Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 7
Synthesis of Substituted Phenothiazines derivatives, Antioxidant, P-Glycoprotein, Cyp Enzyme Activity, HIA and BBB Prediction
Mahesh K Gaidhane1, Ajay M Ghatole2*, Meghasham N Narule3, Pravin K Gaidhane4, Kushal R lanjewar5 and Gunwant P Gadekar62Dhote Bandhu Science College, Department of Chemistry, Gondia, India
3Vidya Vikas Arts, Commerce & Science College, Samudrapur, India
4Govindrao Wanjari Engineering and Technology, Hudkshwar Road, Nagpur, India
5Mohsinbhai Zaweri Mahavidyalaya, Desaiganj, Wadsa, MS, India
6Dhote Bandhu Science College, Department of Zoology, Gondia, India
Ajay M Ghatole, Dhote Bandhu Science College, Department of Chemistry, Gondia, India, Email: ajay.ghatole5@gmail.com
Received: 07-Jul-2020 Accepted Date: Nov 16, 2020 ; Published: 30-Nov-2020
Abstract
As of late, there is an enormous increment of medication safe pathogens, prompting the plan and advancement of more current antibacterial specialists. Different epic subbed phenothiazines derivatives have arranged to concentrate based on pharmacological exercises in drugs revelation. Phenothiazine derivatives subbed in the 2, 3 and 4 positions have a place with a major gathering of tricyclic aromatic combinations. They are in broad use in psychiatry as tranquillizers and neuroleptics. Because of their trademark structure, they show numerous important scientific properties. A short easy synthesis of 8- [2/-(3//, 5//- dimethyl-4//- ethoxy carbonyl pyrrolyl) hydrazine] subbed phenothiazines (5a-j) from 2-arylaminbenzal-2-(3',5'-dimethyl-4'-ethoxycarbonyl pyrrole) hydrazines (4) in the imminence of sulfur and iodine. These combinations show antibacterial and antifungal drills when contrasted and standard drug Norfloxacin and Griseofulvine against Bacterial cultures, for example, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Proteus Vulgaris and fungal cultures, such as Aspergillus niger and Candida albicans. And also, these compounds screened for their antioxidant activity. The synthesized compounds characterized by FTIR, 1H NMR, elemental and chemical properties. The modern Swiss ADME web tool that stretches free admittance to a pool of hasty yet solid clarifying models for physicochemical properties, pharmacokinetics, and therapeutic science.
Keywords
Antioxidant active, Antifungal activity, Pyrrolyl, Phenothiazines
Introduction
We survey the proof to propose that phenothiazines claim characteristic antibacterial and efflux inhibitory properties empowering them to battle drug resistance. We likewise talk about that understanding their mode of action is basic to facilitate the structure of new phenothiazine derivatives or novel specialists for use as an antibiotic adjuvant [1]. Phenothiazine derivatives incorporate compounds described by a tricyclic aromatic ring with sulfur and nitrogen atoms and substituents in the 2 and 10 or 3 and 7 positions. Phenothiazine positions are anticholinergic derivatives substituted in the 2 and 10 known as anti-psychotropic and antihistaminic drugs. They have been studied in many fields of chemical, biological and medical research owing to their pharmacological activity [2]. Studies right now uncovered that numerous compounds, having a place with various pharmacological families, bear huge nanocarriers on antibacterial movement. Instances of such "non-antibiotics" incorporate antihistamines, for example, promethazine and fluphenazine antipsychotic compounds, for example, promazine, chlorpromazine thioridazine, trifluoperazine and triflupromazine, anti-inflammatory specialists, for example, diclofenac and even antihypertensive operators, such as methyl-DOPA and propranolol [3]. Few drugs such as orlistat, lorcaserin, qsymia, contrave, phentermine etc. have approved by Food and Drug Administration as anti-obesity agents while some others such as sibutramine and rimonabant have withdrawn due to their serious side effects [4]. Pyrroles exhibit interesting biological properties1-3. Besides, Phenothiazines are well known CNS depressant compounds and have emerged as an important area of research for the biological activities like antiparkinsonian [5], anticonvulsant [6], New job growing extraneously dynamic of phenothiazine derivatives as incidentally acting CB1 receptor threatening enemy of obesity agent [7]. Antihelmatic [8], antiviral, anti-parasitic activities [9,10]. Anti-proliferative and anti-MDR activities [11], synthetic antipsychotic drugs [12] Antitubercular, antimalarial and analgesic activities [13]. Heterocycles are the biggest class of organic compounds [14] Among them, pyrroles have a recognized situation in the chemistry of living organisms because of their nearby biogenetic association with the porphyrins, the chlorins, and the corrins. Besides, they viewed as advantaged structures by synthetic chemists on account of widespread applications in therapeutic chemistry and materials science [15]. Until this point, antipsychotic and antihistamine specialists from the gathering of phenothiazines have recited most for their antimicrobial activity both in vitro and in vivo [16,17]. Impact of novel phenothiazine derivatives on brain dopamine in Wistar rodents [18]. Swiss ADME permits the review of ADME restrictions of medication aspirants and small molecules and gives statistics that authorizations early hazard calculation in the drug enhancement progression [19]. Exceptionally, swiss ADME affords a point to estimate Lipinski's rule of five [20] for medication semblance of oral bioavailability.
Interpretation of these annotations it thought worthwhile to synthesized and investigate the compounds in which pyrrole derivative have linked with phenothiazine moiety. We report in this paper, the synthesis and pharmacological investigation of 8-[2/-(3//,5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] substituted phenothiazines (5a-j) (Scheme-I).
