Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 5
Synthesis, Molecular Docking and Biological Evaluation of Novel N-(Benzyloxy)-6- Fluro-Chromone-2-Carboxamides and N-(Benzyloxy)-Chromone-3-Carboxamides as Analgesic and Anti-inflammatory Agents
M Vijaya Bhargavi1,2*, P Shashikala1, M Sumakanth2 and J Archana2
1Department of Pharmacy, Faculty of Technology, Osmania University, Hyderabad-500007, Telangana, India
2RBVRR Women's College of Pharmacy, Osmania University, Hyderabad-500007, Telangana, India
- *Corresponding Author:
- M Vijaya Bhargavi
Department of Pharmacy
Faculty of Technology
Osmania University
Hyderabad-500007, Telangana, India
Abstract
Novel N-(Benzyloxy)-6-Fluro-Chromone-2-Carboxamides and N-(Benzyloxy)-Chromone-3-Carboxamides are synthesized from an efficient and straight forward procedure from the reaction of 6-Fluoro-chromone-2-acid and chromone-3-acid with O-(substituted benzyl)-hydroxylamines. The structures of the synthesized compounds are established based on Infrered Radiation (IR), Nuclear Magnetic Resonance (NMR) and MASS spectrometry. Exploration of molecular interaction of the obtained compounds, performed through Discovery Studio v3.5, molecular docking studies with COX-2 enzyme, revealed the high docking scores (Lib Dock) in the range of 113.4-139.0 as compared to celecoxib 145.691. The compounds with high LibDock score were screened for their in vivo analgesic and antiinflammatory activities. Among them, compound 3b displayed good activity and potency.
Keywords
Chromone, Carboxamide, Molecular docking, COX-2 inhibitors, Antiinflammatory activity
Introduction
Inflammation is a complex host response to tissue injury that is controlled by many mediators among which are the prostaglandins (PGs) are important. Cyclooxygenase (COX) enzymes catalyse the synthesis of PGs from arachidonic acid (AA). It exists in two isoforms, COX-1 and COX- 2. Most of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) inhibit both COX-1 and COX-2 at their therapeutic doses. From the literature, it was suggested that inhibition of prostanoids produced by COX-2 can be ascribed to the anti-inflammatory, analgesic and antipyretic effects of NSAIDs. Because of that it is considered a potential target for the treatment of inflammation [1] and the design of selective COX-2 inhibitors should provide relief from the symptoms of inflammation and pain. Chromone (or 1,4-benzopyrone) is a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin. Chromones [2] have attracted considerable attention due to their wide spectrum of pharmacological and biological activities such as antitumor [3], antifungal [4], antiviral [5], and antibacterial [6] agents. Furthermore, it has been already reported that chromone is a potential nucleus for the development of anti-inflammatory drugs [7-12].
Recent reports suggest that N-(benzyloxy)-benzamide and its analogues with the O-benzylhydroxylamine moiety (-CONHOCH2-Ar) possess antibacterial, herbicidal and enzyme inhibiting activities [13-16]. The pharmacophore (-CONHOCH2-) is generally considered to be the bioisosteric analog of (-CONHCH2CH2-) in drug design [17]. To discover a new chromone series with improved potency and COX-2 selectivity, we designed and synthesized a new series of N-(benzyloxy)-6-fluoro-chromone-2-carboxamides and N-(benzyloxy)-chromone-3-carboxamides. Molecular docking studies were used to gain insight into the possible interactions of our newly synthesized compounds with the COX-2 enzyme. The newly synthesized compounds with good Lib Dock scores were evaluated for analgesic and anti-inflammatory activities.
Materials and Methods
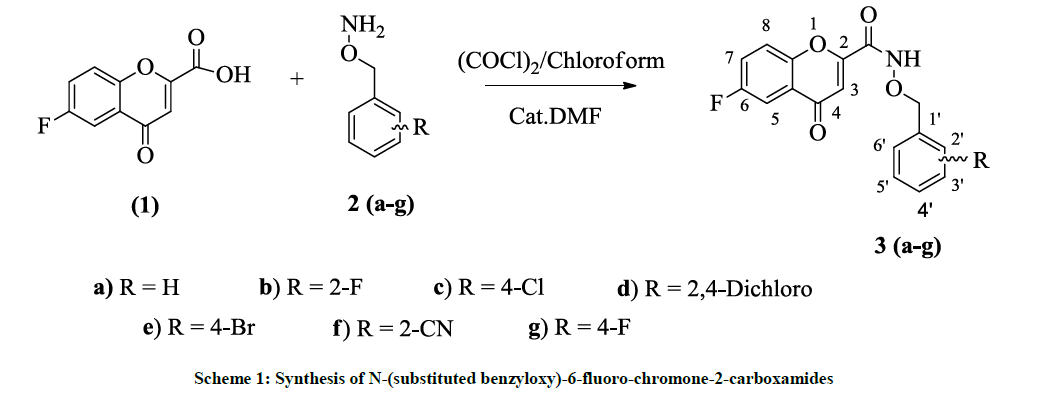
In the present work N-(substituted benzyloxy)-6-fluoro-chromone-2-carboxamides were synthesized from the amide coupling of 6-fluoro-chromone- 2-carboxylic acid [18] with O-substituted benzylhydroxylamines [19] via acid chloride method at RT as shown in scheme 1 [20].
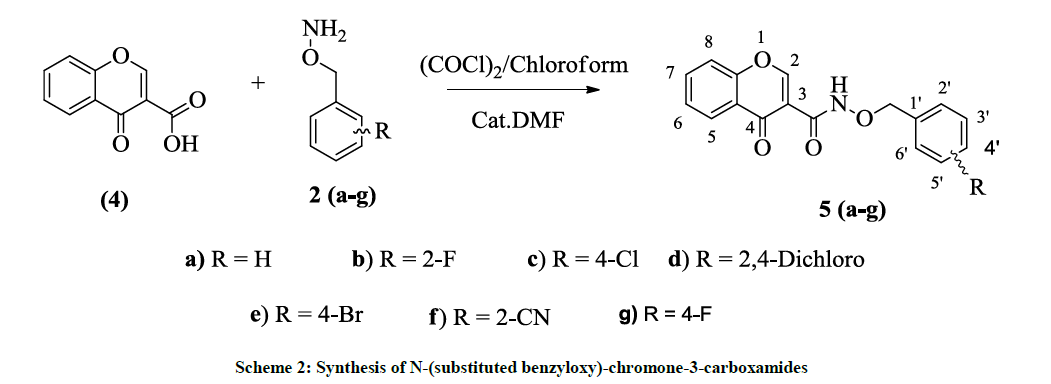
N-(substituted benzyloxy)-chromone-3-carboxamides were synthesized from the amide coupling of chromone-3-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT as shown in scheme 2.
Initially, 6-Fluoro-chromone-2-acid (1) was synthesized from the reaction of 5-Fluoro-2-hydroxyacetophenone and diethyl oxalate in the presence of sodium ethoxide followed by the acid hydrolysis. Chromone-3-acid (4) was synthesized from the reaction o-hydroxyacetophenone in POCl3/DMF followed by the Jones Oxidation. N-Hydroxy phthalimide was alkylated with substituted benzyl halides in the presence of K2CO3 and DMF (Dimethylformamide) at RT. These intermediates are reacted with hydrazine hydrate in ethanol at reflux temperature to give O- (substituted benzyl)-hydroxyl amines 2(a-g). Synthesis of N-(substituted benzyloxy)-6-fluoro-chromone-2-carboxamides 3(a-g) were synthesized from the amide coupling of 6-fluoro-chromone-2-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT. In a similar way, N-(substituted benzyloxy)-chromone-3-carboxamides were also synthesized from the amide coupling of chromone-3-carboxylic acid (4) with O-substituted benzylhydroxylamines 2(a-g) via acid chloride method at RT. These were purified by column chromatography and characterized from their spectra.
