Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 2
Synthesis Of Phenyl Tetrazolyl Hexa Hydropyranochromene Derivatives
Rateesh Vanam, Prasanna Bethanamudi, M Ravinder and Amarnath Velidandi*Amarnath Velidandi, Department of Chemistry, Chaitanya Deemed to be university, Kishanpura, Hanamkonda, Warangal, Telangana State 506 001, India, Email: velidandi@yahoo.co.in
Received: 23-Sep-2020 Accepted Date: Feb 20, 2021 ; Published: 26-Feb-2021
Abstract
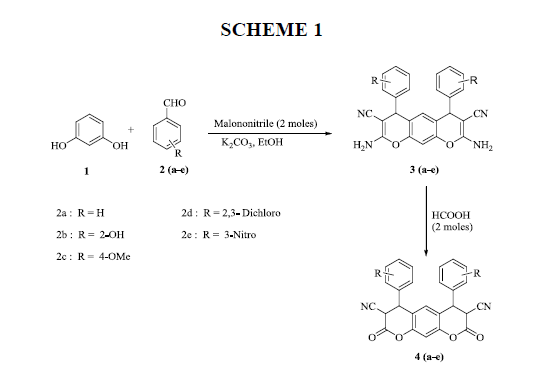
Novel series of bis-pyranotetrazole derivatives have been developed by using resorcinol as starting compound with involvement of substituted aromatic aldehydes (a-e), malononitrile in pot condensation. During the reaction 2,8-diamino-4,6-bis (substituted phenyl)-4,6-dihydropyrano[3,2-g] chromene-3,7-dicarbonitrile 1(a-e) and 2,8-dioxo-4,6-bis (substituted phenyl)-2,3,4,6,7,8-hexahydropyrano [3,2-g] chromene-3,7-dicarbonitrile 2(a-e) formed as intermediates. The synthesized compounds were confirmed by their IR, NMR and Mass spectral data.
Keywords
Bis-Coumarin, Tetrazole, Chromone, Malononitrile, Aromatic Aldehyde
Introduction
An important class of compounds was coumarin derivatives which are possess several types of pharmacological p properties such as anticancer, anti-HIV, anticoagulant, spasmolytic, and antibacterial activity [1]. A large number of structurally novel coumarin derivatives have been reported to show substantial cytotoxic activity in vitro and in vivo. Benzochromenes are significant pharmacophores exhibit pharmacological activities, such as anticancer [2] antimalarial [3], anti-inflammatory [4] and pesticides activities [5]. In addition, chromene moieties are used as fluorescence markers [6] as well as laser dyes [7] in pharmacy and biology.
Tetrazole are useful as ligands, and in medicinal chemistry, as stable bioisosteres of carboxylic acids. Since the acidity of tetrazole group corresponds closely with that of carboxylic acid, replacement of C-terminal amino acid residue with a tetrazole analogue often improves the biological activity of parent peptides. They are used in the construction of potential anti-inflammatory [8], central nervous stimulants [9], hypertensives [10], glycosidase inhibitors [11], antibiotic, antiviral agents [12] anti- cancer [13] and heart diseases [14].
Experimental
Melting points were uncorrected. Infrared spectra were recorded by using a Bruker WM-4(X) spectrometer 577 model. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained on a Bruker WM-400 spectrophotometer in DMSO-d6with tetramethylsilane as reference. Mass spectra (ESI) were taken out on a JEOL SX-102 spectrophotometer. Elemental result was done on a Carlo Erba EA 1108 automatic elemental analyzer. The synthesized compounds were purified by column chromatography and thin-layer chromatography (TLC).
General procedure for the synthesis of 2,8-diamino-4,6-bis(subtitutedphenyl)-4,6-dihydropyrano[3,2-g]chromene-3,7-dicarbonitrile 3(a-e)
To a mixture of resorcinol (1) (0.01mol), substituted aromatic aldehydes 2(a-f) (0.02 mol),andmalononitrile (0.02 mol) dissolved in absolute ethanol. Then added anhydrous K2CO3 (0.04 mol) small portions with continuous stirring at 00C for 30 min.
Then warmed to attain room temperature and stirred for 4 h. After completion of the reaction (monitored by TLC), mixture poured into ice-cold water orange colour solid separated out filtered and dried. The compounds were purified by column chromatography (mobile phase ratio 2:8 ethyl acetate: pet ether).