Results and Discussions
The required starting material 3′,5′-dimethyl-2′,4′-diethoxy carbonyl pyrrole (1) has prepared by the reported method. The compound (1) refluxed with hydrazine hydrate to yield 2-(3′,5′ dimethyl-4′-ethoxy carbonyl pyrrole) acid hydrazide (2). The compound (2) which reacts with 4-hydroxy benzaldehyde in the presence of ethanol for 8 hr to get the desired isosemicarbazide (3). The compound (3) was condensed with different aryl amines by refluxing in ethanol to get Substituted 2-arylamin benzal-2-(3′,5′-dimethyl-4′-ethoxy carbonyl pyrrole) hydrazine (4a-j). The compound (4a-j) treated with sulphur and iodine to yield 8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl Pyrrolyl) hydrazine] substituted Phenothiazines (5a-j) in the scheme I (Figure 1).
Antioxidant activity
Free radical scavenging activity of the test mixes 4a-j and 5a-j were controlled by the 1,1-diphenyl picryl hydrazyl (DPPH) test strategy. Drug stock arrangement (1 mg/mL) was weakened to definite concentrations of 2, 4, 6, 8 and 10 mg mL–1 in methanol. DPPH methanol arrangement (1 mL, 0.3 mmol) was added to 2.5 mL of drug arrangements of various concentrations and permitted to respond at room temperature. After 30 min the absorbance esteems were estimated at 518 nm and changed over into the rate antioxidant activity. Methanol was utilized as the solvent and ascorbic corrosive as the standard. The level of hindrance extrapolated against fixation is delineated. Results are introduced in Table 1. The standard drug utilized was ascorbic acid [21].
| % Inhibition | |||||
|---|---|---|---|---|---|
| Comp. | 20 μg/mL | 40 μg/mL | 60 μg/mL | 80 μg/mL | 100 μg/ml |
| 4a | 12 | 16 | 22 | 27 | 34 |
| 4b | 15 | 14 | 19 | 23 | 31 |
| 4c | 16 | 21 | 25 | 28 | 32 |
| 4d | 14 | 16 | 16 | 22 | 28 |
| 4e | 12 | 18 | 20 | 24 | 28 |
| 4f | 13 | 16 | 22 | 24 | 26 |
| 4g | 14 | 17 | 20 | 22 | 25 |
| 4h | 15 | 15 | 18 | 20 | 24 |
| 4i | 18 | 25 | 28 | 32 | 36 |
| 4j | 20 | 26 | 29 | 34 | 36 |
| 5a | 18 | 22 | 26 | 30 | 34 |
| 5b | 18 | 27 | 30 | 36 | 39 |
| 5c | 25 | 29 | 34 | 37 | 41 |
| 5d | 24 | 28 | 33 | 38 | 45 |
| 5e | 23 | 28 | 34 | 39 | 46 |
| 5f | 28 | 35 | 39 | 46 | 52 |
| 5g | 27 | 40 | 46 | 48 | 52 |
| 5h | 18 | 36 | 41 | 44 | 46 |
| 5i | 28 | 33 | 42 | 48 | 54 |
| 5j | 25 | 29 | 38 | 45 | 48 |
| 2 μg/mL | 4 μg/mL | 6 μg/mL | 8 μg/mL | 10 μg/mL | |
| Ascorbic acid | 10 | 15 | 20 | 33 | 56 |
Table 1: Antioxidant activity of the compounds 4a-j and 5a-j.
Swiss ADME Pharmacokinetics, Physicochemical, Medicinal Properties study
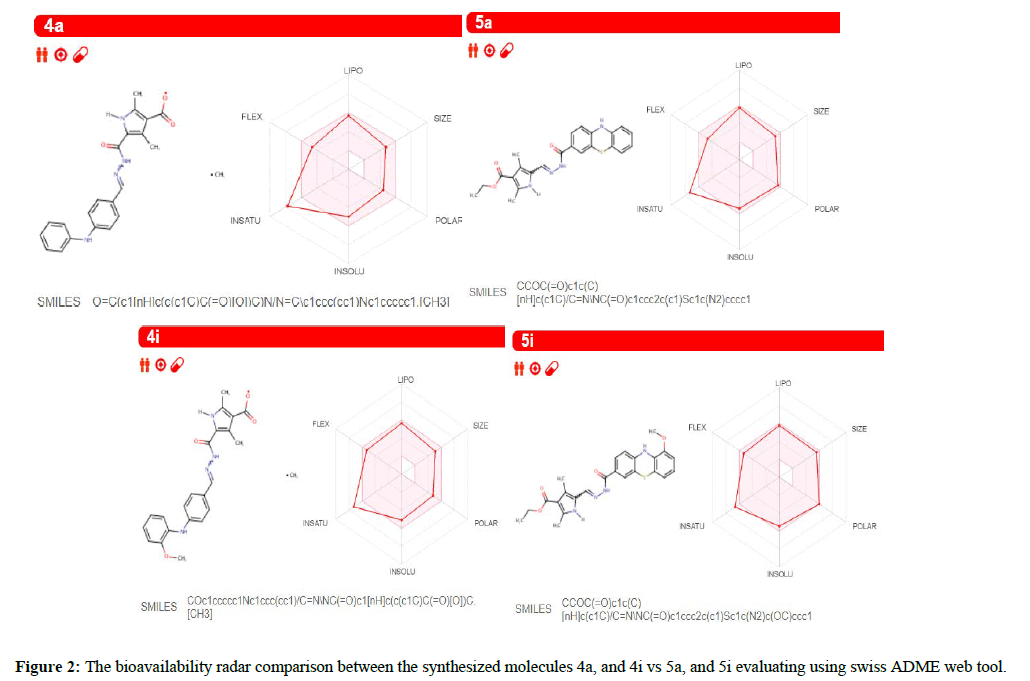
The functioning highlights of these molecules entered in the Swiss ADME site (http://swissadme.ch) using the Chem Axon's Marvin JS structure drawing instrument. Assistant highlights of a pharmacophore sway including bioavailability, transport properties, sympathy to proteins, reactivity, poisonous quality, and metabolic steadiness. Unique to swiss ADME is the bioavailability radar [22] that gives a graphical assessment of the prescription resemblance parameters of an orally accessible bioactive drug. The drug impression chart exhibited as a hexagon (Figures 2) with every one of the vertices dialogues to a restriction that symbolizes a bioavailable drug.
