Research - Der Pharma Chemica ( 2023) Volume 15, Issue 5
SYNTHESIS, CHARACTERIZATION, ANTIHELMINTIC AND INSILICO EVALUATION OF 2,3 DI SUBSTITUTED QUINOLINE DERIVATIVES
Sudha Rani K*, Sowmya A, Pavani J, Dhanunjay M, Yamini Siva lalitha V, Punya Keerthi N, Sai Reethika B and Gopal Reddy NSudha Rani K, Assistant Professor, Department of Pharmaceutical Chemistry, Sri Siddhartha Pharmacy College, Ammavarithota, Nuzvid, India, Email: sudha.k377@gmail.com
Received: 07-Sep-2023, Manuscript No. dpc-23-114948; Accepted Date: Sep 29, 2023 ; Editor assigned: 09-Sep-2023, Pre QC No. dpc-23-114948; Reviewed: 25-Sep-2023, QC No. dpc-23-114948; Revised: 28-Sep-2023, Manuscript No. dpc-23-114948; Published: 30-Sep-2023, DOI: 10.4172/0975-413X.15.5.84-89
Abstract
A Series of Bioactive compounds 2-methyl-1H-Indole (1a) , 2-phenyl-1H-Indole (1b), 2-(2-methylquinolin-3-yl)aniline (2a), 2-(2-phenylquinolin-3- yl)aniline (2b), were Synthesized according to the Literature methods. The Synthesized compounds were characterized by NMR, IR & Mass Spectroscopy. All the compounds have been evaluated for In vitro anthelmintic activity and were compared with their corresponding standards.
Keywords
2, 3-Di Substituted quinolines; Anthelmintic; Albendazole; Earthworms; Indoles
INTRODUCTION
According to the World Health Organization, infectious diseases are the leading cause of death and disease worldwide [1]. Helminthes are considered a major impediment to animal production and human blood loss in the tropics, and contribute to malnutrition, anemia, eosinophilia, and pneumonia, with global incidences of approximately 500 million to 1 billion per year [2]. Ideally, an anthelmintic should have a broad spectrum of activity, have a high cure rate, be free of host toxicity, and be inexpensive [3]. Alcoholic active substances of plant origin are widely used for their anthelmintic effects in children aged 5 to 13 years. Worms that infect the intestines are ex-tapeworms; Tapeworm nematodes [Taeniasolium], e.g. Hookworms [Ancylostoma duodenale], roundworms [Ascaris lumbricoides] and flukes or trematodes [Schistosoma mansoni and Schistosoma haematobolium]. Diseases caused by parasitic infections that cause severe morbidity include lymphatic filariasis, onchocerciasis, and schistosomiasis.
A literature review revealed that the heterocyclic quinoline ring is a versatile nucleus with a myriad spectrum of pharmacological effects such as anthelmintic, antibacterial, has antifungal, antioxidant, anti-inflammatory, anticancer and antiviral properties [4] with these reasonable observations in mind, it has been considered important to produce effective medicines with reduced administration frequency, reduced side effects and improvements that meet these requirements to develop a new anthelmintic.
The aim of this study is to investigate the anthelmintic activity of newly synthesized 2, 3-disubstituted quinolines and their derivatives. Triazolidine-3-has been very effective against various worms for decades. In addition, some worms have been shown to be resistant to major classes of anthelmintic such as benzimidazoles, imidazothiazoles and macro cyclic lactones. The search for these 2, 3-disubstituted quinolines and their derivatives led to the evaluation of prototypical anthelmintic compounds [5].
MATERIALS AND METHODS
Melting points were determined in open glass capillaries using Gallen Kamp (MFB- 600) melting point apparatus and were uncorrected. IR spectra (KBr discs) Bruker analyzers were confirmed by Shimadzu FT-IR Spectrophotometer using KBr pellets technique, Model No.8400S (Japan). 1H and 13C NMR spectra were recorded on Bruker 400 MHzNMR spectrometer (Switzerland) using DMSO as solvent. T.L.C. was run on silica gel G plates using ethyl acetate: n-hexane (7:3) as a developing solvent to assess the progress of reaction and purity of the compounds. All other chemicals used in the present study were of analytical grade.