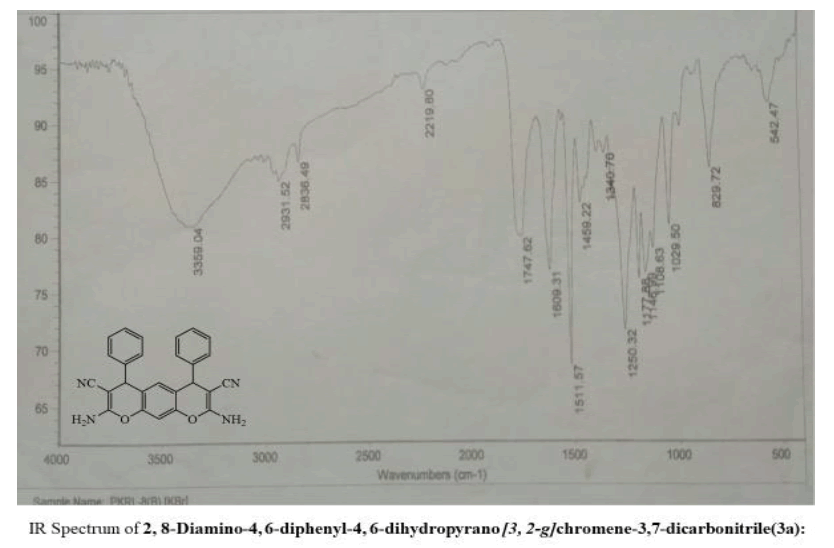
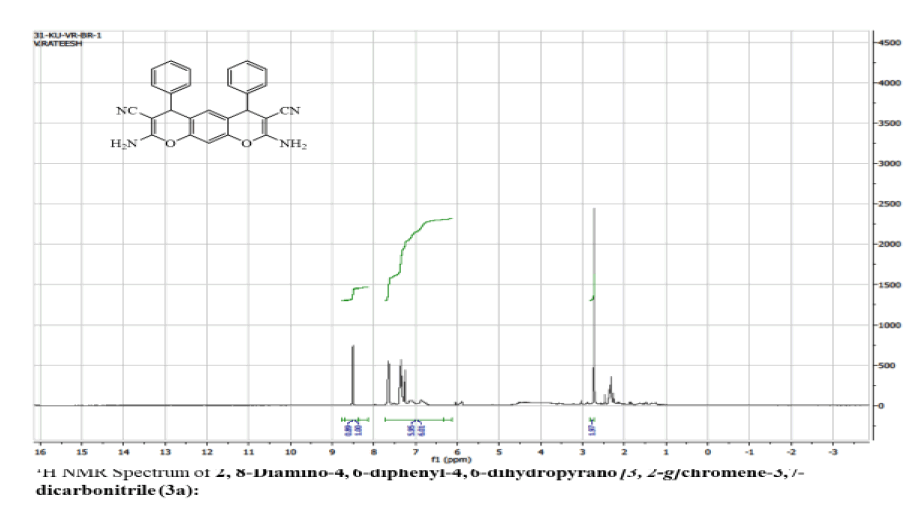
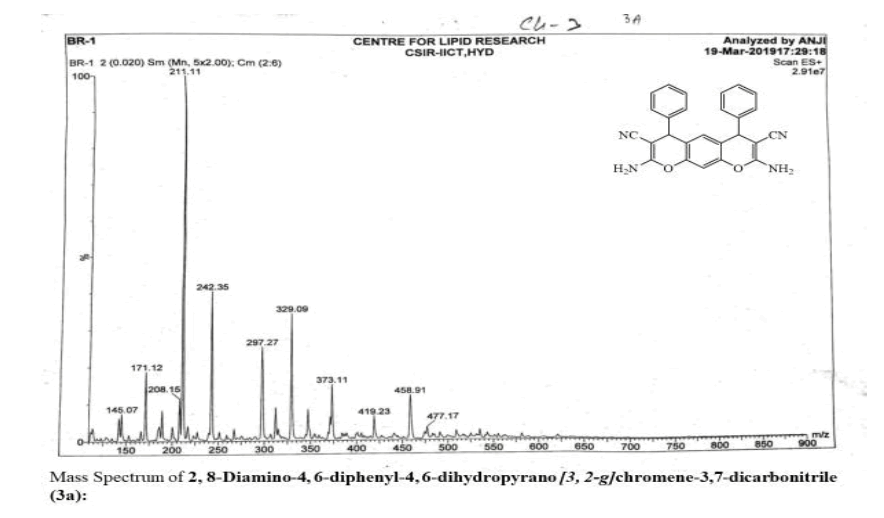
2, 8-Diamino-4, 6-diphenyl-4, 6-dihydropyrano [3, 2-g] chromene-3,7-dicarbonitrile (3a):
Yield: 78 %: m p: 132-134 0C; FT-IR(KBr, ν,cm-1): 3270-3400 (-NH2), 2270 (-CN), 1630 (-C=C-); 1H NMR(300MHz, DMSO-d6, δ, ppm): δ 4.76 (s, 2H, pyran–H), 6.31 (s, 1H ,Ar-H), 6.92 (s, 1H, Ar-H), 7.25 (m, 4H, Ar-H), 7.29 ( m, 2H, Ar-H), 7.41 ( m, 4H, Ar-H), 8.62 (br s, 4H, -NH2). 13C NMR(75 MHz, DMSO-d6, δ, ppm): δ 28.1, 59.5, 113.2, 114.5, 117.0, 125.4, 127.8, 128.9, 129.6, 140.7, 151.2, 154.7, 177.7; MS (EI, m/z (%)): 418.15[M+H]+;Calculated %, of C26H18N4O2: C, 74.63; H, 4.34; N, 13.39 Found: C, 74.62; H, 4.31, N, 13.60.
2,8-diamino-4,6-bis(2-hydroxyphenyl)-4,6-dihydropyrano[3,2-g] chromene-3,7-dicarbo nitrile (3b):
Yield: 62 %: m p: 123-124 0C; FT-IR(KBr, ν,cm-1): 3480 (-OH), 3280-3407 (-NH2), 2170 (-CN), 1632 (-C=C-): 1H NMR(300MHz, DMSO-d6, δ, ppm): δ 4.72 (s, 2H, pyran–H), 6.32 (s, 1H ,Ar-H), 6.8 (s, 1H, Ar-H), 7.1-7.3 (m, 4H, Ar-H), 6.9-7.0 ( m, 4H, Ar-H), 7.6 (br s,4H, -NH2 ), 10.2 (s, 2H, -OH); 13C NMR(75 MHz, DMSO-d6, δ, ppm): δ 24.6, 56.6, 112.2, 115.5, 117.5, 119.5, 122., 127.3, 129.9, 131.2, 150.2, 154.1, 176.2; MS (EI, m/z (%)): 450.13[M+H]+; Calculated% of C26H18N4O4: C 69.33; H 4.03;N 12.44; Found: C 68.59; H 4.18;N 12.22.