The pink location inside the hexagon speaks to the perfect range for each property (lipophilicity: XLOGP3 somewhere in the array of −0.7 and +5.0, size: extremity: TPSA (Topological Polar Surface Area) somewhere in the field of 20 and 130 Å2, MW somewhere in the scope of 150 and 500 g/mol, solubility: log S not higher than 6, adaptability: close to 9 rotatable bonds, and saturation: some portion of carbons in the sp3 hybridization at any rate 0.25). The near recommended run for the compound to go about as bioavailable medication is portrayed in Tables 2 and 3.
| Sr. No. | Flex | Lipo | Size | Polar | Insolu. | Insatu. |
|---|---|---|---|---|---|---|
| 4a | IN | IN | IN | IN | IN | OUT |
| 4b | OUT | BOU | IN | OUT | IN | OUT |
| 4c | OUT | IN | IN | OUT | IN | OUT |
| 4d | OUT | IN | IN | OUT | IN | OUT |
| 4e | BOU | BOU | IN | IN | IN | OUT |
| 4f | BOU | BOU | IN | IN | IN | OUT |
| 4g | BOU | BOU | IN | IN | IN | OUT |
| 4h | BOU | BOU | BOU | IN | IN | OUT |
| 4i | IN | IN | IN | IN | IN | OUT |
| 4j | OUT | IN | IN | IN | IN | OUT |
| 5a | IN | IN | IN | IN | IN | OUT |
| 5b | IN | IN | BOU | OUT | IN | OUT |
| 5c | IN | IN | BOU | OUT | IN | OUT |
| 5d | IN | IN | BOU | OUT | IN | OUT |
| 5e | IN | BOU | IN | IN | BOU | OUT |
| 5f | IN | BOU | IN | IN | BOU | OUT |
| 5g | IN | BOU | IN | IN | BOU | OUT |
| 5h | IN | BOU | BOU | IN | OUT | OUT |
| 5i | IN | IN | IN | BOU | IN | OUT |
| 5j | IN | IN | IN | BOU | IN | OUT |
(Lipo- lipophilicity; Insolu.- Insolubility; Insatu.- Insaturation, IN- Inside the hexagon speaks; OUT- Outside the hexagon speaks, BOU- Boundary of the hexagon speaks).
Table 2: The drug-likness ideal range for every property of a bioavailable drug.
| Sr. No. | MW | HA | AHA | FCsp3 | RTB | HBA | HBD | MR | TPSA |
|---|---|---|---|---|---|---|---|---|---|
| 4a | 390.44 | 29 | 17 | 0.14 | 7 | 4 | 3 | 112.32 | 86.35 |
| 4b | 449.46 | 33 | 17 | 0.17 | 10 | 6 | 3 | 126.35 | 141.4 |
| 4c | 449.46 | 33 | 17 | 0.17 | 10 | 6 | 3 | 126.35 | 141.4 |
| 4d | 449.46 | 33 | 17 | 0.17 | 10 | 6 | 3 | 126.35 | 141.4 |
| 4e | 438.91 | 31 | 17 | 0.17 | 9 | 4 | 3 | 122.53 | 95.58 |
| 4f | 438.91 | 31 | 17 | 0.17 | 9 | 4 | 3 | 122.53 | 95.58 |
| 4g | 438.91 | 31 | 17 | 0.17 | 9 | 4 | 3 | 122.53 | 95.58 |
| 4h | 483.36 | 31 | 17 | 0.17 | 9 | 4 | 3 | 125.22 | 95.58 |
| 4i | 420.46 | 31 | 17 | 0.17 | 8 | 5 | 3 | 118.81 | 95.58 |
| 4j | 434.49 | 32 | 17 | 0.21 | 10 | 5 | 3 | 124.02 | 104.81 |
| 5a | 434.51 | 31 | 17 | 0.17 | 7 | 4 | 3 | 124.58 | 120.88 |
| 5b | 479.51 | 34 | 17 | 0.17 | 8 | 6 | 3 | 133.4 | 166.7 |
| 5c | 479.51 | 34 | 17 | 0.17 | 8 | 6 | 3 | 133.4 | 166.7 |
| 5d | 478.5 | 34 | 17 | 0.17 | 8 | 7 | 2 | 132.44 | 163.8 |
| 5e | 468.96 | 32 | 17 | 0.17 | 7 | 4 | 3 | 129.59 | 120.88 |
| 5f | 468.96 | 32 | 17 | 0.17 | 7 | 4 | 3 | 129.59 | 120.88 |
| 5g | 468.96 | 32 | 17 | 0.17 | 7 | 4 | 3 | 129.59 | 120.88 |
| 5h | 513.41 | 32 | 17 | 0.17 | 7 | 4 | 3 | 132.28 | 120.88 |
| 5i | 464.54 | 33 | 17 | 0.21 | 8 | 5 | 3 | 131.07 | 130.11 |
| 5j | 464.54 | 33 | 17 | 0.21 | 8 | 5 | 3 | 131.07 | 130.11 |
(MW: Molecular weight; HA: Heavy atoms; AHA: Aromatic heavy atom; FCsp3: Fraction Csp3; RTB: Rotatable bonds; HBA: H-bond acceptors; HBD: H-bond donors; MR: Molar refractivity; TPSA: Topological polar surface area).
Table 3: Physicochemical properties of the synthesized molecules 4a-j and 5a-j.
