Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
Synthesis of New Triazole and Aza-β-lactam Compounds Derived from o-(Npropargyl)-sulfonamido Benzoic Acid of Possible Biological Activity
Ahmed W Naser1, Muthanna S Farhan2 and Kany A Abdulqader1*
1Department of Chemistry, College of Science, University of Baghdad, Baghdad, Iraq
2Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, Baghdad, Iraq
- *Corresponding Author:
- Kany A Abdulqader
Department of Chemistry
College of Science
University of Baghdad
Baghdad, Iraq
Abstract
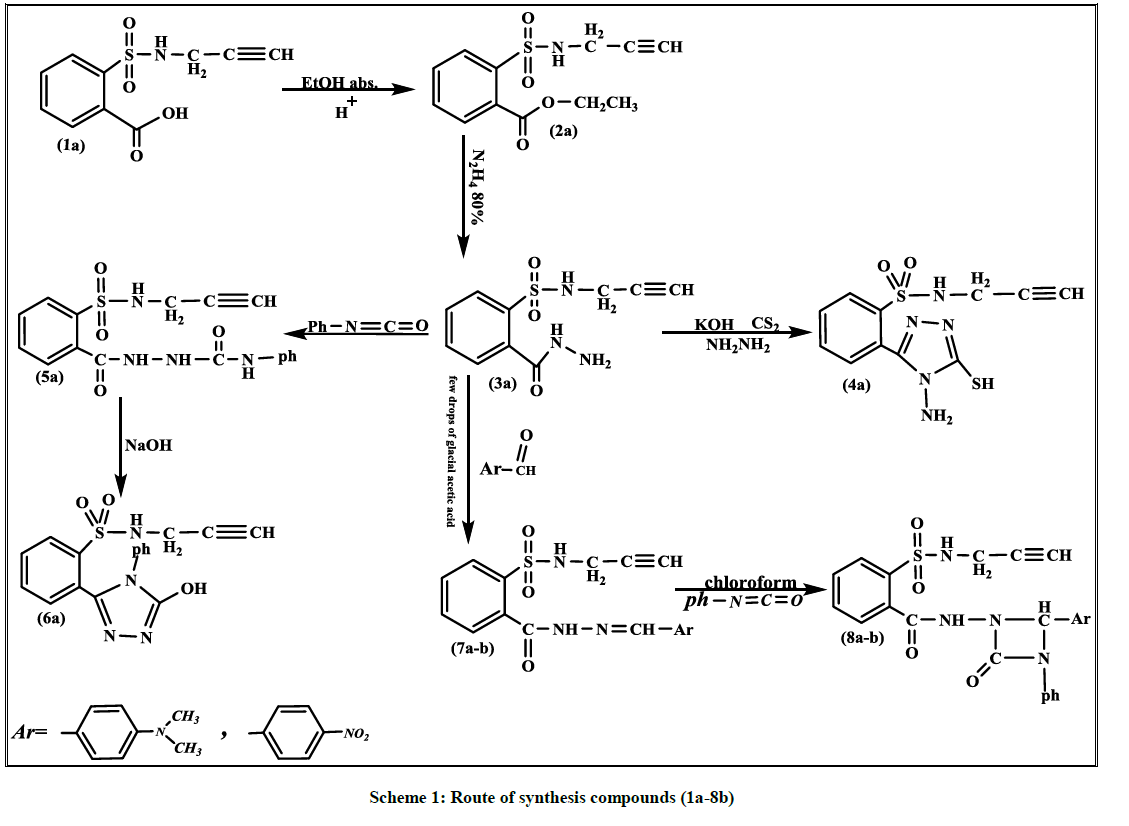
A variety of new triazole and Aza-β-lactam derivatives derived from o-(N-propargyl) sulfonamido benzoic acid (1a) were synthesized. Firstly, reaction of compound (1a) with absolute ethanol, in the presence concentrated H2SO4 gave ethyl-o-(N-propargyl)sulfoamido benzoate (2a). Subsequently, the produced react with 80% hydrazine hydrate gave o-(N-propargyl)sulfonamido benzohydrazide (3a). The cyclization of (3a) with carbon disulfide and hydrazine hydrate (80%) in presence potassium hydroxide gave o-(4-amino-5-mercapto-1,2,4-triazol-3-yl)-N-propargyl benzene sulfonamide compound (4a). N-propargyl-o-[N\-sulfamidobenzoyl] hydrazine carboxamide (5a) was obtained by reaction of (3a) with Phenyl isocyanates. The cyclization of compound (5a) with 30% NaOH gave 3-hydroxy-4-phenyl-1,2,4-triazole compound (6a). The Schiff bases (7a-b) were obtained by condensation of (3a) with 4-(N,N-dimethyl amino)benzaldehyde and p-nitrobenzaldehyde. Aza-β-lactam compounds (8a-b) were prepared by the cycloaddition of Schiff-bases (7a-b) with phenyl isocyanate via [2+2] cycloaddition reaction. The newly prepared compounds were identified by (FTIR), (1H-NMR) and their physical properties were measured. The compounds were tested for their biological activity, some compound was active and others were not.