2,8-diamino-4,6-bis(4-methoxyphenyl)-4,6-dihydropyrano[3,2-g] chromene-3,7-dicarbo- nitrile (3c):
Yield: 73 %: m p: 156-158 0C; FT-IR(KBr, ν,cm-1): 3290-3420 (-NH2), 2168 (-CN), 1630 (-C=C-);1H NMR(300MHz, DMSO-d6, δ, ppm); δ 3.82 (s, 6H, 2 x -OCH3), 4.68 (s, 2H, pyran–H), 6.48 (s, 1H ,Ar-H), 6.83 (s, 1H, Ar-H), 6.94 (m, 4H, Ar-H), 7.21 ( m, 4H, Ar-H), 8.40 (br s,4H,-NH2); 13C NMR(75 MHz, DMSO-d6, δ, ppm): δ 23.5, 28.4, 56.2, 59.0, 112.2, 114.5, 117.0, 130.7, 131.0, 132.6, 152.7, 159.2, 175.3; MS (EI, m/z (%)): 478.16[M+H]+; Calculated %, of C28H22N4O4: C 70.28; H 4.63;N 11.71. Found: C 70.11; H 4.38; N 11.48;
2,8-diamino-4,6-bis(2,3-dichlorophenyl)-4,6-dihydropyrano[3,2-g] chromene-3,7-di carbonitrile (3d):
Yield 68 %: m p:142-144 0C; FT-IR(KBr, ν,cm-1): 3260-3380 (-NH2), 2180 (-CN),1636 (C=C), 1H NMR(300MHz, DMSO-d6, δ, ppm) : δ 4.62 (s, 2H, pyran–H), 6.52 (s, 1H ,Ar-H), 6.82 ( s, 1H, Ar-H), 7.1 (m, 2H, Ar-H), 7.3 (m, 2H, Ar-H), 7.62 ( d, 2H, Ar-H), 8.56 (br s,4H, -NH2 ); 13C NMR(75 MHz, DMSO-d6, δ, ppm): δ 24.5, 59.5, 110.8, 114.8, 117.2, 118.5, 124.4, 126.7, 128.9, 129.6, 131.2, 133.6, 152.7, 177.1; MS (EI, m/z (%)): 556.99[M+H]+;Calculated% of C26H14Cl4N4O2: C 56.14; H 2.54; N 10.07; Found: C 55.59; H 2.21; N 9.8;
2,8-diamino-4,6-bis(3-nitrophenyl)-4,6-dihydropyrano[3,2-g] chromene-3,7-dicarbo nitrile (3e)
Yield:72 %: m p: 186-187 0C; FT-IR(KBr, ν,cm-1): 3280-3400 (-NH2), 2160 (-CN), 1620 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm): δ 4.72 (s, 2H,pyran–H), 6.41 (s, 1H, Ar-H), 6.93 ( s, 1H, Ar-H), 7.52-7.64 (m, 4H, Ar-H), 8.11-8.24 ( m, 4H, Ar-H), 8.65 (br s,4H, -NH2 ), 4.72 (s, 2H,pyran–H): 13C NMR(75 MHz, DMSO-d6, δ, ppm): δ 32.2, 59.5, 110.6, 114.5, 117.0, 121,5, 122.4, 131.2, 132.6, 134.3, 142.2, 147.7, 151.0, 177.3; MS (EI, m/z (%)): 508.11[M+H]+; Calculated% of C26H16N6O6: C 61.42; H 3.17; N 16.53; Found: C 60.90; H 2.98; N 16.22.
General procedure for the synthesis of 2,8-dioxo-4,6-bis(subtitutedphenyl)-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile 4(a-e)
To the compound 1(a-f) (0.01 mol) added a solution of formic acid (0.04 mol) drop wise with stirring at room temperature for 10 min. Than continued the stirring at 500C for 40 min. After completion of the reaction (monitored by TLC), the mixture poured into ice-cold water, yellow colored solid separated out filter it, washed with water than re-crystallized from ethanol to get pure corresponding compounds 4(a-e).