Drug-likeness
The drug resemblance properties of the fused compound are articulated to by the red mutilated hexagon inside the pink shade (Figure 1). Swiss ADME likewise has computational channels that incorporate Ghose [23], Egan [24], Veber [25], and Muegee [26] created by top pharmaceutical organizations and cheminfomaticians to assess the drug resemblance of molecules. The values maintained in Table 4 indicated the violation of the prescribed range for the molecule to act as a drug.
| Sr. No. | Lipinski #violations |
Ghose #violations |
Veber #violations |
Egan #violations |
Muegge #violations |
|---|---|---|---|---|---|
| 4a | 0 | 0 | 0 | 0 | 0 |
| 4b | 0 | 0 | 1 | 1 | 0 |
| 4c | 0 | 0 | 1 | 1 | 0 |
| 4d | 0 | 0 | 1 | 1 | 0 |
| 4e | 0 | 0 | 0 | 0 | 1 |
| 4f | 0 | 0 | 0 | 0 | 1 |
| 4g | 0 | 0 | 0 | 0 | 1 |
| 4h | 0 | 1 | 0 | 0 | 1 |
| 4i | 0 | 0 | 0 | 0 | 0 |
| 4j | 0 | 0 | 0 | 0 | 0 |
| 5a | 0 | 0 | 0 | 0 | 0 |
| 5b | 0 | 1 | 1 | 1 | 1 |
| 5c | 0 | 1 | 1 | 1 | 1 |
| 5d | 0 | 1 | 1 | 1 | 1 |
| 5e | 0 | 0 | 0 | 0 | 1 |
| 5f | 0 | 0 | 0 | 0 | 1 |
| 5g | 0 | 0 | 0 | 0 | 1 |
| 5h | 1 | 2 | 0 | 0 | 1 |
| 5i | 0 | 1 | 0 | 0 | 0 |
| 5j | 0 | 1 | 0 | 0 | 0 |
Table 4: Drug-likeness evaluation of synthesized compounds using swiss ADME.
PAINS, Break and Lead likeness screening
PAINS (pan-assay interference screening) that regularly gives bogus positive chemical properties bring about high-throughput screens. PAINS will, in general, respond vaguely with various biological targets as opposed to explicitly influencing one foreseen objective. Swiss ADME evaluation did not post any PAINS alert (Table 5). In additional verdict model, Brenk [27] measured combinations that are smaller and less hydrophobic and not those described by "Lipinski's standard of 5" to augment open doors for lead streamlining. Lead likeness tests are projected to provide primes with extraordinary kinship in high-throughput screens that consider the recognition and control of new exchanges ahead of the pack headway stage (Table 5). All the synthesized compound 4a-j and 5a-j drifted the break alert and lead likeness criteria.
| Sr. No. | PAINS #alerts | Brenk #alerts | Leadlikeness #violations | Synthetic Accessibility |
|---|---|---|---|---|
| 4a | 0 | 1 | 2 | 3.27 |
| 4b | 0 | 3 | 3 | 3.74 |
| 4c | 0 | 3 | 3 | 3.74 |
| 4d | 0 | 3 | 3 | 3.62 |
| 4e | 0 | 1 | 3 | 3.49 |
| 4f | 0 | 1 | 3 | 3.49 |
| 4g | 0 | 1 | 3 | 3.47 |
| 4h | 0 | 1 | 3 | 3.52 |
| 4i | 0 | 1 | 3 | 3.39 |
| 4j | 0 | 1 | 3 | 3.57 |
| 5a | 0 | 1 | 2 | 3.83 |
| 5b | 0 | 3 | 3 | 4.05 |
| 5c | 0 | 3 | 3 | 4.03 |
| 5d | 0 | 3 | 3 | 4 |
| 5e | 0 | 1 | 2 | 3.86 |
| 5f | 0 | 1 | 2 | 3.83 |
| 5g | 0 | 1 | 2 | 3.84 |
| 5h | 0 | 1 | 2 | 3.88 |
| 5i | 0 | 1 | 3 | 4.04 |
| 5j | 0 | 1 | 3 | 4.01 |
Table 5: Medicinal chemistry evaluation of the synthesized compounds.
P-glycoprotein and CYP enzyme activity prediction
Swiss ADME furthermore permits the valuation for a compound to be a substrate of p-glycoprotein (P-gp) or inhibitor of the cytochrome p450 isoenzymes (CYP isoenzymes). P-gp is largely spread and linked in the intestinal epithelium where it drives xenobiotics [28]. The generations return "Yes" or "No" if the particle under assessment has a higher probability to be substrate or non-substrate of P-gp or inhibitor or non-inhibitor of a given CYP. The screening results classified (Table 6).
| Sr. No. | GI abs. | BBB per. | P-gp sub. | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor |
|---|---|---|---|---|---|---|---|---|
| 4a | High | No | No | Yes | No | No | No | No |
| 4b | Low | No | No | No | Yes | Yes | No | Yes |
| 4c | Low | No | No | No | Yes | Yes | No | Yes |
| 4d | Low | No | No | No | Yes | Yes | No | Yes |
| 4e | High | No | No | No | Yes | Yes | No | Yes |
| 4f | High | No | No | No | Yes | Yes | No | Yes |
| 4g | High | No | No | No | Yes | Yes | No | Yes |
| 4h | High | No | No | No | Yes | Yes | No | Yes |
| 4i | High | No | No | No | No | No | No | No |
| 4j | High | No | No | No | Yes | Yes | No | Yes |
| 5a | High | No | No | No | Yes | Yes | No | Yes |
| 5b | Low | No | No | No | Yes | Yes | No | Yes |
| 5c | Low | No | No | No | Yes | Yes | No | Yes |
| 5d | Low | No | Yes | No | Yes | No | No | No |
| 5e | Low | No | No | No | Yes | Yes | No | Yes |
| 5f | Low | No | No | No | Yes | Yes | No | Yes |
| 5g | Low | No | No | No | Yes | Yes | No | Yes |
| 5h | Low | No | No | No | Yes | Yes | No | Yes |
| 5i | Low | No | No | No | Yes | Yes | Yes | Yes |
| 5j | Low | No | No | No | Yes | Yes | Yes | Yes |
(GIabs.: gastrointestinal absorption; BBBper.: blood-brain barrier permeant; CYP: Cytochromes, P-gp sub.: P-glycoprotein substrate)
Table 6: Pharmacokinetic evaluation of the synthesized compounds.
