Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 3
Synthesis of Fused Triazolo Thiadiazine Moeties Possessing Antiinflammatory Activity
Gejalakshmi S1*, Harikrishnan N2 and Muralidaran P3
1Department of Pharmaceutical Chemistry, Dr. M.G.R, Educational and Research Institute, Vellapanchavadi, Tamil Nadu, India
2Department of Pharmaceutical Analysis, Dr. M.G.R, Educational and Research Institute, Vellapanchavadi, Tamil Nadu, India
3Department of Pharmacology, C.L. Baid Metha College of Pharmacy, Rajiv Gandhi Salai, Thoraipakkam, Tamil Nadu, India
- *Corresponding Author:
- Gejalakshmi S
Department of Pharmaceutical Chemistry
Dr. M.G.R, Educational and Research Institute
Vellapanchavadi, Tamil Nadu, India
Abstract
A vast array of fusing two heterocyclic rings namely 1,2,4-triazolo[34-b]thiadiazine derivatives having excellent pharmacological activity forms an invaluable part of the present inventions. A series triazolo thiadiazine derivative were synthesized by using aromatic acid and ethyl alcohol in alkali by treating aromatic acid with hydrazine hydrate and substituted acetophenone under reflux. The synthesised compounds were undergone spectral and elemental analysis. The active compound was found to be 4-(3-(2-(2,6-dichlorophenylamino)benzyl)-7H-[1,34]thiadiazin-6- yl)phenol equipotent as standard drug diclofenac. Presence of electronegative functional moieties as phenyl ring due to C2 position of thiazole ring possessing large hydrophilic for enhanced anti-inflammatory activity.
Keywords
Triazolothiadiazine, Anti-inflammatory, Acetophenone
Introduction
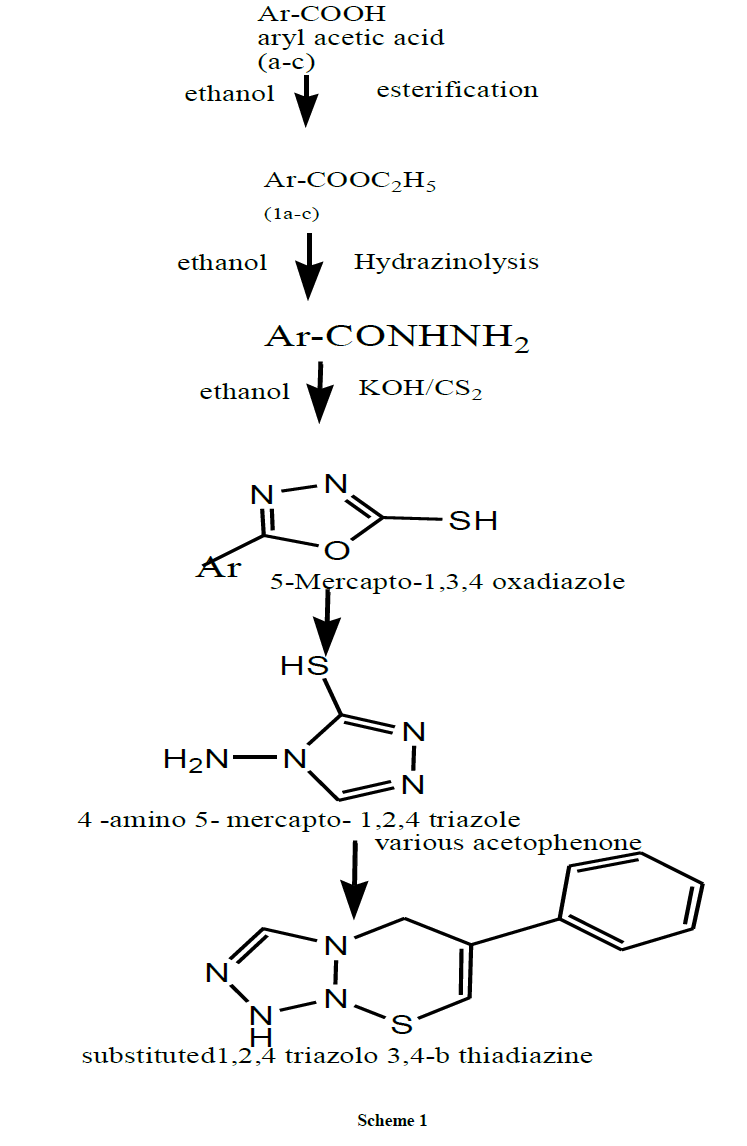
Drug discovery research is a highly creative stimulating work environment where people are driven to succeed by personal and scientific objectives and the desire to contribute to society a well-being [1,2] The first step of drug discovery involves the identification of novel active compounds often called hits which are typically found by screening many compounds for the desired biological properties [3]. Further chemical modification in order to improve biological and physiochemical properties of a given candidate compound library [4]. The final step involves the rendering the lead compounds suitable for use in the clinical trial. This involves the optimisation of synthetic route for bulk production and the preparation of a suitable drug formulation. The present study involves the fusion of two heterocyclic rings namely triazolo-thiadiazine nucleus which incorporates N-C-S moiety linkage, it exhibits a large number of biological activities like antimicrobial [5] antifungal [6] antiinflammatory [7], analgesics [8], antitubercular [9]. Various substituted 1,2,4-triazolo[3,4-b]thiadiazine derivatives were synthesised using various aromatic acid and ethyl alcohol refluxed under strong acidic condition using sodium bicarbonate as neutralizing agent. Due to potency of the compounds in vitro anti-inflammatory activity was performed by HMBC membrane stabilizing method.
