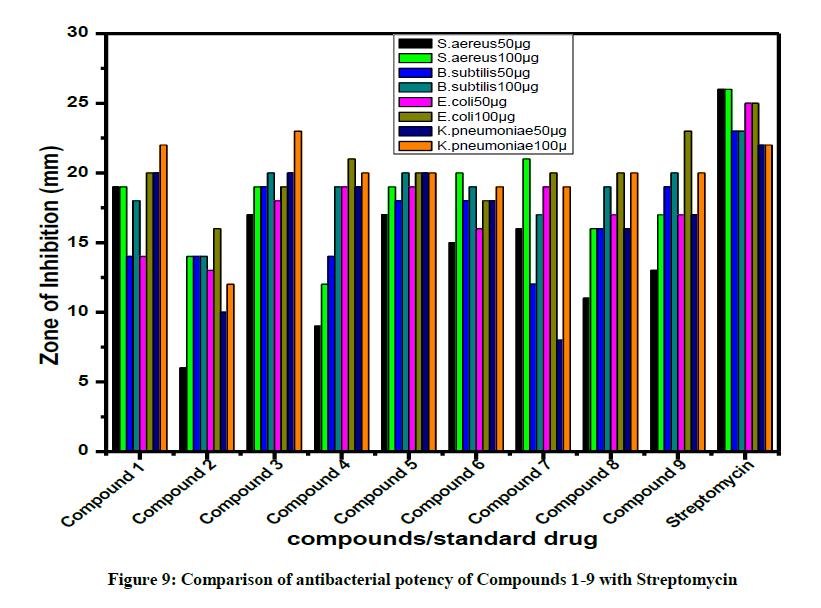
All the compounds were screened for their antibacterial activity by disc diffusion technique. Compounds are screened in-vitro for their anti-microbial activity against E. coli, S. aureus, Klebsiella pneumoniae and B. subtilis with standard drug streptomycin (30 μg) (Figure 9). The zones of inhibition formed for the compounds against organisms were calculated.
The species were obtained from Microbial Type Culture Collection (MTCC), Chandigarh, India. The commercially available Nutrient Agar and Potato dextrose agar (PDA) media used in this study were purchased from Hi Media Pvt. Ltd, Mumbai, India.
Growth media: The antibacterial activities of all test compounds were carried out by disc diffusion method. The compounds were taken in DMSO and used in the concentration of 50 μg and 100 μg /disc.
The target microorganisms were cultured in Mueller–Hinton broth (MHB). After 24 h the suspensions were adjusted to standard sub culture dilution. The Petri dishes containing Muller Hinton Agar (MHA) medium were cultured with diluted bacterial strain.
Disc made of Whatman No.1, diameter 6 mm was pre-sterilized and maintained in aseptic chamber. Each concentration was injected to the sterile disc papers. Then the prepared discs were placed on the culture medium. Standard drug streptomycin (30 μg) was used as a positive reference to determine the sensitivity of each microbial species tested. Then the inoculated plates were incubated at 37 ºC for 24 h. The diameter of the clear zone around the disc was measured and expressed in millimeters as its anti-microbial activity. The antibacterial activity of the compounds 1-9 are summarized in Tables 4-6.