HIA and BBB prediction
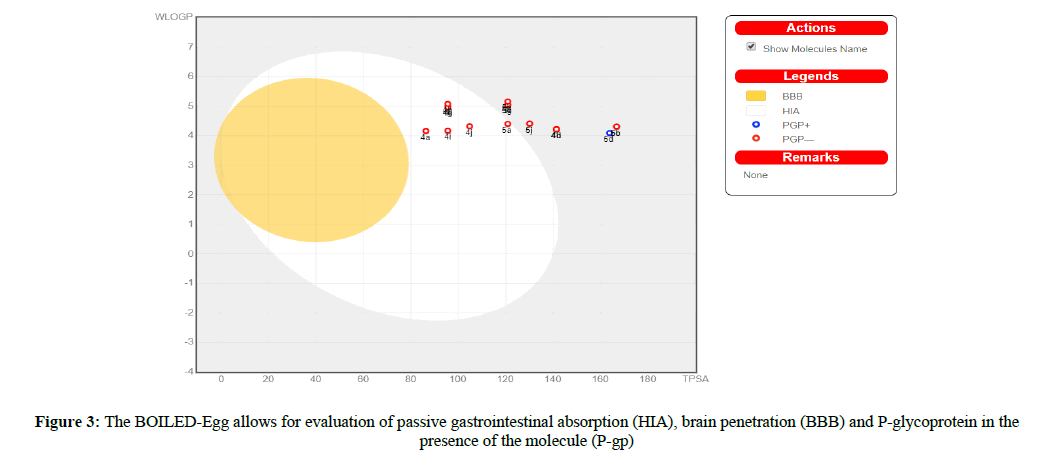
Suitable to P-gp and CYP protein vitality is human gastrointestinal ingestion (HIA) and blood-brain barrier infiltration (BBB). Swiss ADME 'BOILED egg' (Figure 3) grants for appraisal of HIA as a component of the circumstance of the molecules in the WLOGP-versus-TPSA referential.
The white segment of the 'BOILED egg' is for a high chance of reflexive adjustment by the gastrointestinal tract, and the yellow region (yolk) is for a high prospect of cerebrum entrance, Some of the blended particle 4a, e,g, i,j, and 5a under the white segment. With this, the focuses are concealed in blue at whatever point foreseen as adequately effluxes by P-gp (PGP+) and in red anticipated as non-substrate of P-gp (PGP−). HIA and BBB are responsible for water solvency and lipophilicity of the medication. Two topological ways to deal with predict water solvency contained Swiss ADME. The first is an implementation of the ESOL (Delaney, 2004) model, and the resulting one improved from Ali et al. [29,30]. SILICON-IT made Swiss ADME third pointer for solvency. Every single foreseen quality are the decimal logarithm of the molar solvency in water (log S) portrayed in Table 7. Agreement Log p is the normal estimation of all Log P assessed with different lipophilicity criteria (Table 8).
| Sr. No. | ESOL | Ali | Silicos | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log S | Solu. (mg/ml) | Solu. (mol/l) | Class | Log S | Solu. (mg/ml) | Solu. (mol/l) | Class | Log S | Solu. (mg/ml) | Solu. (mol/l) | Class | |
| 4a | -5.03 | 3.65E-03 | 9.34E-06 | MS | -5.97 | 4.16E-04 | 1.07E-06 | MS | -6.88 | 5.18E-05 | 1.33E-07 | PS |
| 4b | -5.41 | 1.75E-03 | 3.89E-06 | MS | -7.56 | 1.23E-05 | 2.73E-08 | PS | -7.3 | 2.26E-05 | 5.04E-08 | PS |
| 4c | -5.06 | 3.89E-03 | 8.65E-06 | MS | -6.99 | 4.57E-05 | 1.02E-07 | PS | -7.3 | 2.26E-05 | 5.04E-08 | PS |
| 4d | -5.06 | 3.89E-03 | 8.65E-06 | MS | -6.99 | 4.57E-05 | 1.02E-07 | PS | -7.3 | 2.26E-05 | 5.04E-08 | PS |
| 4e | -5.59 | 1.12E-03 | 2.56E-06 | MS | -6.86 | 6.04E-05 | 1.38E-07 | PS | -8.54 | 1.25E-06 | 2.85E-09 | PS |
| 4f | -5.59 | 1.12E-03 | 2.56E-06 | MS | -6.86 | 6.04E-05 | 1.38E-07 | PS | -8.54 | 1.25E-06 | 2.85E-09 | PS |
| 4g | -5.59 | 1.12E-03 | 2.56E-06 | MS | -6.86 | 6.04E-05 | 1.38E-07 | PS | -8.54 | 1.25E-06 | 2.85E-09 | PS |
| 4h | -5.91 | 6.01E-04 | 1.24E-06 | MS | -6.92 | 5.77E-05 | 1.19E-07 | PS | -8.73 | 8.90E-07 | 1.84E-09 | PS |
| 4i | -5.1 | 3.32E-03 | 7.89E-06 | MS | -6.13 | 3.08E-04 | 7.33E-07 | PS | -6.98 | 4.40E-05 | 1.05E-07 | PS |
| 4j | -5.07 | 3.69E-03 | 8.50E-06 | MS | -6.37 | 1.85E-04 | 4.27E-07 | PS | -8.06 | 3.78E-06 | 8.69E-09 | PS |
| 5a | -5.39 | 1.75E-03 | 4.03E-06 | MS | -6.89 | 5.54E-05 | 1.28E-07 | PS | -8.04 | 3.99E-06 | 9.18E-09 | PS |
| 5b | -5.46 | 1.64E-03 | 3.43E-06 | MS | -7.68 | 1.00E-05 | 2.09E-08 | PS | -7.37 | 2.04E-05 | 4.26E-08 | PS |
| 5c | -5.46 | 1.64E-03 | 3.43E-06 | MS | -7.68 | 1.00E-05 | 2.09E-08 | PS | -7.37 | 2.04E-05 | 4.26E-08 | PS |
| 5d | -5.35 | 2.16E-03 | 4.52E-06 | MS | -7.43 | 1.77E-05 | 3.70E-08 | PS | -7.37 | 2.04E-05 | 4.26E-08 | PS |
| 5e | -5.99 | 4.77E-04 | 1.02E-06 | MS | -7.55 | 1.33E-05 | 2.83E-08 | PS | -8.62 | 1.13E-06 | 2.41E-09 | PS |