Drugs and chemicals (2.1)
Phenyl Hydrazine (WWW.RESEARCHLAB.IN-B.No:1174220521), Acetophenone (FINAR.B.No:506560212BP), Acetone (SUN.B.No:8189), 2-Amino Benzaldehyde (PALLAV B.No:PC/2186/16-2), Ethanol (CSS.B.No-110605), Potassium Hydroxide- (FISHER). Silica gel-G-(RSEARCH-LAB FINE CHEM industries- b.No:1317310113),
Ethyl acetate-(AVRA), Dimethyl Sulphoxide (AVRA- B.No:N140122180), Sodium Chloride (FINAR-B.No:76274020 Thiosemicarbazide (Sisco-B.No, 5595361).
Chemical synthesis (2.2)
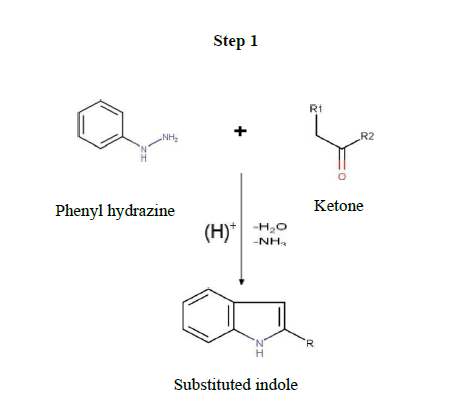
Synthesis of substituted indole derivatives (Step 1)
Equimoles of different ketones and phenyl hydrazine were taken into mortar and pulverized by pestle at room temperature by employing the friction method. Then the mixture was moist with a few drops of ethanol. The progress of the reaction was checked by TLC, and all the reactions were found to be completed in times of 10-12 min. The product was recrystallized from ethanol. The purity was confirmed by thin- layer chromatography and melting point.
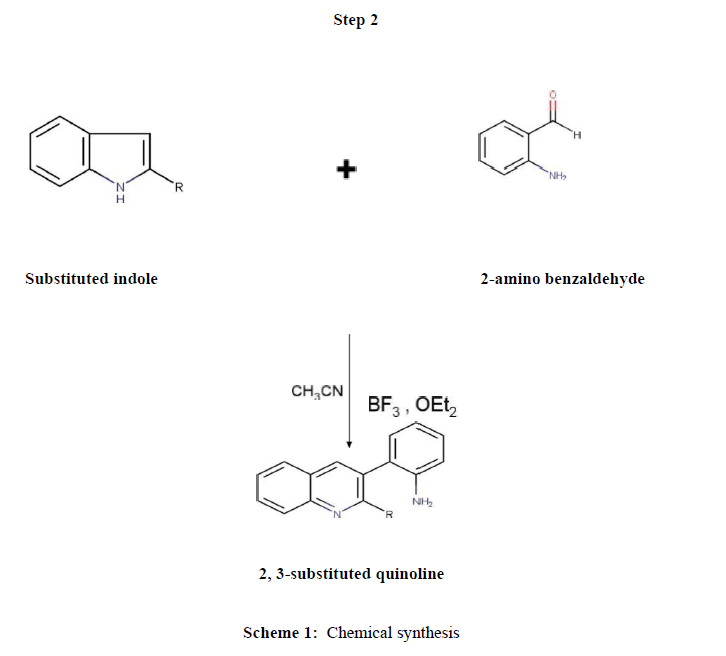
Synthesis of quinoline compounds (Step 2)
Equimoles of substituted indole derivatives and 2-amino benzaldehyde were taken into mortar and pulverized by pestle at room temperature by employing the friction method at room temperature using CH3CN, BF3, and OEt2. The mixture was neutralized and transferred to ice cold water; precipitate was filtered, dried and recrystallized from ethanol. The purity and progress of reaction was confirmed by thin layer chromatography. The procedure was illustrated under (Scheme 1) and the physical data were tabulated in (Table 1)
| Code | Compound | M.F | M.W | % yield | C% | H% | O % | N% | Cl% | S% |
|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 2-(2-methylquinolin-3-yl) aniline | C16H15N2 | 234.30 | 74 | 81.94 | 6.4 | _ | 11.9 | _ | _ |
| 2b | 2-(2-phenylquinolin-3-yl) aniline | C21H16N2 | 295.38 | 76 | 85.31 | 5.41 | _ | 9.47 | _ | _ |
Compound [2a]:2-(2-methylquinolin-3-yl) aniline: Yield- 80%
IR Data: FTIR (γ max, C m-1) 1640 (-C=C stretch), 3080 ( =C-H stretch), 1025 (=C-H bend), 1520 (-C=N ), 3150 (- C- NH2); 1H NMR (500MHZ,CDC13), ծ 7.63(Ar-H), 6.65, 7.14, 7.26, 7.63, 7.71, 8.04, 8.10, 8.43 (C-H), ծ 7.63 (C-NH2); 13C NMR (500MHZ,CDCl3): δ 128.85, 127.54, 130.77, 130.02, 121.20, 116.02, 145.17, 121.27, 128.89, (C-H), δ 129.06, (C-NH2), δ 154.34 (C-CH3), δ 154.34 (C=N),