| Entry | R | Yield % | Melting range (°C) | ESI Mass Observed /(Calculated) in positive mode |
|---|---|---|---|---|
| 1 | Phenyl | 60 | 136-140 | 438.1 -437.51 |
| 2 | Piperanol | 65 | 156-160 | 526.3 -525.53 |
| 3 | 3,4,5-Trimethoxy | 62 | 148-150 | 618.4 -617.7 |
| 4 | 3,5-Dimethyl | 69 | 174-178 | 494.3 -493.62 |
| 5 | 3,5-Dichloro | 66 | 167-169 | 575.2 -575.29 |
| 6 | 3-Fluoro | 71 | 162-165 | 474.1, 496 [M+Na] -473.5 |
| 7 | 4-Fluoro | 63 | 146-149 | 474.1, 495.9 [M+Na] -473.5 |
Table 5: Analytical data of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-carboxylate derivatives Compounds (1-7)
| Test Pathogens | Zone of Inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | |||||||
| Staphylococcus aereus | Bacillus subtilis | Escherichia coli | Klebsiella pneumoniae | |||||
| 50 µg | 100 µg | 50 µg | 100 µg | 50 µg | 100 µg | 50 µg | 100 µg | |
| Compd 1 | 19 | 19 | 14 | 18 | 14 | 20 | 20 | 22 |
| Compd 2 | 6 | 14 | 14 | 14 | 13 | 16 | 10 | 12 |
| Compd 3 | 17 | 19 | 19 | 20 | 18 | 19 | 20 | 23 |
| Compd 4 | 9 | 12 | 14 | 19 | 19 | 21 | 19 | 20 |
| Compd 5 | 17 | 19 | 18 | 20 | 19 | 20 | 20 | 20 |
| Compd 6 | 15 | 20 | 18 | 19 | 16 | 18 | 18 | 19 |
| Compd 7 | 16 | 21 | 12 | 17 | 19 | 20 | 8 | 19 |
| Compd 8 | 11 | 16 | 16 | 19 | 17 | 20 | 16 | 20 |
| Compd 9 | 13 | 17 | 19 | 20 | 17 | 23 | 17 | 20 |
| Streptomycin (30 µg) | 26 | 23 | 25 | 22 | ||||
Table 6: Antibacterial activity of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-carboxylate derivatives
A close survey of the zone of inhibition values indicate that all the compounds exhibited a varied range (6–23 μg) of antibacterial activity against all the tested bacterial strains except 2 which showed moderate activity against S. aureus, B. subtilis, E. coli and K. pneumoniae even at a maximum concentration of 100 μg. The test compounds 1, 3 and 5 displayed a significant antibacterial activity against S. aereus, Bacillus subtilis, E. coli and K. pneumoniae. Compound 4 displayed good antibacterial activity against E. coli and K. pneumoniae. Compounds 6 and 7 with fluoro substituent also exhibited significant antibacterial activity against both the gram positive and gram negative bacteria. Compound 2 with piperanol substituent showed a moderate antibacterial activity against S. aereus, Bacillus subtilis, E. coli and K. pneumoniae. The results of these compounds were promising as antimicrobial therapeutic agents. Hence, the obtained results may be used as a key step for the designing of novel chemical compound with interesting antimicrobial profiles comparable to that of the standard drug.
Antifungal activity by disc diffusion method
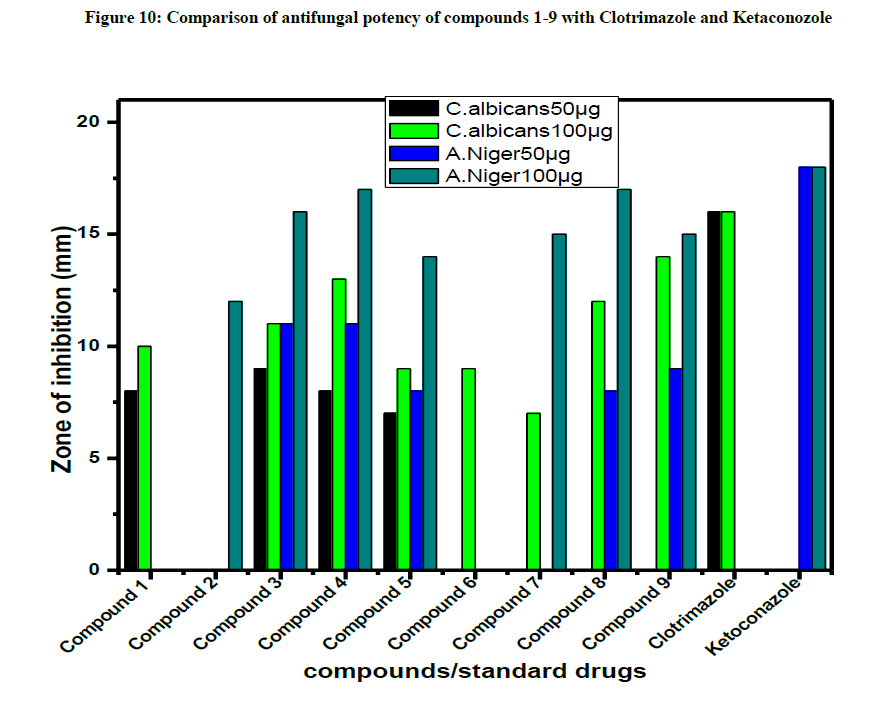
All the compounds were screened for antifungal activities by disc diffusion technique. Compounds are screened in-vitro for their antifungal activity against Candida albicans and Aspergillus Niger and compared with the standard drugs ketoconazole and clotrimazole (Figure 10). The zone of inhibition formed for the compounds against organisms were calculated.