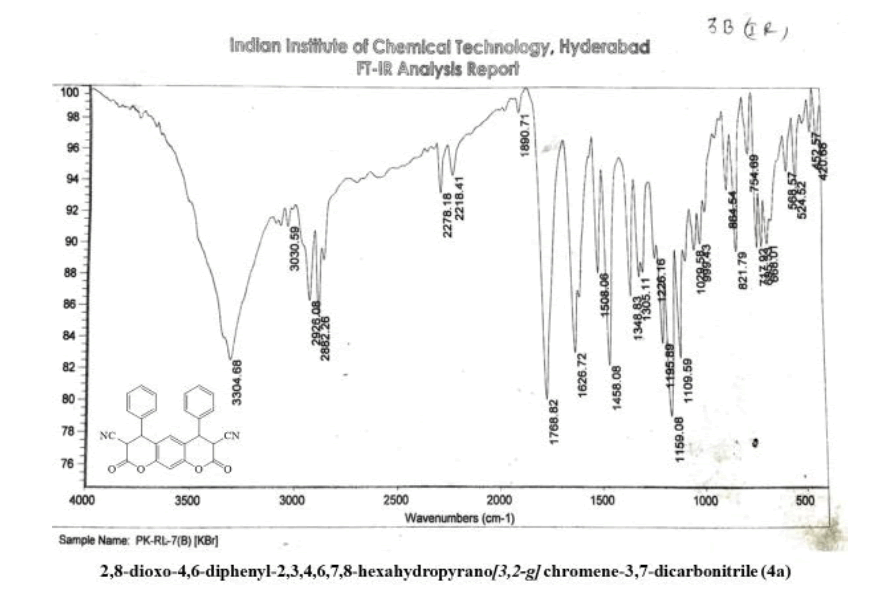
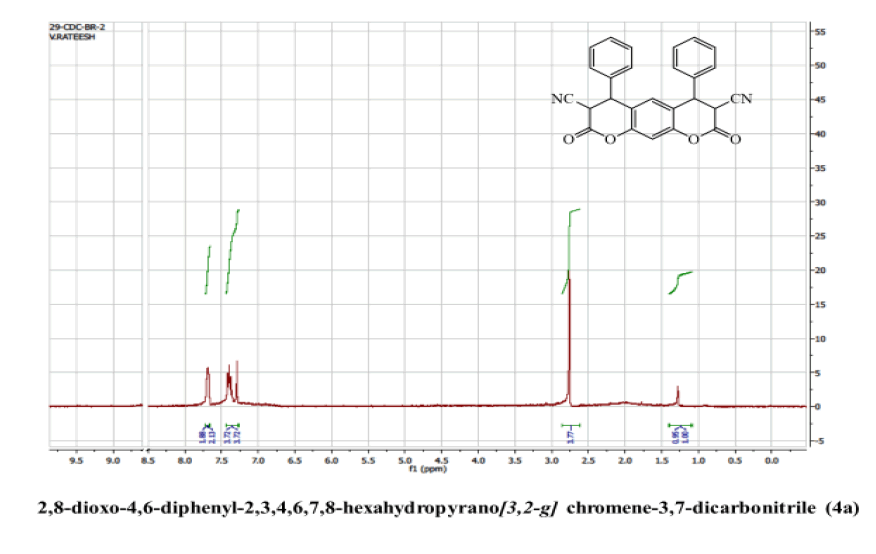
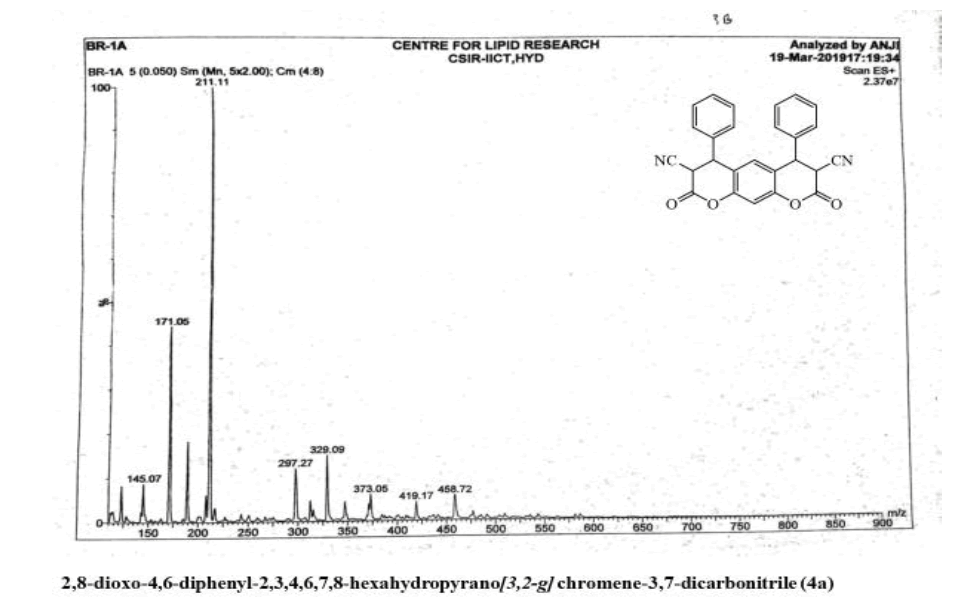
2,8-dioxo-4,6-diphenyl-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile (4a)
Yield: 81%; m p: 192-194 0C; FT-IR(KBr, ν,cm-1): 2190 (-CN), 1690, 1640 (-C=O), 1620 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.13 (d, 2H-CH), 4.71 (d, 2H, pyran-H), 7.12 (s, 1H, Ar-H), 7.22 (s, 1H, Ar-H), 7.31-7.50 (m, 10H, Ar-H); 13C NMR(75 MHz, DMSO-d6, δ, ppm): 35.4, 42.8, 116.2, 118.8, 124.3, 126.2, 128.7, 129.0, 135.9, 143.7, 146.3, 169.6,MS (EI, m/z (%)): 420.11[M+H]+;Calculated % of C26H16N2O4: C 74.28; H 3.84; N 6.66 : Found:C 73.98;H3.62;N6.45
4,6-bis(2-hydroxyphenyl)-2,8-dioxo-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile(4b)
Yield: 75%; m p: 186-188 0C; FT-IR(KBr, ν,cm-1): 2200 (-CN), 1630, 1650 (C=O), 1610 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm); 4.08 (d, 2H, -CH), 4.65 (d, 2H, pyran-H), 6.80-7.02 (m, 6H, Ar-H), 7.10 (s, 1H, Ar-H), 7.22 (m, 1H, Ar-H), 7.21 (m, 1H, Ar-H), 10.5 (s, 2H, Ar-OH); 13C NMR(75 MHz, DMSO-d6, δ, ppm): 28.4, 43.3, 116.4, 116.8, 118.8, 122.3, 124.0, 127.4, 129.2, 132.9, 135.8, 147.8, 156.3, 169.0; MS (EI, m/z (%)): 452.10M+H]+;Calculated % of C26H16N2O6: C 69.02; H 3.56; N 6.19: Found: C 68.89; H 3.42; N 6.05.