Molecular docking studies
Computational studies of the novel chromone carboxamide derivatives were done using Discovery studio v 3.5. The three dimensional structure of celecoxib bound at the COX-2 active site (PDB ID: 3LN1) was retrieved from the Brookhaven Protein Data Bank (PDB), USA (http://www.rcsb.org/pdb). Protein preparation and ligand preparation wizard were used for protein and ligand preparation respectively. Initially, ions, water molecules, all the internal ligands were removed and missing atoms were inserted before minimization of the target protein. Alternate conformations (disorder) were removed.
The bioactive binding poses of inhibitors in the active site of the enzyme was generated by using a LibDock program of Discovery Studio. The ligand poses were placed in to polar and apolar interactions site. MMFF force field was used for energy minimization of the ligands. ‘Hotspots’ are the binding site features. The hotspot map counts for polar and apolar cluster in the active site of the protein which is further used for the alignment of the ligand conformations to the interaction sites of the protein. All the minimized ligand poses and their rankings are based on the ligands score. In accordance with the LibDock score, each pose is assessed for binding energy, by a simple pair-wise method. The ligands with high LibDock scores are considered for estimating binding energies of the protein-ligand complex. The complex pose with the best binding energy is used for further binding mode analysis. For the docking validation, the co-crystallized ligand CEL in the Musmusculus COX-2 binding site is redocked. The binding affinities of the synthesized compounds were compared and analyzed in reference to Celecoxib to identify structural characteristics of the complexes formed by these compounds and the protein.
Antiinflammatory activity-carrageenan-induced rat paw EDMA assay
Antiinflammatory activity of synthesized compounds was assessed by carrageenan-induced rat paw edema method which was described by Winter et al. [21]. The animals were housed under standard environmental conditions, one week before the start and also during the experiment as per the rules and regulations of the institutional ethics committee (Regd No: RBVRR1328/01/2017/CPCSEA). Male albino wistar rats weighing between 190 to 260 g, were used in the current study. The rats were divided into fourteen groups, each group consist of six animals. Group-1 referred as control [0.5% Carboxy Methyl Cellulose (CMC)] and Group-2 received standard (indomethacin) along with the vehicle prior to the administration of carrageenan. Group 3-14 served as test samples, received 20 and 40 mg/kg test samples. All the standard and test compounds were suspended in 0.5% of CMC and administered orally 60 min prior to the injection of 0.1 ml of freshly prepared carrageenan (1%) in physiological solution in to the sub-planter tissue of hind paw of each rat. Plethysmometer apparatus was used to measure the volume prior to the administration of carrageenan. The edema volume was measured 1 h, 3 h, 5 h, and 7 h after the injection also. The increase in volume of the rat paw was adopted as a measure of edema. The antiinflammatory effects of the compounds were estimated as percentage inhibition of the induced inflammation in comparison with control. Statistical analysis was also carried out using a one-way analysis of variance (ANOVA). A significance level of p<0.05 denoted significance in all cases.
The percentage inhibition of edema was calculated using the formula given below:
(Vc -Vt / Vc) × 100
V
Analgesic activity
Hot plate method
The hot-plate test was performed to measure response latencies according to the method described by Eddy and Leimbach (1953) [22]. Swiss albino male wistar rats (170-210 g body weight) were divided into groups of six animals each. Group I served as control; group II served as standard, received aspirin (10 mg/kg); Groups III and XIV served as test samples, received 20 and 40 mg/kg test samples. The animals were placed on the hot plate, maintained at 55 ± 2°C. The pain threshold is considered to be reached when the animals lift and lick their paws or attempt to jump out of the hot plate. The time taken for the rats to react in this fashion was obtained using a stopwatch and noted as basal reaction time. A latency period of 30 sec (cut-off) was defined as complete analgesia and the measurement was terminated if it exceeded the latency period in order to avoid injury. The reaction time was reinvestigated at 30, 60, 120 and 180 min after the treatment and changes in the reaction time were noted.
Tail immersion method
Young Male Albino Wistar rats (170-210 g body weight) are used. The lower 5 cm portion of the tail is marked. This part of the tail is immersed in a cup of freshly filled water of exactly 55°C. Within a few seconds the rat reacts by withdrawing the tail. After each determination the tail is carefully dried. The reaction time is determined before and periodically after either oral or administration of the test substance, e.g., after 30, 60, 90, 120, 180 min. The cut off time of the immersion is 15 sec.
Results and Discussion
All the materials and solvents were used directly unless otherwise stated. All the synthesized compounds were purified by recrystallization or column chromatography on silica gel (60-120 mesh, Spectrochem, Mumbai, India). Melting points were obtained on a Polmon instrument, India (model MP96) and are uncorrected. The IR spectra were measured on a Fourier Transform Infrared Spectroscopy Perkin-Elmer 337 (Perkin Elmer instrument company, Massachusetts, USA). 1H-NMR and 13C-NMR spectra were recorded on Bruker 400 MHz, Switzerland using TMS as an internal standard (Chemical shift in δ, ppm). J-Values are given in Hz. Mass spectral data were obtained with Agilent 6310 ion trap mass spectrometer, USA.
Synthesis of N-(benzyloxy)-6-fluoro-chromone-2-carboxamide (3a)
To a stirred solution of 6-fluoro-chromone-2-carboxylic acid (250 mg, 1.2 mmol) in chloroform (15 ml) a catalytic amount of DMF was added. Oxalyl chloride (1 ml) was added dropwise and stirred for 1 h at RT. Solvents were removed under vacuum, acid chloride was dissolved in CHCl3 (15 ml) and kept aside under N2 atmosphere. O-benzylhydroxylamine (180 mg, 1.46 mmol) was dissolved in chloroform (15 ml). To this triethylamine (0.5 ml) was added. Acid chloride dissolved in chloroform was added dropwise to the oxyamine at RT and stirring was continued for 2 h. After completion of the reaction by TLC reference, water (30 ml) was added and layers were separated, dried over Na2SO4 and concentrated to yield the crude oxyamide and purified by column chromatography using silca gel (60 x 120) by eluting with petether: ethylacetate (5:5).
Yield = 100 mg, white solid, M. P. 154-155°C; IR (KBr): 1663 (O=C-NH), 1705 (4-C=O), 3210 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.05 (s, -OCH2), 7.19 (s, H-8), 7.24 (s, H-3), 7.41-7.48 (m, Ar-H & H-5), 7.8-7.84 (m, H-7), 9.8 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=77.4 (-OCH2), 121.5 (C-5), 123.1 (C-3), 123.3 (C-8), 124.9 (C-7), 128.4 (C-4a), 128.5 (C-2' & C-6'), 128.9 (C-4'), 129.1 (C-3' & C-5'), 135.2 (C-1'), 151.1 (C-8a), 155.1 (C-6), 156.4 (O=C-NH), 157.9 (C-2), 160.4 (4-C=O). DIPMS: m/z at 314 (M+1).