Compound [2b]: 2-(2-phenylquinolin-3-yl) aniline: Yield- 70%
IR Data: FTIR (γ max, C m- 1) 1655 (-C=C stretch), 3050 (= C-H stretch), 1035 (=C-H bend), 1550 (-C=N), 3200 (- C-NH2). 1H NMR (500MHZ, CDCl3): δ 7.40, 7.42, 7.51, 7.63, (Ar-H), δ 4.21, 6.65, 7.14, 7.63, 7.71, 8.04, 8.10, 8.81, (C-H), δ7.63 (C-NH2) ; 13C NMR (500MHZ,CDCl3): δ 128.85, 127.54, 130.77, 130.02, 120.74, 116.02, 145.17, 121.27, 128.89, (C-H), δ 129.06 (C-NH2), δ 156.25 (C-C6H5), δ 154.34 (C=N)
Anthelmintic Activity (3)
Anthelmintic activity was done by Indian adult earthworms of the genus and species, Pheretima posthuma (Family: Megascolecidae). The earthworms were collected from the waterlogged areas of soils in Nuzvid, Andhra Pradesh, India. The earthworms were washed with normal saline to remove all the fecal matter and waste surrounding their body. The earth worms (Pheretima posthuma) 5-8 cm in length and 0.2-0.3 cm width weighing 0.8–3.04 g was used for all experiment protocols. The earthworms resembled the intestinal roundworm parasites of human beings both anatomically and physiologically and hence were used to study the anthelmintic activity.
PROCEDURE
Five groups of approximately equal sized Indian earthworms consisting of six earthworms. Group one serve as 2-(2-methylquinoline-3-yl)aniline; Group two serve as 2-(2- phenylquinoline-3-yl)aniline; Group third serve as control, receive only saline; Group forth serve as standard Albendazole.
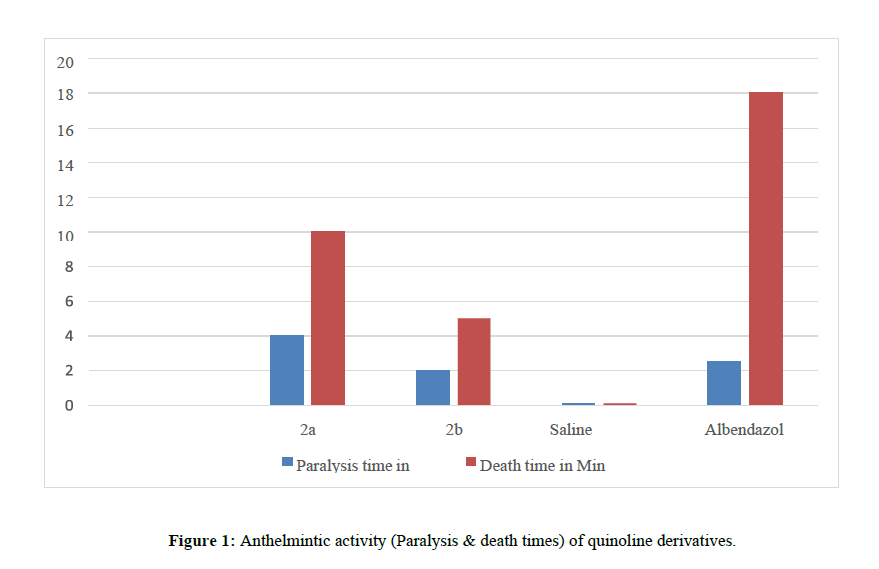
Observations were made for the time taken to paralyze and death of the organism. Paralysis was said to occur when the worms do not review even in normal saline. Death was concluded when worms lost their motility followed by fading away of the body color, and the values are summarized in (Table 2) (Figure 1)
Sl. No. |
Compounds | Dose | Paralysis time in minutes | Death time in minutes |
|---|---|---|---|---|
| Mean±S.E. M | Mean±S.E. M | |||
| 1 | 2a | 100mg/ml | 4±0.52 | 10±0.36 |
| 2 | 2b | 100mg/ml | 2±0.5 | 5±0.72 |
| 3 | Control (Normal saline) | -------- | -------- | |
| 4 | Albendazole | 100mg/ml | 2.51±1.1 | 18±2.1 |
In silico evaluation for drug-likeness and toxicity predictions (4)
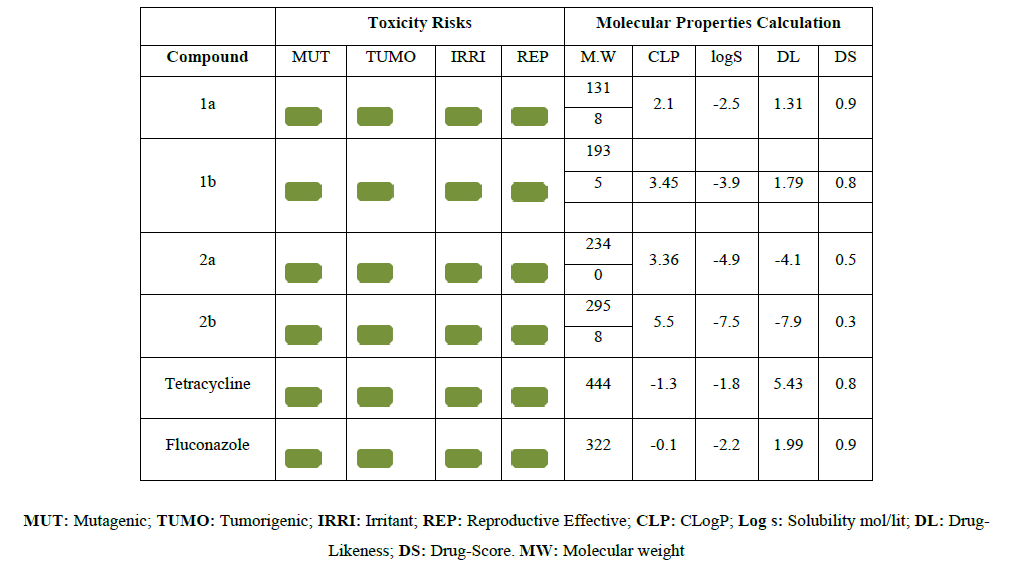