4,6-bis(4-methoxyphenyl)-2,8-dioxo-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile (4c)
Yield: 67 %; m p: 205-206 0C; FT-IR(KBr, ν,cm-1): 2210 (-CN), 1690, 1710 (C=O), 1610 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm): 3.83 (s, 6H,-OCH3), 4.09 (d, 2H,-CH), 4.70 (d, 2H, pyran-H), 6.92 (dd, 4H, Ar-H), 7.10 (s, 1H, Ar-H), 7.15 (s, 1H, Ar-H), 7.20 (dd, 4H, Ar-H);13C NMR(75 MHz, DMSO-d6, δ, ppm): 34.6, 43.8, 53.8, 114.8, 116.2, 119.1, 124.8, 129.2, 135.2, 135.9, 147.7, 156.8, 169.2; MS (EI, m/z (%)): 480.13[M+H]+; Calculated % of C28H20N2O6: C 69.99; H 4.20; N 5.83: Found: C 69.19; H 4.06; N 5.45.
4,6-bis(2,3-dichlorophenyl)-2,8-dioxo-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile (4d)
Yield: 76 %; m p: 172-173 0C; FT-IR(KBr, ν,cm-1): 2190 (-CN), 1640, 1690 (C=O), 1590 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.22 (d, 2H), 4.90 (d, 2H, pyran-H), 6.92 (s, 1H, Ar-H), 7.14 (s, 1H, Ar-H), 7.20-7.4-2 (dd, 4H, Ar-H), 7.71 (d, 2H, Ar-H);13C NMR(75 MHz, DMSO-d6, δ, ppm): 29.2, 43.8, 116.7, 118.8, 124.8, 126.9, 129.0, 134.9, 135.6, 147.6, 158.3, 169.1, MS (EI, m/z (%)): 555.96[M+H]+; Calculated % of C26H12Cl4N2O4: C 55.94; H 2.17; N 5.02: Found: C 55.19; H 1.96; N 4.95.
4,6-bis(3-nitrohenyl)-2,8-dioxo-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile (4e)
Yield: 72 %; m p:180-182 0C; FT-IR(KBr, ν,cm-1): 2210 (-CN), 1640, 1700 (C=O), 1620 (C=C); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.12 (d, 2H), 4.80 (d, 2H, pyran-H), 7.04 (s, 1H, Ar-H), 7.10 (s, 1H, Ar-H), 7.73 (m, 4H, Ar-H), 8.02 (d, 2H, Ar-H), 8.20 (s, 2H, Ar-H);13C NMR(DMSO-d6): 33.4, 43.3, 116.8, 119.2, 121.5, 124.6, 125.2, 129.0, 135.9, 136.7, 143.7, 148.9, 169.2,.MS (EI, m/z (%)): 510.08[M+H]+;Calculated % of C26H14N4O8: C 61.18; H 2.76; N 10.98: Found: C 60.98; H 2.62; N 10.42.
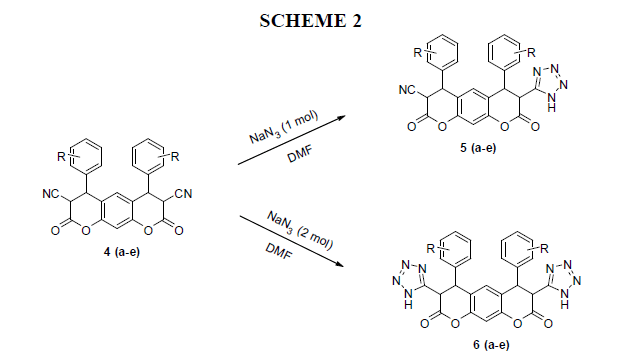
General procedure for the synthesis of 2,8-dioxo-4,6-bis(subtitutedphenyl)-7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3-carbonitrile 5(a-e)
To a stirred solution of dicarbonitrile (1 mmol) and sodium azide (1.3 mmol) in dry DMF was added Aluminium chloride (20 mol %) and the reaction mixture was heated up to 120 ˚C for 4 h. After completion of the reaction (reaction monitored by TLC), the catalyst was removed by filtration and filtrate was treated with ethyl acetate and 4 N HCl and stirred vigorously. The resultant organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with water, brine and dried over sodium sulfate, concentrated to furnish the desired tetrazoles, the obtained products were purified by crystallization using petroleum ether/ethyl acetate (1:1 ratio).