| 5f | -5.99 | 4.77E-04 | 1.02E-06 | MS | -7.55 | 1.33E-05 | 2.83E-08 | PS | -8.62 | 1.13E-06 | 2.41E-09 | PS |
| 5g | -5.99 | 4.77E-04 | 1.02E-06 | MS | -7.55 | 1.33E-05 | 2.83E-08 | PS | -8.62 | 1.13E-06 | 2.41E-09 | PS |
| 5h | -6.31 | 2.54E-04 | 4.94E-07 | PS | -7.61 | 1.26E-05 | 2.45E-08 | PS | -8.8 | 8.04E-07 | 1.57E-09 | PS |
| 5i | -5.47 | 1.57E-03 | 3.38E-06 | MS | -7.06 | 4.07E-05 | 8.77E-08 | PS | -8.13 | 3.40E-06 | 7.33E-09 | PS |
| 5j | -5.47 | 1.57E-03 | 3.38E-06 | MS | -7.06 | 4.07E-05 | 8.77E-08 | PS | -8.13 | 3.40E-06 | 7.33E-09 | PS |
Table 7: Water solubility evaluation of the synthesized compounds.
Materials and Methods
The synthesized compounds are first purified by re-crystallization using appropriate solvents. Melting points were resolved in an open capillary tube and are uncorrected. The IR spectra were recorded on ABB BOMEM FTIR Spectrometer utilizing KBr plate method. The NMR spectra were recorded as Bruker 400MHz NMR Spectrometer in DMSO utilizing TMS as an inner standard. Chemical shift is given in δ ppm. Mass spectra were recorded on GCMS QP 5000 Shimadzu. Thin-layer chromatography was made using pre-coated aluminium plates, coated with silica gel GF254 [E. Merck]. ethyl acetate–acetone (6:4) for 5a, 5b, 5c, 5e; ethyl acetate – chloroform (8:2) for 5d, 5f, 5g, 5h, 5i, 5j Solvent for crystallization; aq. ethanol for (5a –j). The spots were visualized in the iodine chamber. Characterization data of compounds are given in Table 8.
| Compound | Molecule | RF Value | Yield (%) | Analysis (Cal) (found) | ||
|---|---|---|---|---|---|---|
| Formula | C | H | N | |||
| 5a | C23H22O3N4S | 0.36 | 80% | 65.7 | 5 | 12.9 |
| -65.8 | (5.95 | -12 | ||||
| 5b | C23H21O5N5S | 0.24 | 65% | 56.61 | 4.3 | 14.6 |
| -56.9 | (4.20 | -14.7 | ||||
| 5c | C23H21O5N5S | 0.91 | 62% | 56.61 | 4.3 | 18.7 |
| -56.9 | (4.20 | -18.7 | ||||
| 5d | C23H21O5N5S | 0.71 | 68% | 56.61 | 4.3 | 18.7 |
| -56.9 | (4.20 | -18.7 | ||||
| 5e | C23H21O3N4SCl | 0.37 | 70% | 58.9 | 4.4 | 11.9 |
| -58.7 | -4.3 | -11.4 | ||||
| 5f | C23H21O3N4SCl | 0.48 | 58% | 58.9 | 4.4 | 11.9 |
| -58.7 | -4.3 | -11.4 | ||||
| 5g | C23H21O3N4SCl | 0.36 | 55% | 58.9 | 4.4 | 11.9 |
| -58.7 | -4.3 | -11.4 | ||||
| 5h | C24H23O4N4S | 0.87 | 66% | 62.1 | 4.9 | 12.09 |
| -62 | -4.9 | -12.6 | ||||
| 5i | C24H23O4N4S | 0.11 | 92% | 62.1 | 4.9 | 12.09 |
| -62 | -4.9 | -12.6 | ||||
| 5j | C23H20O3N4SBr | 0.92 | 71% | 53.9 | 3.9 (3.4) | 10.9 (10.7) |
| -53.6 | ||||||
Table 8: Characterization of data of newly synthesized compounds.
2-(3',5'-Dimethyl-4'-ethoxy carbonyl pyrrole) acid hydrazide (2) 3,5-Dimethyl-2,4-diethoxy carbonyl pyrrole (1) (0.05 mole), hydrazine hydrate (1.0 mL, 99%), and ethanol (20 mL) were taken in a 100 mL round base flask. The response blend was refluxed for 4hr. on the water bath. The response was checked by thin-layer chromatography. The mixture was dissipated to its half and left-over night. The product precipitated was sieved, washed with water, desiccated and crystallized from ethanol. Yield 70%: M.P.216°C: IR (KBr, cm-1): 3153(NH), 1621(CONH), 1712 (COOC2H5), 1322(-CH3):1 1H-NMR (DMSO, δ in ppm): (m, 3H CONHNH2), 8.9 (s,1H, Pyrrole-NH).