Materials and Methods
Melting point was determined on Vego digital melting point apparatus and was uncorrected. IR spectra were recorded using potassium bromide on a Perkin Elmer FT-IR Spectrophotometer. 1H-NMR spectra were recorded on Bruker Spectrophotometer (400 MHz) in Deuterated Chloroform (CDCl3) using Tetramethylsilane (TMS) as an internal standard. Mass spectra were recorded on LC-MSD Trap-SL 2010A-Shimadzu. Micro analysis was performed on a Perkin Elmer-240 CHN elemental analyzer.
Synthesis of substituted ethyl esters (1a-c)
Various aryl acetic acid (0.01 mol), ethyl alcohol (0.05 mol), conc. sulphuric acid (1.5 ml) were refluxed and the residual mixtures was poured into water and excess acid was neutralised by using sodium bicarbonate. Completion of the reaction was monitored on TLC using silica gel-G coated plates by using ethyl acetate and petroleum ether (1:1) as the eluent and observed in U.V. light.
Synthesis of substituted arylacetic acid hydrazide (2a-c)
The ester of the substituted aromatic acid (0.1 M) was dissolved in 30 ml of ethanol and hydrazine hydrate (0.1 M) with stirring. The resulting mixture was refluxed at 80°C. The contents were allowed to cool and excess ethanol was distilled. The formed crystals were filtered, washed thoroughly with water and allowed to dry. Completion of the reaction was monitored on TLC using silica gel-G coated plates by using ethyl acetate and petroleum ether (1:1) as the eluent and observed in U.V. light.
Synthesis of substituted 5-mercapto-1,2,4-oxadiazole (3a-c)
The aryl acid hydrazide was added in the solution of potassium hydroxide (0.15 M) and ethanol (200 ml) and the solution was cooled using ice. Carbon disulphide (0.15 M) was added to this in small portions with constant stirring. The reaction was agitated for 15 h continuously. Then it was diluted with anhydrous ether. The precipitated 5-mercapto-1,2,4-oxdiazole derivatives were filtered washed with dry ether and dried. Compounds were used in the next steps without further purification.
Synthesis of 3-substituted-4-amino-5-mercapto-1,2,4-triazole (4a-c)
A suspension of the above mercapto-oxadiazole (0.1 m) water (0.06 mol) and hydrazine hydrate (0.05 mol) was prepared and refluxed for 6-7 h with occasional stirring. The colour of the reaction was cooled at room temperature, diluted with water and acidified with hydrochloric acid. The required triazolo was precipitated. The crystals formed were filtered, washed thoroughly with water and allowed to dry. The completion of the reaction was monitored on TLC using silica gel-G coated plates by using ethyl acetate and petroleum ether (1:1) as the eluent and observed in U.V. light.