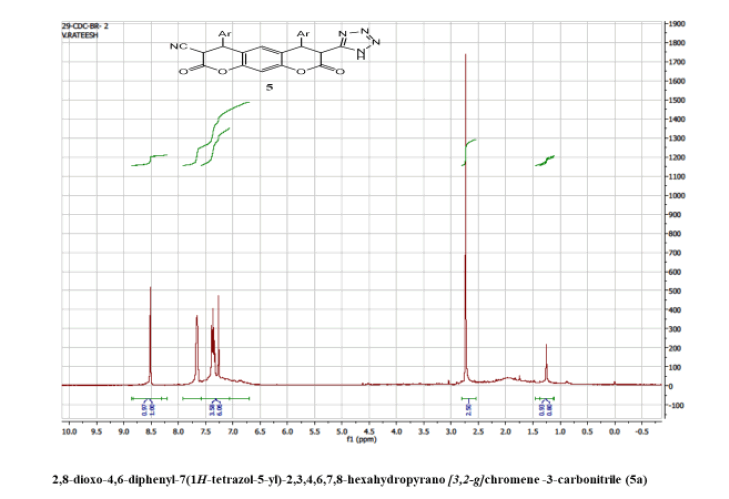
2,8-dioxo-4,6-diphenyl-7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene -3-carbonitrile (5a)
Yield: 64%; m p: 182-184 0C; FT-IR(KBr, ν,cm-1): 3416 (-NH), 3126 (C-H, SP2 Stretching-Ar), 2190 (-CN), 1694(-C=N), 1640 (C=O), 1620 (C=C),1597, 1470, 1320 (N=N); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.02 (d, 1H), 4.31 (d, 1H), 4.70 (d, 1H, pyran-H),5.02 (d, 1H, pyran-H); 7.10 (s, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.41-7.52 (m, 10H, Ar-H); 13C NMR(DMSO-d6): 36.2, 43.8, 50.4, 54.6, 116.2, 119.1, 124.3, 126.6, 128.2, 129.0, 135.7, 143.4, 145.3, 147.2, 146.3, 158.2, 167.6, 170.2: MS (EI, m/z (%)):463.13[M+H]+; Calculated % of C26H17N5O4: C 67.38; H 3.70; N 15.11: Found: C 67.08; H 3.52; N 14.95.
4,6-bis(2-hydroxyphenyl)-2,8-dioxo--7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano [3,2-g] chromene-3-carbonitrile (5b)
Yield: 68 %; m p:196-198 0C; FT-IR(KBr, ν,cm-1): 3520 (-OH), 3410 (-NH), 3115 (C-H, SP2 Stretching-Ar), 2190 (-CN), 1692 (C=N), 1660 (C=O), 1610 (C=C), 1580, 1480, 1310 (N=N); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.10 (d, 1H), 4.52 (d, 1H), 4.83 (d, 1H, pyran-H), 5.10 (d, 1H, pyran-H), 6.85-9.82 (m, 6H, Ar-H), 7.06 (s, 1H, Ar-H), 7.10 (m, 2H, Ar-H), 7.2 (s,1H, Ar-H), 10.5 (s,2H, Ar-OH); 13 C NMR(DMSO-d6): 29.4, 43.2, 44.0, 55.2, 116.5, 119.7, 124.8, 127.6, 129.0, 135.8, 143.4, 145.3, 147.0, 146.3, 155.7, 158.2, 164.6, 169.6; MS (EI, m/z (%)): 495.12[[M+H]+; Calculated % of, C26H17N5O6: C 63.03; H 3.46; N14.14: Found: C 62.98; H 3.22; N 13.98.
4,6-bis(4-methoxyphenyl)-2,8-dioxo--7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano [3,2-g] chromene-3-carbonitrile (5c)
Yield: 65%; m p: 212-214 0C; FT-IR(KBr, ν,cm-1):3420(-NH), 3125 (C-H, SP2 Stretching-Ar), 2200 (-CN), 1690 (C=N), 1650 (C=O), 1620 (C=C), 1590, 1480, 1310 (N=N); 1H NMR(300MHz, DMSO-d6, δ, ppm): 3.80 (s, 6H,-OCH3), 4.02 (d, 1H), 4.33 (d, 1H), 4.75 (d, 1H, pyran-H), 5.22 (d, 1H, pyran-H); 6.92.(m, 4H, Ar-H), 7.06 (s, 1H, Ar-H), 7.10 (s, 1H, Ar-H), 7.21 (m, 4H, Ar-H); 13C NMR(75 MHz, DMSO-d6, δ, ppm): 34.4, 43.4 ,50.0, 54.3, 56.8, 114.2, 116.0, 119.1, 124.2, 130.9.135.8, 136.2, 138.3, 143.4, 147.4, 158.2, 160.7, 167.5, 169.6;MS (EI, m/z (%)): 523.15[M+H]+; Calculated % of, C28H21N5O6: C 64.24; H 4.04; N13.38: Found: C 63.98; H 3.92; N 13.04.
4,6-bis(2,3-dichlorophenyl)-2,8-dioxo--7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano [3,2-g] chromene-3-carbonitrile (5d)
Yield (72 %); m p: 176-177 0C; FT-IR(KBr, ν,cm-1): 3415 (NH), 3128(C-H, SP2 Streching-Ar), 2210 (CN), 1686(C=N), 1650 (C=O), 1610 (C=C), 1560, 1460, 1320(N=N), 1200, 1120 ,650,700; 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.1(d, 1H), 4.4 (d, 1H), 4.7 (d, 1H, pyran-H) ,5.1(d, 1H, pyran-H); 6.9.(s,1H,ArH), 7.04 (s, 1H,ArH), 7.15-7.2(m, 4H,ArH), 7.6 (d,2H,ArH).; 13C NMR(75 MHz, DMSO-d6, δ, ppm):29, 42,44, 54, 116.8, 119, 124, 127,129, 133.,136, 137, 147.4, 159,1 167, 169MS (EI, m/z (%)): 598.97[M+H]+ ;Calculated %, C26H13 Cl4N5O4: C 51.94; H 2.18; Cl,23.59, N11.65: Found: C 51.68; H 2.02;Cl,23.08 N11.04.