Currently, in this work three cheminformatics programs were used to evaluate the drug likeness of compounds, toxicity predictions, to assess the inhibition of the derivatives against 5 subtypes of cytochrome P450. An open-source program OSIRIS Property Explorer was used to predict the fragment-based drug-likeness of title compounds and comparing them with Fluconazole and tetracycline, to assess the occurrence frequency of each fragment in the individual structure. The program estimated the risks of side effects, such as mutagenic, tumorigenic, irritant and reproductive effects, as well as drug-relevant properties including cLogP, LogS (solubility), MW and drug-likeness. Molinspiration cheminformatics used for calculation of important molecular properties like logP, Polar surface area, Number of hydrogen bond donors, Number of hydrogen bond acceptors, Number of rotatable bonds, Volume, Number of violations from rule of five. It was also used to predict bioactive scores for the most important drug targets like GPCR ligand, Kinase inhibitors, Ion channel modulators, nuclear receptors, Protease inhibitors, Enzyme inhibitors [7-9] (Table 3) (Table 4) (Table 5) (Table 6)
| Compound Code | Compound IUPAC Names | Log P | Polar Surface Area | H-Bond Acceptors | H-Bond Donor | Volume |
|---|---|---|---|---|---|---|
| 1a | 2- Methyl-1H-indole | 2.38 | 15.79 | 1 | 1 | 129.58 |
| 1b | 2-Penyl-1H-Indole | 3.83 | 15.79 | 1 | 1 | 184.43 |
| 2a | 2-(2-methylquinoline-3-yl)aniline | 3.54 | 38.91 | 2 | 2 | 223.14 |
| 2b | 2-(2-Phenylquinoline-3-yl)aniline | 4.99 | 38.91 | 2 | 2 | 277.99 |
Compound |
GPCR Ligand | Ion channel modulator | Kinase inhibitor | Nuclear receptor ligand | Protease inhibitor | Enzyme inhibitor |
|---|---|---|---|---|---|---|
| 1a | -0.17 | -0.19 | -0.55 | -1.02 | -1.16 | -0.31 |
| 1b | -0.02 | 0.05 | 0.62 | -0.07 | -0.35 | 0.11 |
| 2a | 0.14 | 0.16 | 0.15 | -0.48 | -0.14 | 0.24 |
| 2b | 0.31 | 0.28 | 0.53 | 0.01 | -0.07 | 0.42 |
Compound |
Aqueous solubility | LogIGC5 0 | AMES | CYP3A 4 | CYP2D 6 | CYP2C1 9 | CYP2C 9 | CYP1A 2 |
|---|---|---|---|---|---|---|---|---|
| 1a | 3.7 | 0.99 | Inactive | - | - | - | - | + |
| 1b | 3.2 | 1.2 | Inactive | - | + | + | - | + |
| 2a | 5.6 | 1.09 | Active | + | - | + | + | + |
| 2b | 5.2 | 1.8 | Active | + | - | + | + | + |
| Tetracycline | 3.31 | 0.23 | Inactive | - | - | - | - | - |