Employing the similar procedure as mentioned for 3a, compounds 3b-3g were obtained as solids.
6-Fluoro-N-(2-fluorobenzyloxy)-chromone-2-carboxamide (3b)
Yellow solid, M. P. 146-148°C; IR (KBr): 1637 (-CONH), 1692 (-C=O), 3216 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.15 (s, - OCH2), 7.11-7.19 (m, H-5', H-3 & H-8), 7.45-7.50 (m, H-5, H-7 & H-3', H-6'), 7.83 (dd, H-4', J=4.2Hz, J=6.0Hz), 9.6 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=70.7 (-OCH2), 109.4 (C-5), 109.9 (C-3'), 115.4 (C-3), 121.5 (C-8), 123.3 (C-7), 124.5 (C-4a), 124.9 (C-5'), 131.1 (C-1'), 132.1 (C-6'), 151.4 (C-4'), 154.9 (C-8a), 156.5 (C-2'), 157.9 (O=C-NH), 159.6 (C-6), 160.4 (C-2), 162.1 (4-C=O). DIPMS: m/z at 332 (M+1).
N-(4-Chlorobenzyloxy)-6-fluoro-chromone-2-carboxamide (3c)
Off-white solid, M. P. 161-162°C; IR (KBr): 1632 (O=C-NH), 1701 (4-C=O), 3181 (-CONH) cm-1. 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.01 (s, -OCH2), 6.82 (s, H-8), 7.38-7.42 (m, H-2', H-6' & H-3, H-5), 7.73-7.81 (m, H-7 & H-3', H-5'), 12.59 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=76.4 (-OCH2), 109.7 (C-5), 110.1 (C-3), 121.5 (C-8), 123.3 (C-7), 124.9 (C-4a), 128.3 (C-3' & C-5'), 130.8 (C-2' & C-6'), 133.2 (C-4'), 134.3 (C-1'), 151.4 (C-8a), 154.9 (O=C-NH), 157.9 (C-6), 160.4 (C-2), 176.2 (4-C=O). DIPMS: m/z at 348 (M+1), 370 (M+Na).
N-(2,4-Dichlorobenzyloxy)-6-fluoro-chromone-2-carboxamide (3d)
White solid, M. P. 182-184°C; IR (KBr): 1641 (-CONH), 1690 (-C=O), 3186 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.08 (s, - OCH2), 6.81 (s, H-8), 7.51 (s, H-3), 7.66-7.71 (m, H-5), 7.73-7.77 (m, H-7, H-3' & H-5', H-6'), 12.61 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=73.4 (-OCH2), 109.7 (C-5), 110.1 (C-3), 121.5 (C-8), 123.0 (C-7), 123.3 (C-4a), 124.9 (C-5'), 127.5 (C-6'), 128.8 (C-3'), 132.8 (C-2'), 134.1 (C-4'), 134.3 (C-1'), 151.4 (C-8a), 154.9 (O=C-NH), 156.5 (C-6), 157.9 (C-2), 160.4 (4-C=O). DIPMS: m/z at 382 (M+), 384 (M+2).
N-(4-Bromobenzyloxy)-6-fluoro-chromone-2-carboxamide (3e)
Light brown solid, M. P. 124-125°C; IR (KBr): 1634 (O=C-NH), 1740 (4-C=O), 3195 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=4.96 (s, -OCH2), 6.82 (s, H-8), 7.44-7.46 (m, H-3 & H-6), 7.61-7.63 (m, H-2' & H-6'), 7.72-7.8 (H-7 & H-3', H-5'), 12.6 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=76.4 (-OCH2), 109.9 (C-5), 121.7 (C-3), 123.0 (C-8), 123.3 (C-7), 124.9 (C-4a), 131.1 (C-3' & C- 5'), 131.3 (C-2' & C-6'), 134.8 (C-4'), 151.4 (C-1'), 154.9 (C-8a), 156.6 (O=C-NH), 157.9 (C-6), 160.4 (C-2), 176.3 (4-C=O). DIPMS: m/z at 393 (M+1), 394 (M+2).
N-(2-cyanobenzyloxy)-6-fluoro-chromone-2-carboxamide (3f)
Light orange solid, M. P. 174-175°C; IR (KBr): 1623 (-CONH), 1686 (-C=O), 3209 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.28 (s, -OCH2), 7.46-7.58 (m, H-5', H-3 & H-8), 7.67-7.74 (m, H-5, H-7 & H-3', H-6'), 7.82 (dd, H-4', J=3.2Hz, J=2.8Hz), 9.56 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=74.6 (-OCH2), 109.7 (C-5), 110.0 (C-3'), 112.0 (C-3), 117.2 (C-8), 121.5 (C-7), 123.3 (C-4a), 124.9 (C- 5'), 129.5 (C-1'), 130.9 (C-6'), 133.3 (C-4'), 151.4 (C-8a), 154.9 (C-2'), 156.5 (O=C-NH), 157.9 (C-6), 160.4 (C-2), 162.2 (4-C=O). DIPMS: m/z at 339 (M+1), 361 (M+Na).
N-(4-Fluorobenzyloxy)-6-fluoro-chromone-2-carboxamide (3g)
Light yellow solid, M. P. 121-122°C; IR (KBr): 1633 (O=C-NH), 1704 (4-C=O), 3177 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=4.96 (s, -OCH2), 6.82 (s, H-8), 7.22-7.27 (m, H-3' & H-5'), 7.52-7.56 (m, H-3 & H-5), 7.72-7.8 (H-7, H-2' & H-6'), 12.59 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=76.5 (-OCH2), 109.7 (C-5), 109.9 (C-3), 115.1 (C-8), 115.3 (C-7), 121.5 (C-4a), 123.3 (C-3' & C- 5'), 124.9 (C-2' & C-6'), 131.6 (C-4'), 151.43 (C-1'), 155.0 (C-8a), 157.9 (O=C-NH), 160.3 (C-6), 160.9 (C-2), 163.4 (4-C=O). DIPMS: m/z at 332 (M+1), 354 (M+Na).
Synthesis of N-(benzyloxy)-chromone-3-carboxamide (5a)
To a stirred solution of chromone-3-carboxylic acid (4) (400 mg, 1.2 mmol) in chloroform (15 ml) catalytic amount of Dimethylformamide (DMF) was added. Oxalyl chloride (1.2 ml) was added dropwise and stirred for 1 h at RT. Solvents were removed under vacuum, acid chloride was dissolved in CHCl3 (15 ml) and kept aside under N2 atmosphere. O-benzylhydroxylamine (2a) (283 mg, 2.33 mmol) was dissolved in chloroform (15 ml). To this triethylamine (0.8 l) was added. Acid chloride dissolved in chloroform was added dropwise to the oxyamine at RT and stirring continued for 3 h. After completion of the reaction by Thin Layer Chromarography (TLC) reference, water (30 l) was added and layers separated, dried over Na2SO4 and concentrated to yield the crude oxyamide and purified by column chromatography using silca gel (60 x 120) by eluting with petether:ethylacetate (6: 4).