4-Hydroxybenzal-2-(3′, 5′dimethyl-4′ ethoxy carbonyl pyrrolyl) hydrazines (3) A mixture of 2-(3′,5′-dimethyl-4′-ethyl carbonyl pyrrole) acid hydrazide (2) (0.01 mole) and p-hydroxy aldehyde in appropriate amounts in excess of DMF was magnetically stirred for 8 hours. The resulting mix was permitted to stand for 1 hour keeping the internal temperature between 5–10 °C. The mixture was refluxed for 3 hours. The solvent was detached under vacuum to obtain the crude product which was swept away with water followed by ethanol (10ml) and crystallized from appropriate solvents (70% aqueous ethanol).M.P. 187°, yield 72%. ; IR (KBr, cm-1): 3393.0 (OH), 3306.3 (N-H), 1693.8 (C=O, ester), 1682 (C=N, azomethine), 1573.5 (-CONH). 1H-NMR (DMSO, δ in ppm): 2.10 (s, 6H, 2 x CH3), 3.31(m, 5H, COO CH2CH3), 3.42 (s, 1H, CH=N), 8.93(s, 1H, 2-OH), 8.1(4H, ArH); 13C NMR (300MHz, DMSO-d6) 36.93, 39.20, 39.48, 39.76, 40.03, 77.74, 78.18, 78.62, 116.13, 117.4, 118.9, 131.09, 132.51, 158.60, 163.02.
General Procedure for (4a-j)
Substituted 2-Arylaminebenzal-2-(3′, 5′dimethyl-4′ethoxycarbonylpyrrolyl) hydrazines (4a-j) A mixture of 4-hydroxy benzal-2-(3′, 5′dimethyl-4′ ethoxy carbonyl pyrrolyl) hydrazines (3) (0.05mole) and substituted aromatic primary amine (0.05mole) in absolute ethanol (100ml) was heated under reflux in the presence of anhydride ZnCl2 (0.5g) for 3 hr. on a water bath. On cooling a solid mass parted out which was wash away repeatedly with acidified water to remove inorganic materials. It was filtered off, dried and crystallized from ethanol. (M. P. 170° yield 72 %.). IR (KBr, cm-1): 3342.6 (N–H-pyrrole), 1792.9 (CONH), 1712 (COOC2H5), 1650 (-C=O, ester). 1649 (N-H-Bridge) 1322(-CH3); 1H-NMR (DMSO, δ in ppm): 2.56(s, 6H, 2 x CH3), 4.28 (q, 5H, COOCH2CH3), 3.54 (s, 1H, -CONH); 13C NMR (300MHz, DMSO-d6), 11.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 165.0.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] phenothiazines (5a) A mixture of (4) (0.01mole) sulphur (0.1 moles) and Iodine (0.5 g) was rapidly heated at 1800c in an oil bath and this temperature was maintained for 2 hr. The hot melt was rapidly poured into a mortar and crushed to a fine powder. It was washed with water dried and crystallized from ethanol containing animal charcoal. (M. P. 215° yield 80%.). IR (KBr, cm-1): 3344.6 (N–H-pyrrole), 3320 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COOCH2CH3), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54(s, 1H, -CONH); 13C NMR(300MHz, DMSO-d6) 12.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 2-nitro phenothiazines 5b (M. P. 224° yield 65%.). IR (KBr, cm-1): 3342.6 (N – H-pyrrole), 3323 (N-H-phenothiazine), 2965 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1555 (-NO2), 1514 (-NO2), 1332 (-CH3), 785 (C-S); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, -CONH); 13C NMR(300MHz, DMSO-d6) 14.3, 13.5, 13.6, 22.0, 37.9, 38.2, 34.5, 39.4, 40.0, , 58.5, 76.8, 77.2, 77.6, 111.8, 159.1, 126.2, 137.3, 162.2, 162.1.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 3-nitro phenothiazines 5c (M. P. 212° yield 62%). IR (KBr, cm-1): 3342.6 (N – H-pyrrole), 3324 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COOC2H5), 1650 (-C=O, ester), 1552 (-NO2), 1332 (-CH3), 785 (C-S); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, -CONH); 13C NMR (300MHz, DMSO-d6), 11.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 165.0.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 4-nitro phenothiazines 5d (M. P. 267° yield 68%.). IR (KBr, cm-1): 3346.(N – H-pyrrole), 3324 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650(-C=O, ester), 1553 (-NO2), 1336 (-CH3), 785 (C-S); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (1H, s, -CONH); 13C NMR (300MHz, DMSO-d6) 11.3, 13.4, 13.9, 27.0, 38.9, 34.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 164.6.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 2-chloro phenothiazines 5e (M. P. 238° yield 70%.). IR (KBr, cm-1): 3442.6 (N – H-pyrrole),3327 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COOC2H5), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S), 706 (-Cl); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s,1H, -CONH); 13C NMR(300MHz, DMSO-d6), 11.3, 13.4, 13.9, 27.0, 38.9, 34.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 165.3.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 3-chloro phenothiazines 5f (M. P. 245° yield 58%.). IR (KBr, cm-1): 3342.6 (N – H-pyrrole),3320 (N-H-phenothiazine), 2960 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S) 736 (-Cl); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, -CONH); 13C NMR(300MHz, DMSO-d6), 11.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 165.1.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 4-chloro phenothiazines 5g (M. P. 217° yield 55%.). IR (KBr, cm-1): 3348.6(N – H-pyrrole),3326 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S),726 (-Cl); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, -CONH); 13C NMR(300MHz, DMSO-d6), 11.3, 13.4, 12.9, 25.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 123.2, 137.3, 164.2, 165.3.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 2-methoxy phenothiazines 5h (M. P. 209° yield 66%.). IR (KBr, cm-1): 3452.6 (N – H-pyrrole), 3352 (N-H-phenothiazine), 2969 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COOCH2CH3), 3.54 (s, 1H, -CONH); 13C NMR(300MHz, DMSO-d6), 11.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2, 165.0.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 4-methoxy phenothiazines 5i (M. P. 251° yield 92%.). IR (KBr, cm-1): 3362.6 (N – H-pyrrole),3390 (N-H-phenothiazine), 2967 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1339 (-CH3), 785 (C-S). 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, -CONH), 13CNMR(300MHz,DMSOd6), 11.3, 13.4, 13.9, 27.0, 38.9, 39.2, 39.5, 39.7, 40.0, 40.3, 58.5 ,6.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.3, 162.2.