Keywords
1,2,4-Triazole, Aza-β-lactam, Hydrazine carboxamide, Antibacterial
Introduction
The heterocyclic compounds are requisite to life in different ways and are widely distributed in nature [1]. The chemistry of heterocyclic compounds is one of the most complex branches of organic chemistry [2]. It is evenly interesting for its theoretical implications, for the variety of its synthetic procedures, and for the industrial and physiological significance of heterocyclic compounds. Also the studies of heterocyclic compounds have been an important field for a long time in medicinal chemistry [3]. A number of heterocyclic derivatives which having nitrogen or (and) sulphur atom and it can be viewed as a privileged scaffold for experimental drug design [4]. Triazoles and their substituted derivatives are important and continuous concern in the chemistry of five-member heterocyclic compounds. Four and Five-membered nitrogen heterocycle compounds such as triazoles and Aza-β-lactams are significant structural fragments, and also considered as biologically active compounds [5- 10], corrosion inhibitors [11], pesticides [12], dyes [13], acid-base indicator [14], and other industrial chemicals [15]. 2-Azetidinones have been used as intermediates to the synthesis of alpha-amino acid and hydantoins [16].
Materials and Methods
All chemicals were purchased from Fluka, BDH and Merk. Melting points were recorded using electro thermal melting point apparatus. The FT-IR spectral data were recorded on a Shimadzu FTIR-8400 S spectrophotometer in the Department of Chemistry, College of Science, University of Baghdad. 1H-NMR spectrum was recorder on central laboratory of Isfahan University and Sharif University of technology, 400 MHz, using Deuterated Chloroform (CDCl3) or Dimethyl Sulfoxide (DMSO).
Synthesis of ethyl-o-(N-propargyl)sulfoamido benzoate (2a) [17]
To the solution of compound o-(N-propargyl) sulfonamido benzoic acid (1a) (0.5 g, 0.000 mol) in absolute ethanol (30 ml), conc. sulfuric acid (2 ml) was added and the mixture was refluxed for 8 h, then concentrated the solution and neutralized by sodium bicarbonate. The formed white precipitate was filtered and recrystallized from ethanol to give compound (2a). Yield 61%, m.p. 110-112°C, IR (KBr) cm-1: 3274 (C-H), Acetylinic, 2125 (C≡C), 1737 (C=O), 1056 (C-O) Ester. 1H-NMR (DMSO-d6), (δ ppm): 1.4 (s, 3H, CH3), 2.45(s, 1H, CH acetylinic), 3.95 (s, 2H, CH2), 4.55 (O-CH2), 7.3.94 (s, 1H, NH-C=O), 7.65-8.2 (m, 4H, Ar-H).
Synthesis of o-(N-propargyl)sulfoamido benzohydrazide (3a) [18]
To a solution of compound (2a) (0.5 g, 0.004 mol) in absolute ethanol (20 ml), (0.05 mol) of hydrazine hydrate was added and the mixture was refluxed 4 h. After cooling, the formed white precipitate was filtered and recrystallized from ethanol to give compound (3a). Yield 70%, m.p. 78- 80°C, IR (KBr) cm-1: 3330-3242 NH, NH2, 1656-1625 (C=O) amide. 1H-NMR (DMSO-d6), (δ ppm): 1.45 (s, 2H, NH2), 2.98 (s, 1H, CH acetylinic), 3.91 (s, 2H, CH2), 5.94 (s, 1H, NH-C=O), 7.54-7.86 (m, 4H, Ar-H).