Yield = 210 mg, White solid, M. P. 165-167°C; IR (KBr): 1616 (O=C-NH), 1677 (4-C=O), 3208 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.04 (s, -OCH2), 7.35-7.5 (m, H-6 and H-2' to H-6'), 7.56 (d, H-8, J=), 7.71-7.8 (m, H-7), 8.24 (dd, H-5, J=4.2Hz, J=6.0Hz), 8.98 (s, H- 2), 11.45 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz) : δ (ppm)=78.6 (-OCH2), 115.5 (C-3), 118.4 (C-8), 123.8 (C-6), 126.3 (C-4a), 126.4 (C-5), 128.6 (C-2' & C-6'), 128.7 (C-4'), 129.1 (C-3' & C-5'), 134.8 (C-7), 135.2 (C-1'), 155.9 (C-8a), 161.0 (O=C-NH), 162.1 (C-2), 176.2 (4- C=O). DIPMS: m/z at 296.2 (M+1).
Employing the similar procedure as mentioned for 5a, compounds 5b-5g was obtained as solids.
N-(2-Fluorobenzyloxy)-chromone-3-carboxamide (5b)
Yellow solid, M. P. 146-147°C; IR (KBr): 1676 (-CONH), 1740 (-C=O), 3236 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.12 (s, - OCH2), 7.18-7.21 (m, H-5' & H-6'), 7.34-7.36 (m, H-6), 7.48-7.58 (m, H-3' & H-7, H-8), 7.54-7.79 (m, H-4'), 8.24 (dd, J=1.6Hz, J=1.6Hz, H-5), 8.98 (s, H-2), 9.6 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=70.7 (-OCH2), 115.5 (C-3), 115.6 (C-3'), 118.4 (C-8), 123.8 (C-6), 124.1 (C-4a), 124.2 (C-5'), 126.3 (C-5), 126.4 (C-1'), 130.7 (C-6'), 130.8 (C-4'), 131.5 (C-7), 134.9 (C-8a), 156.0 (C-2'), 161.1 (O=C-NH), 162.1 (C-2), 176.2 (4-C=O). DIPMS: m/z at 314.2 (M+1).
N-(4-Chlorobenzyloxy)-6-fluoro-chromone-2-carboxamide (5c)
Off-white solid, M. P. 178-180°C; IR (KBr): 1673 (O=C-NH), 1739 (4-C=O), 3241 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.01 (s, -OCH2), 7.33-7.34 (m, H-2' & H-6'), 7.40-7.42 (m, H-3' & H-5'), 7.49-7.53 (m, H-6), 7.56-7.58 (m, H-8), 7.75-7.79 (m, H-7), 8.23 (dd, J=1.6Hz, J=1.6Hz, H-5), 8.97 (s, H-2), 11.46 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=76.7 (-OCH2), 115.4 (C-3), 118.4 (C-8), 123.8 (C-6), 126.3 (C-4a), 126.5 (C-5), 128.8 (C-3' & C-5'), 130.4 (C-2' & C-6'), 133.7 (C-4'), 134.6 (C-1'), 134.9 (C-7), 156.0 (C-8a), 161.1 (O=C-NH), 162.1 (C-2), 176.2 (4-C=O). DIPMS: m/z at 330.7 (M+H), 332.7 (M+H+2).
N-(2,4-Dichlorobenzyloxy)-chromone-3-carboxamide (5d)
White solid, M. P.180-182°C; IR (KBr): 1637 (-CONH), 1674 (-C=O), 3073 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)= 5.16 (s, - OCH2), 7.28 (dd, J=1.6Hz, J=2Hz, H-5'), 7.42 (d, H-6', J=2Hz), 7.50-7.59 (m, H-6, H-7 & H-8), 7.76-7.79 (m, H-3'), 8.24 (dd, J=1.6Hz, J=1.6Hz, H-5), 8.97 (s, H-2), 11.54 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=74.8 (-OCH2), 115.4 (C-3), 118.4 (C-8), 123.8 (C-6), 126.3 (C-4a), 126.5 (C-5), 127.3 (C-5'), 129.4 (C-6'), 131.6 (C-3'), 131.8 (C-2'), 134.9 (C-4'), 134.9 (C-7), 135.1 (C-1'), 156.0 (C-8a), 161.2 (O=C-NH), 162.2 (C-2), 176.2 (4-C=O). DIPMS: m/z at 365.2 (M+H), 367.2 (M+H+2).
N-(4-Bromobenzyloxy)-6-fluoro-chromone-2-carboxamide (5e)
Light brown solid, M. P.155-157°C; IR (KBr): 1672 (O=C-NH), 1740 (4-C=O), 3241 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.00 (s, -OCH2), 7.34-7.36 (m, H-2' & H-6'), 7.51-7.59 (m, H-3', H-6 & H-7, H-8), 7.78-7.79 (m, H-5'), 8.24 (dd, H-8, J=1.2Hz, J=1.2Hz), 8.97 (s, H-2), 11.47 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=76.7 (-OCH2), 115.4 (C-3), 118.4 (C-8), 122.8 (C-6), 123.8 (C-4a), 126.3 (C-5), 126.5 (C-3' & C-5'), 130.7 (C-2' & C-6'), 131.7 (C-4'), 134.2 (C-1'), 134.9 (C-7), 156.0 (C-8a), 161.1 (O=C-NH), 162.1 (C-2), 176.2 (4-C=O). DIPMS: m/z at 375.2 (M+H), 377.2 (M+H+2).
N-(2-Cyanobenzyloxy)-chromone-3-carboxamide (5f)
Orange solid, M. P. 173-174°C; IR (KBr): 1692 (-CONH), 1740 (-C=O), 3219 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.12 (s, - OCH2), 7.46-7.51 (m, H-5' & H-6'), 7.57-7.59 (m, H-6), 7.64-7.80 (m, H-3', H-4' & H-7, H-8), 8.24 (dd, J=1.6Hz, J=1.6Hz, H-5), 8.98 (s, H-2), 11.58 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=75.7 (-OCH2), 112.7 (C-3), 115.4 (C-3'), 117.1 (C-8), 118.4 (C-6), 123.8 (C- 4a), 126.3 (C-5'), 129.1 (C-5), 130.3 (C-1'), 132.9 (C-6'), 133.0 (C-4'), 134.9 (C-7), 138.7 (C-8a), 156.0 (C-2'), 161.4 (O=C-NH), 162.2 (C-2), 176.2 (4-C=O). DIPMS: m/z at 321.3 (M+H).
N-(4-Fluorobenzyloxy)-chromone-3-carboxamide (5g)
Light yellow solid, M. P. 120-122°C; IR (KBr): 1690 (-CONH), 1742 (-C=O), 3222 (-CONH) cm-1; 1H-NMR (CDCl3, 400 MHz): δ (ppm)=5.10 (s, -OCH2), 7.42-7.48 (m, H-5' & H-6'), 7.51-7.54 (m, H-6), 7.66-7.82 (m, H-3', H-4' & H-7, H-8), 8.22 (dd, J=1.6Hz, J=1.6Hz, H-5), 8.96 (s, H- 2), 11.55 (s, O=C-NH); 13C-NMR (DMSO-d6, 100.6 MHz): δ (ppm)=75.1 (-OCH2), 112.5 (C-3), 115.8 (C-3'), 117.3 (C-8), 118.2 (C-6), 123.5 (C-4a), 126.6 (C-5'), 129.3 (C-5), 130.1 (C-1'), 132.7 (C-6'), 133.2 (C-4'), 134.6 (C-7), 138.6 (C-8a), 156.0 (C-2'), 161.6 (O=C-NH), 162.5 (C-2), 176.6 (4-C=O). DIPMS: m/z at 314.2 (M+1).