4,6-bis(3-nitrophenyl)-2,8-dioxo--7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano [3,2-g] chromene-3-carbonitrile (5e)
Yield: 66%; m p:190-192 0C; FT-IR(KBr, ν,cm-1): 3380 (-NH), 3250 (C-H, SP2 Stretching-Ar), 2200 (CN), 1680 (C=N), 1670 (C=O), 1620 (C=C), 1600, 1480, 1330 (N=N); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.08 (d, 1H), 4.38 (d, 1H), 4.67 (d, 1H, pyran-H), 5.22 (d, 1H, pyran-H); 7.14 (s, 1H, Ar-H), 7.22 (s, 1H, Ar-H), 7.70 (m, 4H, Ar-H), 8.11 (m, 4H, Ar-H); 13C NMR(75 MHz, DMSO-d6, δ, ppm): 33.2, 43.4, 49.0, 56.2, 116.6, 119.0, 121.4, 124.6, 130.2, 133.3.,135.4, 143.2, 147.3, 148.8, 158.8, 166.5, 168.0MS (EI, m/z (%)): 553.10[M+H]+; Calculated % of, C26H15 N7O8: C 56.43; H 2.73; N, 17.72: Found: C 56.08; H 2.19; N, 17.04.
General procedure for the synthesis of 4,6-bis(subtitutedphenyl)-3,7-di(1H-tetrazol-5-yl)-3,4,5,7-tetrahyropyrano[3,2-g] chromene-2,8-dione(6a)
To a stirred solution of dicarbonitrile (1 mmol) and sodium azide (2.6 mmol) in dry DMF was added Aluminium chloride (20 mol %) and the reaction mixture was heated up to 120°C for 4 h. After completion of the reaction (reaction monitored by TLC), the catalyst was removed by filtration and filtrate was treated with ethyl acetate and 4 N HCl and stirred vigorously. The resultant organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with water, brine and dried over sodium sulfate, concentrated to furnish the desired tetrazoles, the obtained products were purified by crystallization using petroleum ether/ethyl acetate (1:1 ratio).
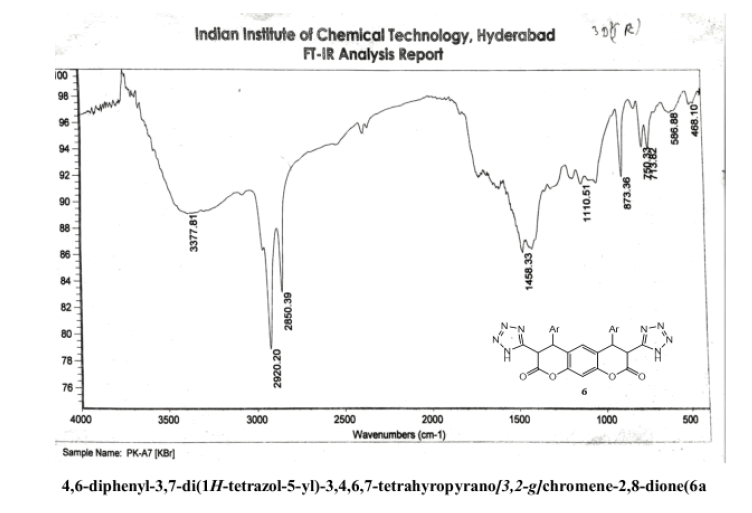
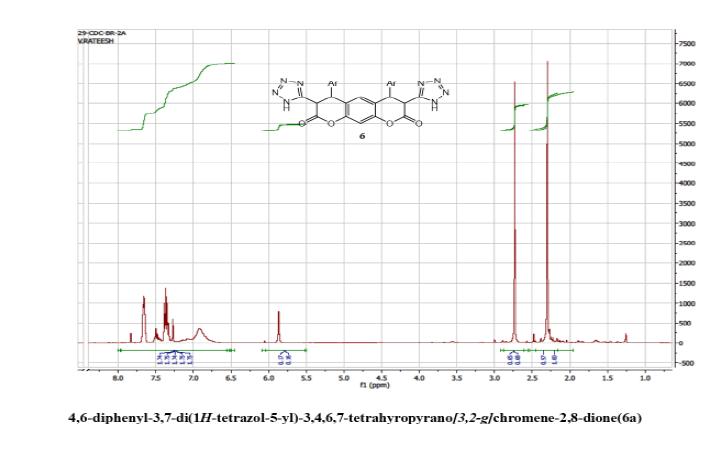
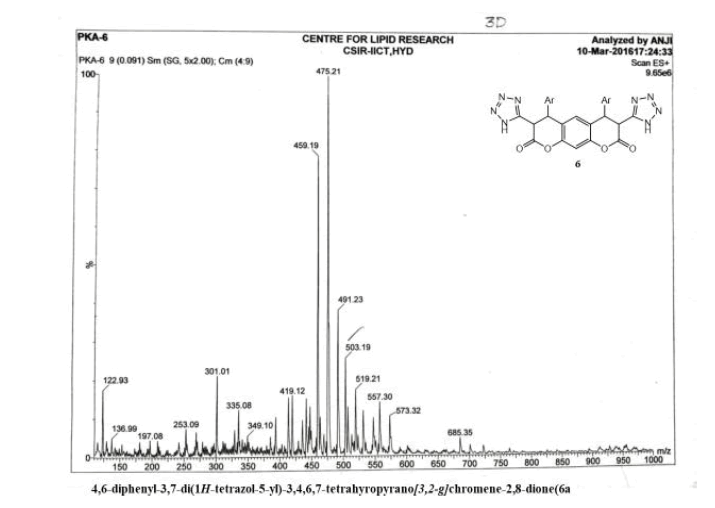
4,6-diphenyl-3,7-di(1H-tetrazol-5-yl)-3,4,5,7-tetrahyropyrano[3,2-g] chromene-2,8-dione(6a)
Yield: 68%; m p: 196-198 0C; FT-IR(KBr, ν,cm-1): 3452 (-NH), 3100 (C-H, SP2 Stretching-Ar), 2180 (-CN), 1686(-C=N), 1680 (C=O), 1630 (C=C),1545, 1450, 1340 (N=N); 1H NMR(300MHz, DMSO-d6, δ, ppm): 4.2 (d, 2H, pyran-H), 4.8 (d, 2H, pyran-H),; 7.10(s, 1H, Ar-H), 7.18 (s, 1H, Ar-H), 7.36-7.49 (m, 10H, Ar-H); 13C NMR(75 MHz, DMSO-d6, δ, ppm): 52.4, 57.6, 118, 122.1, 124.3, 125.9, 128.2, 132.8, 144.4, 146.3, 147.2, 156.8, 167.6, 170.2: MS (EI, m/z (%)): 506.15[M+H]+; Calculated % of C26H18N8O4: C 61.66; H 3.58; N 22.18: Found: C 60.08; H 3.42; N 22.05.