Synthesis of o-(4-amino-5-mercapto-1,2,4-triazol-3-yl)-N-propargyl benzene sulfonamide (4a) [19]
A mixture of compounds (3a) (0.5 g, 0.002 mol), (0.003 mol) carbon disulfide and potassium hydroxide (0.2 g, 0.003 mol) was dissolved in (15 ml) absolute ethanol and refluxed for 4 h in water bath. After that excess carbon disulfide was removed by distillation. Hydrazine hydrate (0.006 mol) was added to the solution and refluxed for 3 h. After cooling, acidification by 20% HCl, the formed precipitate was filtered and recrystallized from ethanol to give compound (4a). Yield 80%, m.p. 133-134°C, IR (KBr) cm-1: 3519-3408 NH, NH2, 1631 (C=N). 1H-NMR (DMSO-d6), (δ ppm): 2.82 (s, 1H, CH acetylinic), 3.45 (s, 1H, NH-SO2), 3.8 (s, 2H, CH2), 5.6 (s, 2H, NH2), 7.80-8.03 (m, 4H, Ar-H), weak signal at 14.79 (s, 1H, SH).
Synthesis of N-propargyl-o-[N\-sulfoamido benzoyl] hydrazine carboxamide (5a) [20]
A mixture of compound (3a) (0.5 g, 0.002 mol) and phenyl isocyanate (0.002 mol) was refluxed in absolute ethanol (15 ml) for 6 h. After cooling the formed precipitate was filtered, dried and recrystallized from ethanol to give the compound (5a). Yield 85%, m.p. 134-136°C, IR (KBr) cm-1: 3298 (N-H), 1672 (C=O). 1H-NMR (DMSO-d6), (δ ppm): 3.09 (s, 1H, CH acetylinic), 3.91 (s, 2H, CH2), 7.25-8.33 (m, 10H, Ar-H and SO2-NH), 9.30 (s, 1H, NH).
Synthesis of o-(4-phenyl-5-hydroxy-1,2,4-triazole -3-yl)-N-propargyl benzene sulfonamide (6a) [21]
A solution of compound (5a) (0.5 g, 0.001 mol) in (18 ml) 30% NaOH was refluxed for 4 h. The reaction mixture was cooled and acidified with conc. HCl. the precipitate was filtered and recrystallized from ethanol, to give compound (6a). Yield 80%, m.p. 279-280°C, IR (KBr) cm-1: 3500-3373. O-H overlap with N-H, 1633 C=N. 1H-NMR (DMSO-d6), (δ ppm): 2.95 (s, 1H, CH acetylinic), 3.45 (s, 1H, NH-SO2), 3.86 (s, 2H, CH2), 7.68-8.02 (m, 9H, Ar-H), weak signal at 11.34 (s, 1H, OH).
General procedure for the synthesis of Schiff’s bases (7a-b) [22]
A mixture of compound (3a) (0.5 g, 0.002 mol), with appropriate aromatic aldehydes namely 4-(N,N-dimethyl amino)benzaldehyde and pnitrobenzaldehyde in (20 ml) absolute ethanol and few drops of glacial acetic acid were refluxed for 5 h. The formed precipitate after cooling was filtered, dried and recrystallization from ethanol to give compounds (7a-b) respectively.
o-[(N-propargyl)sulfamoyl]-N-[4-(N,N-dimethylamino)benzaldine]benzamide (7a): Yield 75%, m.p. 188-191°C, IR (KBr) cm-1: 3120 (N-H), 1602 (C=N), 1226 (C-N). 1H-NMR (DMSO-d6), (δ ppm): 2.07 (s, 1H, CH acetylinic), 3 (s, 6H, (CH3)2-N), 3.81 (s, 2H, CH2), 6.75 (s, 1H, NH-C=O), 7.54-7.86 (m, 8H, Ar-H), 8.76 (CH=N).
o-[(N-propargyl)sulfamoyl]-N-(p-nitrobenzaldine)benzamide (7b): Yield 61%, m.p. 220-222°C, IR (KBr) cm-1: 3160 (N-H), 1596 (C=N), (1552) asym. (1346) sym. (NO2). 1H-NMR (DMSO-d6), (δ ppm): 2.04 (s, 1H, CH acetylinic), 2.9 (s, 6H, (CH3)2-N), 4.15 (s, 2H, CH2), 5.94 (s, 1H, NH-C=O), 7.3-8.1 (m, 8H, Ar-H), 8.77 (CH=N).