Name of the organism: Candida albicans (MTCC-227) and Aspergillus Niger ((MTCC-281)
Growth media: Potato dextrose agar (PDA) was used for fungal cultures. The culture medium was inoculated with the fungal strains separately suspended in Potato dextrose broth. Potato dextrose agar medium was poured onto the plates containing the extracts, mixed and were allowed to solidify. The synthesized compounds were applied on sterile disc. Standard antifungal agents were used as positive control and fungal plates were incubated at 37°C for 72 h. The diameters of zone of inhibition observed were measured. The antifungal activity of the compounds 1-9 are given in Table 7.
| Test pathogens | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Candida albicans | Aspergillus Niger | |||
| 50 µg | 100 µg | 50 µg | 100 µg | |
| Compd 1 | 8 | 10 | --- | --- |
| Compd 2 | --- | --- | --- | 12 |
| Compd 3 | 9 | 11 | 11 | 16 |
| Compd 4 | 8 | 13 | 11 | 17 |
| Compd 5 | 7 | 9 | 8 | 14 |
| Compd 6 | --- | 9 | ---- | --- |
| Compd 7 | --- | 7 | --- | 15 |
| Compd 8 | --- | 12 | 8 | 17 |
| Compd 9 | --- | 14 | 9 | 15 |
| Clotrimazole (20 µg) | 16 | - | ||
| Ketoconazole (20 µg) | - | 18 | ||
“—” indicates no inhibition at 50 μg and 100 μg concentration
Table 7: Antifungal activity of ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-carboxylate derivatives
The test compounds 3 & 4 displayed a moderate antifungal activity against C. albicans and A. Niger but all other compounds do not exhibit activity against both fungi.
Molecular docking studies
Malaria remains to be a global health problem, and international efforts to eradicate have been made some headways. However, the emergence of drug-resistant parasites threatens this progress. Malaria is a parasitic disease that kills over half a million people each year, posing a huge burden to public health [14]. Antimalarial therapeutics acting via novel mechanisms are urgently required. Many of the clinical symptoms of malaria develop during the erythrocytic stage of infection. During this stage, multiple metabolic pathways are initiated within the parasites, which present wide range of potential drug targets.
Among the essential metabolic pathways that occur within erythrocytes, is hemoglobin digestion; host hemoglobin is degraded into free amino acids that are absolutely required for parasite survival [15,16]. Antimalarial drug resistance represents a major threat to global health. While progress towards a malaria vaccine continues [17,18], the continued development of antimalarial agents that work via novel mechanisms is absolutely required.
Target Protein structure preparation
The X-ray crystal structure of parasitic disease protein complex (PDB ID: 4ZX3) was obtained from the RCSB Protein Data Bank (PDB) [19]. All docking calculations were performed using the Induced Fit Docking module of the Schrödinger suite [20]. It performs flexible protein-ligand docking and searches for favorable interactions between one typically small ligand molecule and a typically larger receptor molecule, usually a protein. All computational work was performed using various modules of Schrödinger Suite 2011 using Red Hat Enterprise Linux 5.0 interface running on Pentium D workstation.
Hydrogen bonds play a major role in the structure and function of biological molecules, the ligand-receptor interactions were analyzed on the basis of hydrogen bond interactions. In this study, we have used glide docking to study the binding orientations and expect binding affinities of the piperidine compounds. The results obtained from this study would be helpful in both understanding the inhibitory mode of piperidine derivatives as well as in accurately predicting the activities of newly designed inhibitors on the basis of docking scores. They would also provide some beneficial clues in structural modification for designing new inhibitors against the treatment of malaria.