Molecular docking
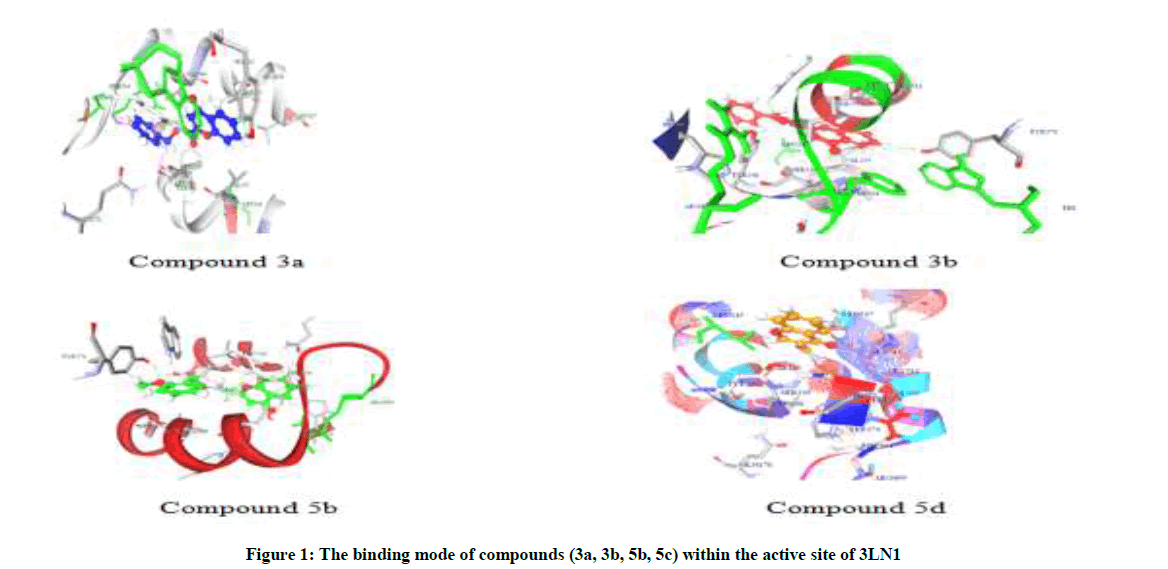
Molecular docking Studies are used to study the binding modes and affinities of our synthesized compounds with Musmusculus COX-2 (PDB ID: 3LN1). All the ligands were targeted to celecoxib bound at the COX-2 site. The best ligand conformation is chosen on the base of Lib Dock score and highly interacting amino acid residues of the ten conformations generated for each compound, the compound with the highest Lib Dock score is taken for interaction analysis of the hydrogen bonding. Lib Dock scores of all the compounds along with their electrostatic energy and vanderwaals energy depicted in Table 1 and Figures 1-5.
| Compound | Electrostatic Energy | Vanderwaals Energy | Libdock Score |
|---|---|---|---|
| 3a | -32.063 | 1.704 | 138.501 |
| 3b | -31.681 | 1.461 | 139.008 |
| 3c | -34.716 | 1.478 | 114.684 |
| 3d | -36.839 | 2.372 | 133.934 |
| 3e | -33.268 | 1.757 | 114.337 |
| 3f | -29.398 | 2.788 | 114.207 |
| 3g | -35.862 | 1.298 | 113.886 |
| 5a | -31.032 | 1.78 | 127.938 |
| 5b | -33.074 | 2.205 | 135.96 |
| 5c | -36.126 | 2.056 | 124.324 |
| 5d | 21.644 | 1.737 | 134.007 |
| 5e | 19.904 | 1.678 | 132.604 |
| 5f | 16.909 | 1.879 | 134.083 |
| 5g | 13.3 | 2.818 | 113.408 |
| Celecoxib | 4.465 | 4.762 | 145.691 |
Table 1: Lib Dock scores of the compounds
The binding site of the protein is the hydrophobic pocket comprising of amino acids Arg 106, Arg 499, Val 509, Leu 517, Tyr 371, Trp 373, Tyr 341, Leu 338, Phe 504, Leu 345. All the synthesized compounds were positioned in a similar orientation to that of celecoxib and showed strong hydrogen bonding interactions between the oxygen atom of the chromone ring present at 2nd position and the amino acid residues such as Arg 106, Tyr 341 and Phe 504 of protein which was similar to that of celecoxib.
In Vivo anti inflammatory activity
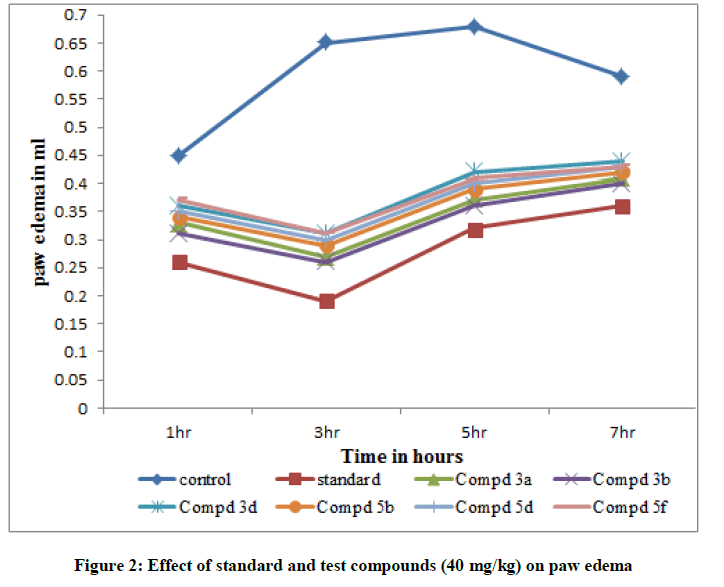
The compounds with good Libdock score was selected for antiinflammatory activity using carrageenan-induced rat paw edema model. Analgesic activity was measured by hot plate method and tail immersion method (Tables 2-5).