General procedure for synthesis of 1,3-diazetidine-4-one(Aza-β-lactam)derivatives (8a-b) [23]
A mixture of Schiff bases (7a-b) (0.5 g, 0.002 mol) and phenyl isocyanate (0.002 mol) in (15 ml) chloroform was refluxed for 6 h. The solvent was removed and the residue treated with mixture of (1:1) ethyl acetate and petroleum ether. The result precipitate was filtered and dried to give compounds (8a-b) respectively (Scheme 1).
N-[2-oxo-3-phenyl-4-[4-(N,N-dimethylamino)phenyl-1,3-diazetidin-1-yl]-o-[(N-propargyl) sulfonamido] benzamide (8a): Yield 87%, m.p. 134-135°C, IR (KBr) cm-1: 3305 (N-H), 1714 (C=O) lactam, 1649 (C=O) Amide, 1228 C-N.
N-[2-oxo-3-phenyl-4-[(p-nitro)phenyl-1,3-diazetidin-1-yl]-o-[(N-propargyl)sulfamido]benzamide (8b): Yield 82%, m.p. 128-129°C, IR (KBr) cm-1: 3328 (N-H), 1706 (C=O) lactam, 1649 (C=O) Amide, (1500) asym., (1313) sym. (NO2).
Antibacterial assay
Some of the prepared compounds were tested against two strain of gram positive bacteria Bacillus, staphylococcus aureus & one strain Gram negative bacteria Klebsiella pneumonia. The agar and petri dishes were sterilized by autoclaving for (15 min) at 121°C. The agar plates were surface inoculated uniformly from the broth culture of the tested microorganisms. The suitably spaced apart holes in solidified medium were made all (6 mm) in diameter, in which were filled with 100 μl of prepared compounds (1 mg of the compound dissolved in 1 ml of DMSO solvent), ciprofloxacin was used as standard. The incubation of these plates was for 24 h at 37°C [24].
Results and Discussion
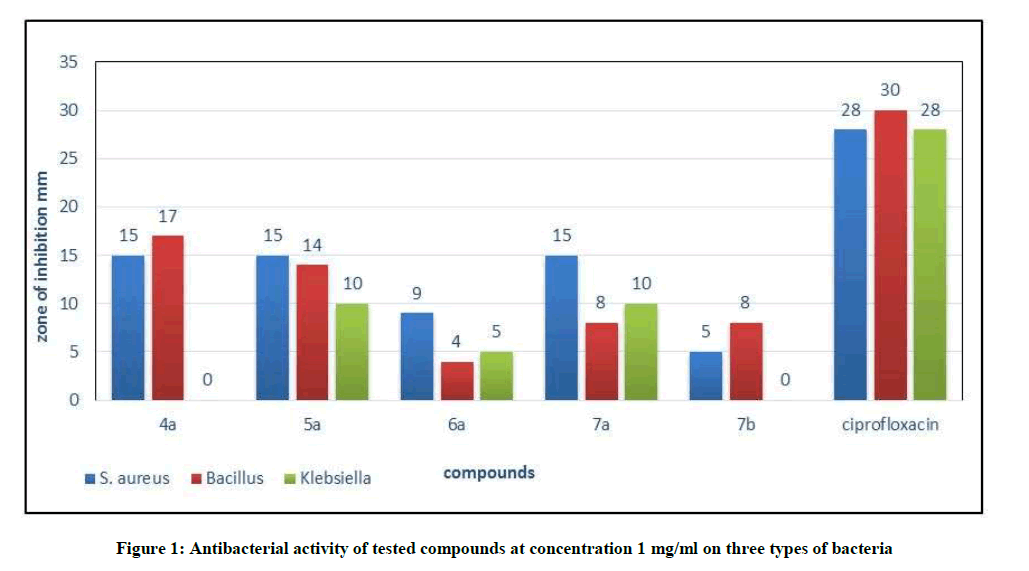
o-(N-propargyl) sulfonamido benzoic acid (1a) as starting material was prepared according to the literature method [25]. It was reacted with absolute ethanol, in the presence concentrated H2SO4 gave compound (2a) then it react with 80% hydrazine hydrate gave o-(N-propargyl) sulfoamido benzohydrazide (3a). The disappearance of (C=O ester) stretching band at (1737) cm-1 and the appearance of new stretching bands at (3242-3330) cm-1, 1656 cm-1 which are due to (NH-NH2) and (C=O amide) respectively are attributed to the formation of benzohydrazide derivative (3a), while 1H-NMR spectra showed disappearance of signals 1.4 (s, 3H, CH3), 4.55 (O-CH2), and appearance of signals at 1.45 (s, 2H, NH2), 5.94 (s, 1H, NH-C=O) ppm. The cyclization of (3a) with carbon disulfide in presence potassium hydroxide then added hydrazine hydrate gave the corresponding 1,2,4-triazole compound (4a). The absence of the stretching band at (1656) cm-1 for C=O amide and appearance of stretching band for (C=N) at 1631 cm-1, it was good evidence for the formation of triazole derivative. 