Docking study was performed in order to analyze the suitability of the crystal conformation of the drug molecule in the protein environment. Molecular docking was performed maintaining the important hydrogen bonds between the drug molecule and its protein target.
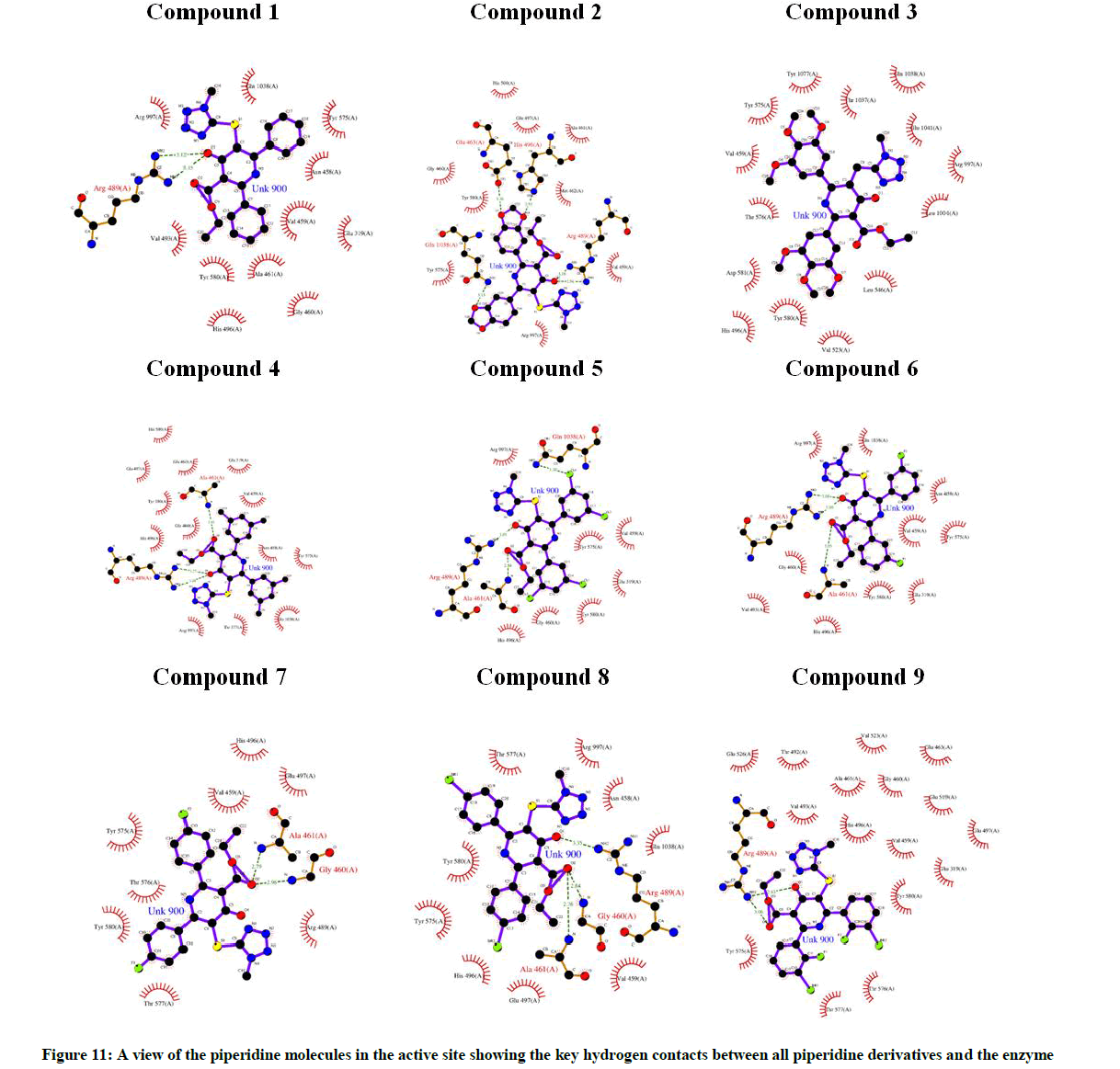
The oxygen atoms of keto group and carboxylate group in all the piperidine moieties, play an important role of hydrogen bond interaction with the amino acid residues of ARG489, ALA461, GLY460, GLN1038, HIS496, GLU463, LYS518 & TYR580, respectively. Mainly, amino group in the active site residues of target protein plays crucial role in hydrogen bonding with keto and carboxylate groups of piperidine derivatives of ligand. The glide energy values of all the docked compounds of the piperidine derivatives are maximum when compared to the co-crystal compound of the targeted protein (Table 8). The study reveals that Compound 7 has good binding capability with the active site amino acids of anti-malarial drug resistance protein.