| Group | Treatment | Dose (mg/kg) orally | Mean paw edema volume ± SEM (ml) | |||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 5 h | 7 h | |||
| Group – I | Control | 1% CMC | 0.45 ± 0.024 | 0.65 ± 0.029 | 0.68 ± 0.027 | 0.59 ± 0.029 |
| Group – II | Standard | 10 | 0.26 ± 0.032** | 0.19 ± 0.031*** | 0.32 ± 0.038*** | 0.36 ± 0.03*** |
| Group –III | 3a | 20 | 0.38 ± 0.028** | 0.37 ± 0.061*** | 0.47 ± 0.007** | 0.49 ± 0.007* |
| Group –IV | 3a | 40 | 0.33 ± 0.029** | 0.27 ± 0.056*** | 0.37 ± 0.045** | 0.41 ± 0.034** |
| Group –V | 3b | 20 | 0.37 ± 0.027* | 0.36 ± 0.039** | 0.46 ± 0.01** | 0.48 ± 0.016* |
| Group –VI | 3b | 40 | 0.31 ± 0.023* | 0.26 ± 0.058*** | 0.36 ± 0.042** | 0.40 ± 0.028* |
| Group –VII | 3d | 20 | 0.40 ± 0.036* | 0.41 ± 0.035* | 0.5 ± 0.062* | 0.52 ± 0.018* |
| Group –VIII | 3d | 40 | 0.36 ± 0.038** | 0.31 ± 0.065*** | 0.42 ± 0.041* | 0.44 ± 0.040* |
| Group –IX | 5b | 20 | 0.39 ± 0.02* | 0.38 ± 0.047** | 0.49 ± 0.032* | 0.50 ± 0.017 |
| Group –X | 5b | 40 | 0.34 ± 0.038* | 0.29 ± 0.059** | 0.39 ± 0.047** | 0.42 ± 0.027* |
| Group –XI | 5d | 20 | 0.39 ± 0.013 | 0.40 ± 0.041* | 0.48 ± 0.056* | 0.51 ± 0.014 |
| Group –XII | 5d | 40 | 0.35 ± 0.002* | 0.30 ± 0.055** | 0.40 ± 0.047* | 0.43 ± 0.030* |
| Group –XIII | 5f | 20 | 0.40 ± 0.014 | 0.40 ± 0.043** | 0.49 ± 0.046* | 0.51 ± 0.013 |
| Group –XIV | 5f | 40 | 0.37 ± 0.006* | 0.31 ± 0.071** | 0.41 ± 0.053* | 0.43 ± 0.031* |
CMC: Carboxy Methyl Cellulose; values are expressed as mean ± SEM (n=6) and analyzed by ANOVA using Graph pad prism 7; ** P<0.001; **p<0.01; *p<0.05 when compared to control group
Table 2: Anti-inflammatory activity by Carrageenan Induced Rat Paw Edema Method
| Compound | Dose | % Inhibition of acute inflammation | |||
|---|---|---|---|---|---|
| 1 h | 3 h | 5 h | 7 h | ||
| Control | 1% CMC | ||||
| Standard | 10 | 44 | 70.7 | 52.9 | 38.9 |
| 3a | 20 | 15.6 | 43.1 | 30.9 | 16.9 |
| 3a | 40 | 26.7 | 58.5 | 45.6 | 30.5 |
| 3b | 20 | 17.8 | 44.6 | 32.4 | 18.6 |
| 3b | 40 | 31.1 | 60 | 47.1 | 32.2 |
| 3d | 20 | 11.1 | 36.9 | 26.5 | 11.9 |
| 3d | 40 | 20 | 52.3 | 38.2 | 32.2 |
| 5b | 20 | 13.3 | 41.5 | 27.9 | 25.4 |
| 5b | 40 | 24.4 | 55.4 | 42.6 | 28.8 |
| 5d | 20 | 13.3 | 38.5 | 29.4 | 13.6 |
| 5d | 40 | 22.2 | 53.8 | 41.2 | 27.1 |
| 5f | 20 | 11.1 | 38.5 | 27.9 | 13.6 |
| 5f | 40 | 17.8 | 52.3 | 39.7 | 27.1 |
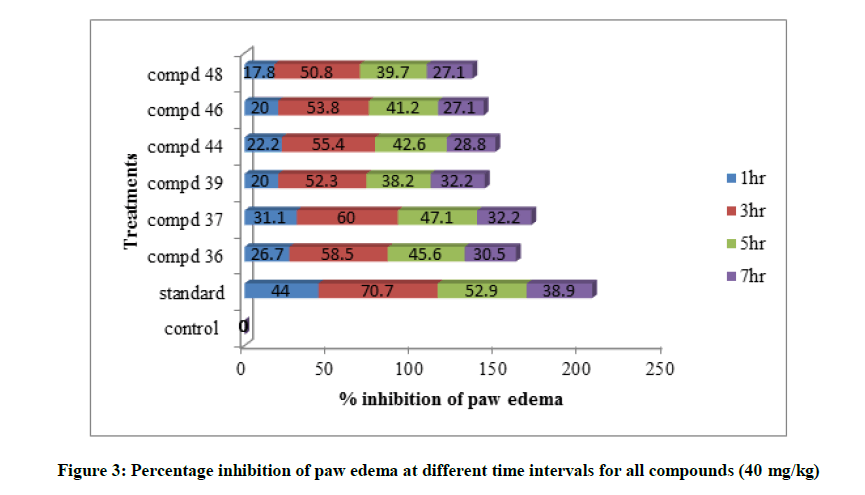
Table 3: % Inhibition of acute inflammation (carrageenan-induced paw edema)
| Compound | Dose | 0 min | 30 min | 60 min | 90 min | 120 min | 180 min |
|---|---|---|---|---|---|---|---|
| Control | 1% CMC | 5.50 ± 0.22 | 6.0 ± 0.26 | 6.16 ± 0.17 | 6.66 ± 0.21 | 6.5 ± 0.22 | 6.33 ± 0.21 |
| Standard | 10 | 6.67 ± 0.21* | 10 ± 0.81** | 13.50 ± 1.69** | 23.17 ± 2.32*** | 15.83 ± 1.74** | 13.33 ± 1.68** |
| 3a | 20 | 6.0 ± 0.26 | 6.5 ± 0.22 | 8.67 ± 0.33** | 12.67 ± 0.33** | 7.17 ± 0.17* | 6.83 ± 0.48 |
| 3a | 40 | 6.33 ± 0.21* | 7.17 ± 0.31* | 10.83 ± 1.01** | 18.50 ± 2.23** | 11.50 ± 1.25* | 9.33 ± 0.42* |
| 3b | 20 | 5.67 ± 0.21 | 7.0 ± 0.26* | 9.17 ± 0.54** | 13.33 ± 1.14** | 7.33 ± 0.33** | 6.67 ± 0.33 |
| 3b | 40 | 6.50 ± 0.22* | 7.67 ± 0.33* | 11.0 ± 0.68** | 18.83 ± 2.49** | 12.33 ± 1.33* | 9.67 ± 1.15* |
| 3d | 20 | 5.50 ± 0.22 | 6.16 ± 0.31 | 8.00 ± 0.44* | 11.67 ± 1.28* | 7.17 ± 0.31* | 6.5 ± 0.22 |
| 3d | 40 | 5.67 ± 0.34 | 7.00 ± 0.26* | 8.33 ± 0.42* | 16.17 ± 2.68* | 10.83 ± 1.11* | 7.50 ± 0.43* |
| 5b | 20 | 5.83 ± 0.30 | 6.83 ± 0.40 | 8.33 ± 0.33** | 12.16 ± 1.37** | 7.17 ± 0.31* | 6.33 ± 0.49 |
| 5b | 40 | 6.17 ± 0.31* | 7.16 ± 0.30 | 10.33 ± 1.22* | 17.17 ± 2.54** | 11.17 ± 1.28* | 8.67 ± 0.71* |