1H-NMR spectrum of compound (4a) showed characteristic signals 5.6 (s, 2H, NH2), weak signal at 14.79 (s, 1H, SH). Carboxamide derivative (5a) was prepared by the reaction of compound (3a) and phenyl isocyanate, the product was identified by FT-IR spectrum which shows absence of stretching band of (NH-NH2) at (3242- 3330) cm-1 and the appearance of new absorption bands due to (NH) at (3220) cm-1 and (C=O) at (1672) cm-1, while 1H-NMR spectrum showed a signal at 9.30 (s, 1H, NH). The reaction of (5a) with 30% NaOH under refluxing intermolecular cyclization in which giving the desired hydroxyl triazole compound (6a). The FT.IR spectrum shows The absence of the carbonyl group (C=O) stretching band at 1672 cm-1 and the appearance of the (OH overlap with NH) stretching band at (3373-3000) cm-1 and stretching band of (C=N) at (1633) cm-1 indicate the formation of compound (6a), while in 1H-NMR spectrum we have 7.68-8.02 (m, 9H, Ar-H) and weak signal at 11.34 (s, 1H, OH). Schiff-bases (7a-b) were prepared by condensation of hydrazine derivative (3a) with some aromatic aldehydes namely [4-(N,N-dimethyl amino)benzaldehyde and p-nitrobenzaldehyde] with few drops of glacial acetic acid. The absence of the (NH2) stretching bands at (3242-3330) cm-1 and the appearance (C=N) stretching band at (1596-1602) cm-1 indicate the formation of Schiff-bases, while 1H-NMR spectrum showed singlet signal for (CH=N) at 8.76 ppm. The reaction of (7a-b) with phenyl isocyanate via [2+2] cycloaddition reaction gave the corresponding 1,3-diazetidine-2-one (Aza-β-lactam) derivatives (8a-b). The FT-IR spectrum showed the appearance of carbonyl group (C=O aza-β-lactam) at (1706-1714) cm-1. Table 1 and Figure 1 show the inhibition zones in (mm) of the mentioned compounds. In comparison with standard drug (Ciprofloxacin), the bacterial activity at concentration 1 mg/ml showed that compound (4a) compound has good activity against Gram-positive bacterium Staphylococcus aureus and Bacillus and no activity against Gram-negative bacterium Klebsiella pneumonia. While (5a and 7a) shows moderate activity against Gram-positive bacterium S. aureus and Bacillus and Gram-negative bacterium K. pneumonia. Compounds (6a and 7b) showed no to low activity against Gram-positive bacterium S. aureus, Bacillus and no activity against Gram-negative bacterium K. pneumonia.