Results and Discussions
The compound 2,8-dioxo-4,6-bis(subtitutedphenyl)-7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano[3,2-g] chromene-3-carbonitrile 3(a-e) was obtained after multiple reaction steps starting from resorcinol. The resorcinol was condensed with malanonitrile and aromatic aldehydes (a-e) under basic conditions gave the corresponding 2,8-diamino-4,6-bis(subtitutedphenyl)-4,6-dihydropyrano[3,2-g]chromene-3,7-dicarbonitrile 1(a-e) further these compounds are treated with formic acid, deamination takes place to produced corresponding 2,8-dioxo-4,6-bis(subtitutedphenyl)-2,3,4,6,7,8- hexahydropyrano[3,2-g] chromene-3,7-dicarbonitrile 2(a-e) and these compounds are converted into corresponding 2,8-dioxo-4,6- bis(subtitutedphenyl)-7(1H-tetrazol-5-yl)-2,3,4,6,7,8-hexahydropyrano[3,2-g]chromene-3-carbonitrile 3(a-e) with sodium azide in good yields (Scheme 1). The pure compounds were obtained by re-crystallization from an ethanol as solvent. All the synthesized compounds were confirmed based on elemental analyses, IR and NMR spectral data.
Conclusion
The formation of 8-(1H-Tetrazol-5-ylmethyl)-naphthalen-2-ol (3) from (7-Hydroxy-naphthalen-1-yl)-acetonitrile (2), was confirmed by their spectral data.
The IR spectra shown the loss of sharp absorption band at around 2250 cm −1 due to –CN group and a band is appeared for -NH group around 3345 cm −1 confirmed the construction of tetrazole, whereas in proton spectra (1H NMR) a singlet is appeared at δ 16.18 due to the tetrazole-NH proton and in carbon spectra (13C NMR) the tetrazole carbon was observed around at δ 158.81.
Summary
In this, we report the novel synthesis of Pyranotetrazole relative compounds 2,8-dioxo-4,6-bis(subtitutedphenyl)-7(1H-tetrazol-5-yl)-2,3,4,6,7,8- hexahydropyrano[3,2-g] chromene-3-carbonitrile 5(a-e) and 4,6-bis(subtitutedphenyl)-3,7-di(1H-tetrazol-5-yl)-3,4,5,7-tetrahyropyrano[3,2-g] chromene-2,8-dione(6a) by using resorcinol, substituted aldehyde and malononitrile. The key intermediates namely 2,8-diamino-4,6- bis(subtitutedphenyl)-4,6-dihydropyrano[3,2-g]chromene-3,7-dicarbonitrile 3(a-e) and 2,8-dioxo-4,6-bis(subtitutedphenyl)-2,3,4,6,7,8- hexahydropyrano[3,2-g]chromene-3,7-dicarbonitrile 4(a-e) prepared in this synthesis. Above reported compounds conformed by spectral data.
References
- I Kostova, G Momekov, M Zaharieva et al., Eur J Med Chem. 2005, 40: p. 542.
- M Ough, A Lewis, EA Bey et al., Cancer Biol Ther. 2005, 4: p. 95.
- VF De Andrade-Neto, MOF Goulart, JF Da Silva Filho et al., Bioorg Med Chem Lett. 2004, 14: p. 1145.
- DO Moon, YH Choi, ND Kim et al., Int Immunopharmacol. 2007, 7: p. 506.
- AM Shestopalov, YM Emelianova and VN Nesterov. Russ Chem Bull. 2003, 52: p. 1164.
- ER Bissell, AR Mitchell and REJ Smith. Org Chem. 1980, 45: p. 2283.
- GA Reynolds andKH Drexhage. Opt Commun. 1975, 13: p. 222.
- PF Juby,TW Hudyma and M Brown. J Med Chem. 1968, 11: p. 111.
- RM Herbst, CW Roberts, HTF Givens et al., J Org Chem. 1952, 17: p. 262.
- RD Smith, JV Duncia, RJ Lee et al., Methods Neurosci. 1993, 13: p. 258.
- TD Heightman, P Ermert, D Klein et al., Chim Acta 1995, 78: p. 514.
- SJ Wittenberger. A review. Org. Prep. Proced Int. 1994, 26: p. 499.
- JJ McGuire, CA Russell, WE Bolanowska et al., Cancer Res. 1990, 50: p. 1726.
- JA Zablocky, M Miyano, NR Sashidhar et al., J Med Chem. 1992, 35: p. 4914.