Synthesis of substituted 1,2,4-triazolo-[3,4-b]thiadiazine (p1-p6)
To a suspension of (0.01 mol) triazole in absolute ethanol, (1.99 g, 0.01 mol) of substituted bromo acetophenone and 2-chloroacetophenone were added to separately. The resulting mixture was refluxed at 80°C for 2 h. The content were allowed to cool to room temperature and then neutralised with ammonia. The precipitate was filtered off, washed thoroughly with water and allowed to dry. The solid obtained was recrystallized from Dimethyl Sulphoxide (DMSO) water (1:1). Completion of the reaction was monitored on TLC using silica gel-G coated plates by using ethyl acetate and petroleum ether (1:1) as the eluent and observed in U.V. light. All the synthesised compounds were analysed and elemental study was performed (Table 1).
| Compound code | Molecular formula | Ar | M.P. (°C) % yield |
IR Data cm-1 |
Hd-NMR data ppm | Mass data M |
Elemental analysis % analysis calculated | HMBC membrane method |
|---|---|---|---|---|---|---|---|---|
| P1 | C23H17Cl2N5S | Phenyl | 178-180 65% |
3393.03 (N-H str.) 1557.95 (N-H def.) 1671.90 Ar overtone 746.27 (C-Cl) 687.54 (C-Br) 1445.13 (C-N str.) 1276.62 Methylene C-H def. | 5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 3.381 (s, 4H, CH2), 6.34-7.4 (m, 1, 1H, Ar-H) | 490.67 | C-56.69 (57.27) H-3.58 (3.55) N-14.48 (14.52) |
57.36 |
| P2 | C23H16BrCl2N5S | Bromo phenyl |
173-175 64% |
3447.13 (N-H str.) 3735.68 (N-N Str.) 1558.40 N-H fermel resonance overtone Ar overtone 834.17 (C-Cl) 616.10 (C-r) 1252.92 asymmetric Ar-o-Ar assymetric 1303.49 (Methylene C-H def.) | 5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 2.35 (s, 4H, CH2), 6.34-7.4 (m, 1, 1H, Ar-H) | 442.65 | C-67.98 (67.43) H-4.68 (4.95) N-16.18 (16.38) |
59.52 |
| P3 | C23H17Cl2N5OS | Phenol | 218-220 78% |
o-H str. 3853.71 N-H str. 3446.44 C-Cl 820.91 Methylene C-H def. 1394.93 |
5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 3.381 (s, 4H, CH2), 6.34-7.4 (m, 1, 1H, Ar-H) | 482.67 | C-57.98 (58.24) H-3.28 (3.58) N-22.41 (22.64) |
61.68 |
| P4 | C24H19Cl2N5OS | Methoxy phenyl | 230-235 62% |
N-H str. 3409.55 Aromatic ring overtone 2284.37 C=C Str. 1571.32 C-N Str. 1411.79 C-Cl str. 834.53 C-Br str. 616.38 | 5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 2.35 (s, 4H, CH2), 4.14 (m, 1, 1H, Ar-H) | 423.34 | C-56.69 (59.27) H-3.58 (3.66) N-14.98 (14.52 |
57.36 |
| P5 | C24H21N5S | Dimethyl phenyl | 170-175 66% |
N-H str. 3446.98 N-H def. 1113.56 Aromatic ring overtone 1616.49 N-H (oop) def. 756.57 | 5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 2.35 (s, 4H, CH2), 6.34-7.4 (m, 1, 1H, Ar-H) | 490.65 | C-67.88 (67.43) H-4.48 (4.95) N-16.48 (16.38) |
27.56 |
| P6 | C22H20CBrN5S | Dimethyl benzene amine | 160-165 71% |
N-H Str. 3419.86 C-H def. 1374.26 c-Br Str. 618.05 | 5.0 (s, 1H, OH, 4.0 (s, 1H, NH), 2.35 (s, 4H, | 445.62SSS | C-58.98 (58.24) H-3.88 (3.58) N-28.41 (22.64) |
56.38 |
Table 1: Analytical characterization of (p1-p6) compounds and in vitro anti-inflammatory activity
Scheme [10]
In vitro anti-inflammatory activity [11]
The synthesised six compounds were screened for in vitro anti-inflammatory activity by HRBC membrane stabilization method. Blood was collected from healthy volunteer. The collected blood was mixed with equal volume of sterilized Alsievier’s solution (2% dextrose, 0.8% sium citrate, 0.5% citric acid and 0.42% sodium chloride in water). The blood was centrifuged at 3000 rpm and packed cell were washed with iso saline (0.85% pH 7.2) and a 10% (v/v) suspension was made with isosaline. The assay mixture contained the drug, 1 ml phosphate buffer (0.15 M, pH 7.4) and 2 ml of hyposaline and 0.5 ml of HRBC suspension. Diclofenac was used as reference drug. Instead of hyposaline 2 ml of distilled water was used in the control. All the assay mixture were incubated at 37°C for 30 min and centrifuged at 3000 rpm. The absorbance of the supernatants were estimated using U.V. Spectrophotometer at 560 nm. The values are tabulated.