| Compound No. | Zone of inhibition in (mm), concentration (1 mg /ml) | ||
|---|---|---|---|
| G+ Staphylococcus | G+ Bacillus | G− Klebsiella pneumonia | |
| 4a | 15 | 17 | - |
| 5a | 15 | 1 4 | 10 |
| 6a | 9 | 4 | 5 |
| 7a | 15 | 8 | 10 |
| 7b | 5 | 8 | - |
| ciprofloxacin | 28 | 30 | 28 |
Table 1: Antibacterial activity of compounds (4a-7a)
Conclusion
Some new hetero cyclic compound derived from o-(N-propargyl)sulfonamido benzoic acid were synthesized. The antibacterial activity of some compounds against Gram-positive and Gram-negative bacteria. Showed that compound (4a) has good activity against Gram-positive bacteria, in which compounds (5a, 6a, 7a and 7b) has moderate to low activity against Gram-positive and Gram-negative bacteria.
Acknowledgment
As the authors of this work, we would love to express our ample thankfulness and gratitude to the Department of Chemistry, College of Science, University of Baghdad and College of Pharmacy, for their magnificent support and providing us with facilities to conduct this research on all accounts.
References
- N.B. Patel, F.M. Shaikh, Sci. Pharm., 2010, 78, 753.
- K. Hamak, H. Eissa, Org. Chem. Curr. Res., 2013, 23, 567.
- K. Starcevic, M. Kralj, K. Ester, I. Sabol, Bioorg. Med. Chem., 2007, 15, 4419.
- M.S. Al-Rawi, H.A. Hassan, D.F. Hassan, R.M. Abdulla, Int. J. Sci. Tech., 2013, 8, 48.
- F.A. Hassan, K.W. Younus, Res. J. Bio. Sci., 2012, 7(1), 48-51.
- S.P. Pardeshi, S.V. Patil, R. Patil, V.D. Bobade, J. Chem. Pharma. Res., 2014, 6(4), 675-681.
- A.J. Atia, S.S. Al-Mufrgeiy, Am. J. Chem., 2012, 2(3), 150-156.
- T. Taj, R.R. Kamble, T. Gireesh, B.V. Badami, J. Chem. Sci., 2011, 123(5), 657-666.
- O. Bekircan, E. Menteşe, S. Ülker, Arch. Pharm. Chem. Life Sci., 2014, 347, 387-397.
- H.M. Abdullah, I.K. Jassim, M.N. Safi, Kerbala, J. Pharm. Sci., 2012, 4, 115-135.
- S. Sripriya1, C. Subha, A. Selvaraj, IOSR-JAC., 2013, 6(2), 25-29.
- S.G. Agalave, S.R. Maujan, V.S. Pore, Chem. Asian J., 2011, 6, 2696-2718.
- J.C. Er, M. K. Tang, C.G. Chia, H. Liew, M. Vendrell, J. Chem. Sci., 2014, 4, 2168-2176.
- V.N. Bulut, C. Duran, A. Gundogdu, Bull. Chem. Soc. Ethiop., 2010, 24(3), 457-460.
- S. Cassani, S. Kovarich, P.P. Roy, L. Van der Wal, J. Haz. Mat., 2013, 258-259, 50-60.
- M. Meusel, M. Gütschow, Org. Prep. Proced. Int., 2004, 36(5), 391-443.
- F. Hussein, K.M. Hello, J. Chem., 2002, 26(1), 35-41.
- A.F. Abdulla, MSc. Thesis, University of Baghdad, College of Science, 2015.
- Z. Shunxue, Z. Anxiong, C.L.Q. Bin, D. Hengshan, Molecules., 2003, 6, 92-95.
- E.S. El-Tamaty, M.E. Abdel-Fattah, I.M. El-Deen, Indian J. Chem., 1996, 35, 1067.
- A.M. Hussein, MSc. Thesis, University of Baghdad, College of Science, 2015.
- A.W. Naser, Iraqi National J. Chem., 2013, 50, 199.
- B. Furniss, A.H. Hannaford, P. Smith, A. Tatchell, Vogel’s Text Book of Practical Organic Chemistry, 5th Ed., Addison Wesley Longman, 1998, 1077.
- S. Narsimha, T.R. Kumar, Med. Chem. Res., 2014, 23, 5321-5327.
- A.M. Majeed, MSc. Thesis, University of Baghdad, College of Science, 2015.