| 5d | 20 | 5.67 ± 0.21 | 6.33 ± 0.42 | 8.17 ± 0.31** | 11.83 ± 1.24* | 7.0 ± 0.37 | 6.67 ± 0.21 |
| 5d | 40 | 5.67 ± 0.21 | 7.0 ± 0.26* | 8.83 ± 0.75* | 16.33 ± 2.69* | 11.0 ± 1.23* | 8.50 ± 0.67* |
| 5f | 20 | 5.33 ± 0.21 | 6.50 ± 0.22 | 7.50 ± 0.22* | 11.0 ± 1.39* | 6.67 ± 0.49 | 6.50 ± 0.22 |
| 5f | 40 | 5.67 ± 0.33 | 6.83 ± 0.31 | 8.67 ± 0.80* | 15.50 ± 2.40* | 9.67 ± 0.67* | 8.0 ± 0.44* |
CMC: Carboxy Methyl Cellulose; values are expressed as mean ± SEM; (n= 6) and analyzed by ANOVA using Graph pad prism 7; *** P<0.001; **p<0.01; *p<0.05 when compared to control group
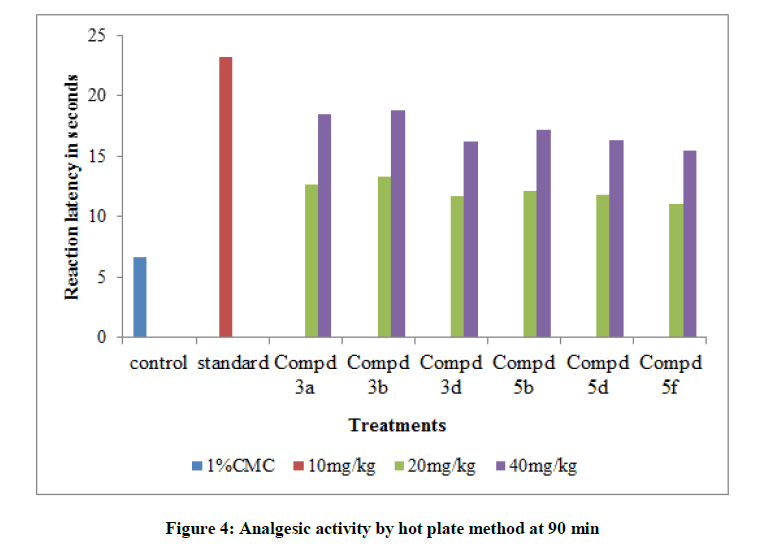
Table 4: Analgesic Activity by Hot Plate Method
| Compound | Dose | 0 min | 30 min | 60 min | 90 min | 120 min | 180 min |
| (mg/kg) | |||||||
| Control | 1% CMC | 4.0 ± 0.26 | 4.17 ± 0.31 | 4.0 ± 0.37 | 4.0 ± 0.37 | 3.67 ± 0.33 | 4.33 ± 0.33 |
| Standard | 10 | 4.50 ± 0.22 | 6.17 ± 0.48* | 9.17 ± 1.14** | 11.33 ± 0.21** | 7.83 ± 0.79* | 6.67 ± 0.21 |
| 3a | 20 | 4.17 ± 0.17 | 5.50 ± 0.22* | 6.17 ± 0.30* | 7.33 ± 1.15* | 5.67 ± 0.33** | 4.67 ± 0.33 |
| 3a | 40 | 4.50 ± 0.22 | 6.33 ± 0.61* | 7.50 ± 0.22** | 8.50 ± 0.91** | 6.16 ± 0.70* | 5.50 ± 0.22* |
| 3b | 20 | 4.33 ± 0.21 | 5.67 ± 0.21* | 6.33 ± 0.42* | 7.50 ± 1.05* | 5.67 ± 0.55* | 4.67 ± 0.33 |
| 3b | 40 | 4.67 ± 0.21 | 6.50 ± 0.62* | 7.67 ± 0.80** | 9.0 ± 0.92** | 6.33 ± 0.61* | 5.50 ± 0.21* |
| 3d | 20 | 4.17 ± 0.30 | 5.17 ± 0.30 | 5.83 ± 0.48 | 6.83 ± 0.87* | 5.50 ± 0.43* | 4.16 ± 0.48 |
| 3d | 40 | 4.17 ± 0.30 | 5.67 ± 0.67 | 7.17 ± 0.70* | 8.0 ± 1.09* | 5.50 ± 0.43* | 4.67 ± 0.49 |
| 5b | 20 | 4.17 ± 0.17 | 5.33 ± 0.21* | 6.0 ± 0.37* | 7.16 ± 1.04* | 5.50 ± 0.34* | 4.50 ± 0.22 |
| 5b | 40 | 4.50 ± 0.22 | 6.17 ± 0.48* | 7.33 ± 0.55* | 8.33 ± 1.13* | 6.0 ± 0.73* | 5.17 ± 0.30 |
| 5d | 20 | 4.33 ± 0.33 | 5.33 ± 0.33* | 6.0 ± 0.25* | 7.0 ± 0.82* | 5.67 ± 0.71* | 4.50 ± 0.67 |
| 5d | 40 | 4.33 ± 0.33 | 6.0 ± 0.58 | 7.33 ± 0.71* | 8.17 ± 1.01* | 5.83 ± 0.48* | 5.0 ± 0.26 |
| 5f | 20 | 4.33 ± 0.42 | 5.33 ± 0.42* | 6.0 ± 0.58* | 7.0 ± 0.86* | 5.67 ± 0.56* | 4.33 ± 0.61 |
| 5f | 40 | 4.33 ± 0.42 | 5.83 ± 0.48 | 7.17 ± 0.54* | 8.0 ± 0.94* | 5.67 ± 0.49* | 5.0 ± 0.37 |
CMC: Carboxy Methyl Cellulose; values are expressed as mean ± SEM (n=6) and analyzed by ANOVA using Graph pad prism 7; *** P<0.001; **p<0.01; *p<0.05 when compared to control group
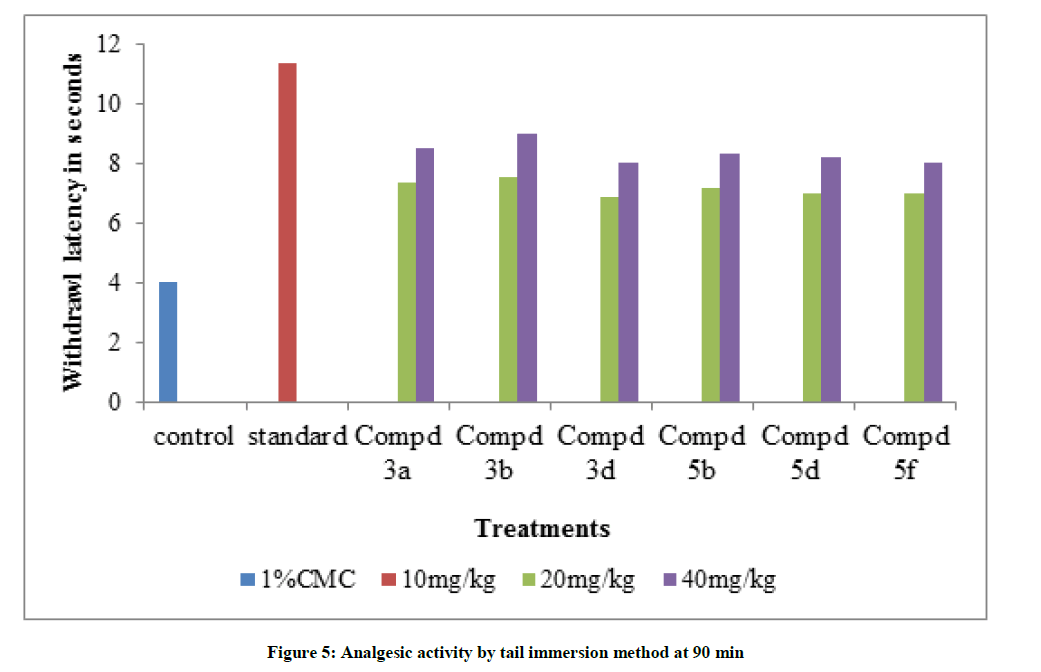
Table 5: Analgesic activity by Tail Immersion method
Discussion
N-(substituted benzyloxy)-6-fluoro-chromone-2-carboxamides were synthesized from the amide coupling of 6-fluoro-chromone-2-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT. And N-(substituted benzyloxy)-chromone-3-carboxamides were synthesized from the amide coupling of chromone-3-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT. All the compounds were characterized by IR, 1H-NMR, 13C-NMR and mass spectroscopy. Molecular docking studies are performed to find the binding affinity of synthesized compounds with 3LN1. Compounds with good Lib Dock score were evaluated for analgesic and antiinflammatory activities. From the overall docking and interaction analysis, the best conformation of the compound 3b showed good Lib Dock score and good hydrogen bond interactions. Hydrogen bonds are formed with the amino acid tyrosine 371 between H with the F11 atom of compound 3b with a hydrogen bond distance of 2.288000 A°. Other hydrogen bonds are also formed between the Arg 106 with the F11 atom of compound 3b with a hydrogen bond distance of 2.037000 A°.