Results and Discussion
The role of this work was to synthesis series of novel substituted triazolo-thiadiazine with potent anti-inflammatory activity. A series of six triazolo thiadiazine derivatives were synthesised by using substituted aromatic acid and ethyl alcohol under strong acidic condition using alkaline sodium bicarbonate as a neutralising agent followed by treating esters of aromatic acid with hydrazine hydrate and substituted acetophenone under reflux condition (Scheme 1) [10]. The synthesised compounds were confirmed by various spectral methods and screened for in vitro anti-inflammatory. From the tabulated data it was clear that majority compounds possessed moderate to strong activity due to C2 position of thiazole ring possessing large hydrophilic, electronegative functional moieties like substituted phenyl ring for enhanced anti-inflammatory activity of triazolo thiadiazine derivatives. From the above conclusion it was finalised compound 6c possessed pronounced anti-inflammatory activity compared to other series of synthesised compounds due to more electronegative nature.
Conclusion
The present work pioneered us to synthesize many triazolothiadiazine derivatives. The most active compound was found to be 4-(3-(2-(2,6- dichlorophenylamino)benzyl)-7H-[1,34]thiadiazin-6-yl)phenol equipotent to that of standard drug diclofenac. Presence of electronegative functional moieties like substituted phenyl ring due to C2 position of thiazole ring possessing large hydrophilic for enhanced anti-inflammatory activity of thiazole derivatives. A series of 6 synthesised compounds were studied for in vitro anti-inflammatory activity by HMBC membrane stabilizing methods and results were compared with standard drug Diclofenac. From the in vitro studies further development of in vivo studies were planned to carried out for the most potent compound4-(3-(2-(2,6-dichlorophenylamino)benzyl)-7H-[1,34]thiadiazin-6-yl)phenol.
Acknowledgement
The authors greatly acknowledge their thanks to management Dr. M.G.R. Educational and research institute for providing the facilities to carry out this work.
References
- W.O. Foye, D.A. Williams, T.L. Lemke, Foye’s Principles of Medicinal Chemistry, 6th Edi., Lippincott Williams & Wilkins publication, New York, 2002, 1-9
- M.E. Wolff, Burger’s Medicinal chemistry and Drug Discovery, Vol. IV, 6th Edi., Wiley-Inter Sciences, New York, 1995, 1-5
- R.H. Khan, R.A.K. Srivastava, R.C. Rastogi, Indian J. Pharm. Sci., 1987, 49(2), 48‐51.
- L.B. Allen, J.H. Huffmann, R.W. Sidwell, Antimicrob. Agents Chemother., 1973, 3, 534‐535.
- B.F. Sabrina, S.C. Marilia, B.J.S.B. Nubia, S.G. Marcelo, M.C. Marlene, B.K. Warner, Eur. J. Med. Chem., 2007, 42, 1388‐1395.
- C. Hart, Am. Chem. Soc., 1999, 2, 20‐31.
- N. Demirbas, A. Demibras, S.A. Karaoglu, E. Celik, Arkivoc., 2005, 1, 75‐91.
- F.P. Invidiata, D. Simoni, F. Scintu, N. Pinna, Farmaco., 1996, 51, 659-664.
- S.M. El-Khawass, M.A. Khalli, A.A. Hazza, A. Bassiouny, N.F. Loutfy, Farmaco., 1989, 44, 703‐709.
- N. Demirbas, A. Demibras, A.S. Karaoglu, E. Celik, Arkivoc., 2005, 1, 75-91.
- B. Tozkoparan, S.P. Aytac, G. Aktay, Archiv Der Pharmazie.,2009, 342(5), 291-298.