8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] 2-bromo phenothiazines 5j (M. P. 2180 yield 71%.). IR (KBr, cm-1): 3362.6 (N – H-pyrrole), 3332 (N-H-phenothiazine), 2961 (C-H-Aromatic stretch), 1792.9 (CONH), 1714 (COO CH2CH3), 1650 (-C=O, ester), 1332 (-CH3), 785 (C-S), 518 (-Br); 1H-NMR (DMSO, δ in ppm): 2.56 (s, 6H, 2 x CH3), 4.28 (q, 5H, COO CH2CH3), 3.54 (s, 1H, CONH); 13CNMR(300MHz,DMSOd6), 11.3, 13.4, 13.9, 37.09, 38.9, 39.5, 39.7, 40.0, 40.3, 58.5, 76.8, 77.2, 77.6, 111.8, 119.1, 126.2, 137.
Conclusion
A series of 8-[2/-(3//, 5//-dimethyl-4//-ethoxy carbonyl pyrrolyl) hydrazine] substituted phenothiazines (5a-j) from 2-arylamin benzal-2-(3′,5′-dimethyl-4′-ethoxy carbonyl pyrrole) hydrazines (4a-j) in presence of sulphur and iodine. These compounds were screened for their Antioxidant Activity.
Acknowledgment
I am highly thankful to the principal, VVACSC, Samudrapur for providing the necessary facility for the completion of this research work. The authors are also thankful to the Head, Department of Pharmaceutical Science Nagpur University for screening activities, Head RSIC, CDRI, Lucknow for providing the spectral data of the compounds.
References
[1] JV Alvar. Leishmaniasis Worldwide and Global Estimates of Its Incidence. 2012, Plos one.
[2] JS Arpinskat. Medical research owing to their pharmacological activity. 1996, p. 12.
[3] R. Brenk, D Schipani, James et al., Chem. Med. Chem., 3(3): p. 435-44.
[4] AP Chamoun-Emanuelli. Agents and Chemotherapy, 2013, 57(6): 2571-2581.
[5] YC Chen. Acta Anaesthesiol Taiwan. 2010, 48(1): p. 3-7.
[6] A Daina, O Michielin & V Zoete. Sci Rep. 2017. 7: p. 42717.
[7] J Delaney. J. Chem. Inf. Comput. Sci. 2004, 44(3): p. 1000-1005.
[8] W Egan, K Merz and J Baldwin. Journal of Medicinal Chem., 2000. 43(21): p. 3867-3877.
[9] J Fagerberg, E Karlsson, J. Ulander et al., Pharmaceutical Research. 2015. 32(2): p. 578-589.
[10] MG Gaidhane. Gurukul Int. Multidisci. Res. J. 2016. p. 206-215.
[11] AL Garcia. PLoS One. 2014. 9(11).
[12] A. Ghatole, M Gaidhane, K Lanjewar et al., Bulg. J. sci. edu. 29(2): p. 206-244.
[13] A Ghatole, K Lanjewar and M Gaidhane. J. Pharm. Res. 2012. 5(5): p. 2758-2762.
[14] A Ghatole, A., Lanjewar, K., & Gaidhane, M. (2014). Antimicrobial Activities; Ionic Liquid And Microwave Assisted Synthesis Of Ring-Substituted 3-(3-Bromo-4-Oxo-4h-Chromen-2-Yl)-4h-Chromen-4-One. World Journal of Pharmaceutical Research, 3(3), 4336-4350.
[15] A Ghatole, K Lanjewar and M Gaidhane. Int. J. Pharm Sci, 2012. 6(2): p. 142-146.
[16] A Ghose, V Viswanadhan and J Wendoloski. J Comb Chem. 1999, 1(1) 55-68.
[17] CS Gopi. J. Chem. 2019, 9(4): p. 255-289.
[18] MG Gracio. Int J Antimicrob Agents. 2003, 22(3): p. 347-51. doi:10.1016/s0924-8579(03)00204-8
[19] FP Grimsey. FEMS Microbiology Reviews. 2019, 43(6): p. 577–590.
[20] SC Hibinoa. Natural Products Reports. 2001, 18: p. 66-81.
[21] MF Kucukdisli. Beilstein J. Org. Chem. 2014, 10: p. 466-470.
[22] C. Lipinski, F Lombardo, B. Dominy et al., Adv Drug Deliv Rev. 2001, 46: p. 3-26.
[23] JA McGee. American J. Hosp. Pharm. 36(5): p. 633-640.
[24] I Muegge, S Heald and D Brittelli. J. Med. Chem. 2001, 44(12): p. 1841-1846.
[25] G Ogu and J Maxa. Proc Bayl Univ Med Cent. 2000, 13(4): p. 421-423.
[26] IJ Onakpoya. BMC Med. 2016, 14: p. 1-11.
[27] MM Sharma. Scientific Report. 2018, 8(1650): p. 1-18.
[28] SP Sinha. Chem Inform. 2012, 2(4): p. 1130-1137.
[29] R Sundberg. New York, USA: Academic Press. 1997.
[30] D Veber, S Johnson, H Cheng et al., J Med Chem. 2002, 45(12): p. 2615-2623.