| Compounds | Docking score | Glide energy (kcal/mol) |
|---|---|---|
| Compd 1 | -2.774 | -48.988 |
| Compd 2 | -4.063 | -58.014 |
| Compd 3 | -3.591 | -54.494 |
| Compd 4 | -3.165 | -56.937 |
| Compd 5 | -4.452 | -59.818 |
| Compd 6 | -5.527 | -60.396 |
| Compd 7 | -5.148 | -68.253 |
| Compd 8 | -4.214 | -54.583 |
| Compd 9 | -4.629 | -64.076 |
| Native | -7.461 | -52.934 |
Table 8: Induced fit docking score and glide energy calculations
In the docking, more number of interactions are noted with maximum glide energy values in Compounds 2, 5 & 8, respectively. In all these complexes, the non-bonded interaction limits are varied from 2.5 Å to 3.5 Å, which reveals that the ligands may result in a strong inhibition. Hydrogen bond interactions of piperidine compounds with amino acids at the active site of anti-malarial drug resistance protein are shown in Table 9. The piperidine molecules docked in the active site of the targeted protein and the key hydrogen contacts are shown in Figure 11.
| Compound | Residues | Interaction | Distance(D…A) (Ǻ) |
|---|---|---|---|
| Compd 1 | ARG489 | *N-H…O *N-H…O |
3.15 3.12 |
| Compd 2 | ARG489 | *N-H…O *N-H…O |
3.16 2.96 |
| HIS496 | *N-H…O | 2.92 | |
| GLU463 | *O-H…O | 3.26 | |
| GLN1038 | *N-H…O | 3.13 | |
| Compd 3 | Hydrophobic | ||
| Compd 4 | ARG489 | *N-H…O *N-H…O |
3.27 3.04 |
| ALA461 | *N-H…O | 2.93 | |
| Compd 5 | ARG489 | *N-H…O | 3.01 |
| ALA461 | *N-H…O | 2.84 | |
| GLN1038 | *N-H…Cl | 3.3 | |
| Compd 6 | ARG489 | *N-H…O *N-H…O |
3.08 3.06 |
| ALA461 | *N-H…O | 3.05 | |
| Compd 7 | ALA461 | *N-H…O | 2.79 |
| GLY460 | *N-H…O | 2.96 | |
| Compd 8 | ARG489 | *N-H…O | 3.35 |
| ALA461 | *N-H…O | 2.76 | |
| GLY460 | *N-H…O | 2.86 | |
| Compd 9 | ARG489 | *N-H…O *N-H…O |
3.06 2.93 |
| Native | ALA461 | *O...H-N *N-H...O |
2.69 3.02 |
| GLY460 | *N-H...O | 2.97 | |
| LYS518 | *N-H...O | 3.02 | |
| TYR580 | *O-H...O *O...H-N |
2.91 2.75 |
Table 9: Hydrogen bond interactions between active site residues and piperidine derivatives
Conclusion
A series of novel ethyl 5-(1-methyl-1H-tetrazol-5-ylthio)-4-oxo-2,6-substituted diphenylpiperidine-3-carboxylate derivatives (1-9) were synthesized in moderate yields and their structures were characterized using various spectral techniques viz., IR, 1H- NMR, 13C-NMR, 2D NMR & Mass spectroscopy. The antimicrobial activity results indicated that some of the tested compounds showed promising antibacterial activity in comparison to antifungal activity against pathogenic microbial strains. These observations of the compounds may promote with better pharmacological profile than standard drugs and serve as template for the construction of drugs to combat bacterial and fungal infection. The study on docking provides the binding modes of the docked compounds, and the hydrogen bond formation for enhancing the activity of this class of compounds.