The compounds with good Lib Dock score (3a, 3b, 3d, 5b, 5d, 5f) were evaluated for analgesic and anti-inflammatory activities. Antiinflammatory activity was performed by paw edema method and analgesic screening was done by hot plate method and tail immersion method. All the compounds were tested at a doses of 20 mg/kg and 40 mg/kg.
All the tested compounds showed maximum inhibition of carrageenan induced rat paw edema at 3rd hr. Indomethacin (10 mg/kg) showed maximum of 70.7% inhibition of edema. In all the tested compounds, compound 3b shows the maximum antiflammatory activity with 40 mg/kg. It shows 60.0% inhibition of edema at 3rd h. Maximum analgesic activity was observed at 90 min. In this also compound 3b 6-Fluoro-N-(2- fluorobenzyloxy)-chromone-2-carboxamide shows maximum activity by hot plate method and tail immersion method. Amides of chromone-2-acids showed good analgesic and antiinflammatory activities when compared with amides of chromone-3-acids. Variations in substitutions of the chromone moieties had a significant impact on the activity. Substitution of the chromone moiety at the 6- position with negatively charged group like fluorine produced best activity.1,2,4-oxadiazoles containing aryl groups at C-3 position also enhances the activity. In both the series 1,2,4-oxadiazole which is substituted with pyridine moiety at 3rd position show maximum Lib Dock score and maximum analgesic and antiinflammatory activities.
Conclusion
Herewith, we report the simple and efficient method for synthesis of novel chromone-2-carboxamides and chromone-3-carboxamides. N- (substituted benzyloxy)-6-fluoro-chromone-2-carboxamides were synthesized from the amide coupling of 6-fluoro-chromone-2-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT. And N-(substituted benzyloxy)-chromone-3-carboxamides were synthesized from the amide coupling of chromone-3-carboxylic acid with O-substituted benzylhydroxylamines via acid chloride method at RT. All the compounds were characterized by IR, 1H-NMR, 13C-NMR and mass spectroscopy. Molecular docking studies are performed to find the binding affinity of synthesized compounds with 3LN1. Compounds with good Lib Dock score were evaluated for analgesic and antiinflammatory activities. Among all compounds 6-Fluoro-N-(2-fluorobenzyloxy)-chromone-2-carboxamide showed good analgesic and antiinflammatory activity.
Acknowledgement
Authors express their heartful thanks to Head-Department of University College of Technology, Osmania University and management of RBVRR Women’s College of Pharmacy for their constant support and for providing necessary facilities.
References
- G.R. Kurumbail, M.A. Stevens, K.J. Gierse, J.J. Mc Donald, A.R. Stegeman, Y.J. Pak, D. Gildehaus, M.J. Miyashiro, D.T. Pennung, Nature.,1996, 384, 644-648.
- A. Gaspar, M.J. Matos, J. Garrido, E. Uriarte, F. Borges, Chem. Rev., 2014, 114(9), 4960-4992.
- W. Huang, M.Z. Liu, Yan Li, G.F. Yang, Eur. J. Med. Chem., 2005, 40(9), 882-887.
- V.S. Dofe, A.P. Sarkate, D.K. Lokwani, S.H. Kathwate, C.H. Gill, Research on Chemical Intermediates., 2017, 43(1), 15-28.
- T.Z. Woldemariam, A.I. Khan, A. Burke, N. Mahmood, Antiviral Res., 1994, 25(3-4), 235-244.
- N. Aggarwal, V. Sharma, H. Kaur, M.P.S. Ishar, Int. J. Med. Chem., 2013.
- C.F. Silva, D.C. Pinto, A.M. Silva, Chem. Med. Chem., 2016, 11(20), 2252-2260.
- H. Liu, R. Xu, L. Feng, W. Guo, N. Cao, C. Qian, P. Teng, L. Wang, X. Wu, Y. Sun, J. Li, PLoS One., 2012, 7(8).
- J.A. Hutter, M. Salman, W.B. Stavinoha, N. Satsangi, R.F. Williams, R.T. Streeper, S.T. Weintraub, J Nat. Prod., 1996, 59(5), 541-543.
- S. Choodej, D. Sommit, K. Pudhom, Bioorg. Med. Chem. Lett., 2013, 23(13), 3896-3900.
- Y.N. Sun, W. Li, S.Y. Yang, J.S. Kang, J.Y. Ma, Y.H. Kim, J Funct Foods.,2016, 21, 232-239.
- F. Fatima Bousejra-ElGarah, B. Lajoie, J.P. Souchard, G. Baziard, J. Bouajila, S. El Hage, Med. Chem. Res., 2016, 25(11), 2547-2556.
- B.J. Ludwig, F. Dursch, M. Auerbach, K. Tomeczek, F.M. Berger, J. Med. Chem., 1967, 10, 556-563.
- S.A. Glover, G.P.A. Hammond, J. Org. Chem., 1998, 63, 9684-9689.
- M.A. Bailen, R. Chinchilla, D.J. Dodsworth, C. Najera, Tetrahedron. Lett., 2001, 42, 5013-5016.
- N.D. Kokare, R.R. Nagawade, V.P. Ranea, D.B. Shindea, Tetrahedron. Lett., 2007, 48, 4437-4440.
- H. Sauter, W. Kramer, U. Schirmer (Eds.), Modern Crop Protection Compounds, Wiley, 2007, 457-491.
- S. chimichi, M. Boccalini, B. Cosimelli, Tetrahedron., 2002, 58, 4851-4858.
- M. Dai, X. Fu, F. He, L. iang, Y. Li, F. Liang, L. Liu, Y. Mi, Y.C. Xu, G. Xun, X. Yan, Z. Yu, J.Y. Zhang, 2011, WO 2011/020861A1.
- W. Ming-Zhong, X. Han, L. Tuan-Wei, Q. Feng, Y. Shu-Jing, W. Su-Hua, L. Zheng-Ming, Eur. J. Med. Chem.,2011, 46, 1463-1472.
- C.A. Winter, E.A. Risley, G.W. Nuss, Proc. Soc. Exp. Biol. Med., 1962, 111, 544.
- N.B. Eddy, D. Leimbach, J. Pharmacol. Exp. Ther., 1953, 107 (3): 385-393.