Acknowledgement
The authors wish to thank the management of Gland Pharma limited for providing facility to carry out this work and take this opportunity to acknowledge R&D team of Gland Pharma and also the authors would like to thank Bioinformatics Infrastructure Facility (BIF), Guindy Campus, University of Madras sponsored by DBT, Government of India, for using the commercial software packages to carry out the computational studies.
References
- M. Arpi, G. Ragini, J. Anshu, Green Chem. Lett. Rev., 2013, 6, 151-182.
- A.T. Balaban, D.C. Oniciu, A.R. Katritzky, Chemical Rev., 2004, 104(5), 2777-2812.
- M. Brichacek, J.T. Njardarson, Org. Biomol. Chem., 2009, 7(9), 1761-1770.
- R.A. Sheldon, Catalysis: the key to waste minimization, J. Chemical Technol. Biotechnol., 1997, 68, 381-388.
- V.V. Dabholkar, F.Y. Ansari, Green Chem. Lett. Rev., 2010, 3(3), 245-248.
- S.D. Diwakar, S.S. Bhagwat, M.S. Shingare, C.H. Gill, Bioorg. Med. Chem. Lett., 2008, 18(16), 4678-4681.
- K.S. Yeung, Z. Qiu, Z. Yang, Bioorg. Med. Chem. Lett., 2013, 23(1), 209-212.
- Y.P. Luo, Q. Gong, Q.Chen, G.F. Yang, Chin. J. Chem., 2013, 26(9), 1545-1548.
- M. Bertinaria, M.A. Shaikh, C. Buccellati, Chem. Med. Chem., 2012, 7(9), 1647-1660.
- R. Romagnoli, P.G. Baraldi, M.K. Salvador, J. Med. Chem., 2012, 55(1), 475-488.
- C. Trecant, A. Dlubala, P. George, P. Pichat, I. Ripoche, Y. Troin, Eur. J. Med. Chem., 2011, 46(9), 4035-4041.
- A.S. Gundugola, K.L. Chandra, E.M. Perchellet, A.M.Waters, J.P.H. Perchellet, S. Rayat, Bioorg. Med. Chem. Lett., 2010, 20(13), 3920-3924.
- Mohammad Asif, Biological Potentials of Substituted Tetrazole Compounds, Pharmaceutical Methods vol 5, Issue 2 (2014).
- WHO, World Malaria Report, World Health Organization, Geneva, Switzerland, 2014, 1-242.
- J. Liu, E.S. Istvan, I.Y. Gluzman, J. Gross, D.E. Goldberg, P. Natl. Acad. Sci. U. S. A., 2006, 103, 8840-8845.
- P.J. Rosenthal, Hydrolysis of erythrocyte proteins by proteases of malaria parasites, Curr. Opin. Hematol., 2002, 9, 140-145.
- L. Foquet, C.C. Hermsen, G.J. van Gemert, E. Van Braeckel, K.E. Weening, R. Sauerwein, P. Meuleman, G. Leroux-Roels, J. Clin. Invest., 2014, 124, 140-144.
- M.P.G. Jepsen, P.S. Jogdand, S.K. Singh, M. Esen, M. Christiansen, S. Issifou, A.B. Hounkpatin, U. Ateba-Ngoa, P.G. Kremsner, M.H. Dziegiel, S. OlesenLarsen, S. Jepsen, B. Mordmueller, M. Theisen, J. Infect. Dis., 2013, 208, 479-488.
- N. Drinkwater, N.B. Vinh, S.N. Mistry, R.S. Bamert, C. Ruggeri, J.P. Holleran, S. Loganathan, A. Paiardini, S.A. Charman, A.K. Powell, V.M. Avery, S. Mc Gowan, P.J. Scammells, Eur. J. Med. Chem., 2016, 110, 43-64.
- Schrodinger Suite 2011, Maestro, version 9.2, Schrodinger, LLC, New York, NY, 2011.