| Fluconazole | 1.8 | 0.15 | Inactive | - | - | - | - | - |
+Inhibitor, - Non inhibitor, AQ-aqueous, IGC 50-Environmental toxicity
The Online Chemical Modeling Environment (OCHEM) a unique and a web-based platform which supports all the steps required to create a predictive model: one such model developed was cytochrome P450 with 5 subtypes the compounds was evaluated to assess their Inhibition on the subtypes of cytochrome P450.
RESULTS AND DISCUSSION
The derivatives synthesized were evaluated by three online software's-OSIRIS, MOL INSPIRATION and OCHEM. OSIRIS results predict that compound 2a have high drug score 0.46 and compound 2b have low drug score 0.33.toxicity predictions inferred that all compounds are safe. From the OCHEM results, all the synthesized compounds were found to inhibit the subtype CYP1A2 of cytochrome P450. Mol inspiration results inferred that all the derivatives satisfy Lipinski rule of five to behave as a drug and found to have kinase and enzyme inhibition properties.
Physicochemical and analytical data were tabulated for synthesized compounds 2a-2b as shown in (Table 1) The Structures of the compounds were characterized through IR and IH and 13C NMR Spectral data, whereas results of anthelmintic studies tabulated in (Table 5) reveals that compound 2a was found to be the most potent Compound in the series which when compared to the standard, compounds 2a showed high activity, other compounds 2b showed significant anthelmintic activity.
CONCLUSION
In conclusion, we have developed a simple, benign and expeditious synthesis of biologically significant quinoline derivatives with good yields under mortar and pestle grinding method and fully characterized the products by IR.1HNMR, Mass spectral and Elemental analysis. The synthesis of quinoline derivatives by mortar and pestle grinding method (yield 70%-76%) All the synthesized compounds were evaluated for their anti-microbial, anti-fungal and anthelmintic activities. By Mol inspiration software we found that compounds is having better bioactive score against enzyme inhibition and protease inhibition. All the synthesized compounds were found to inhibit the sub-type CYP1A2 of cytochrome P450 in OCHEM software.
ACKNOWLEDGEMENT
The authors are thankful to the authorities of Sri Siddhartha Pharmacy College, Nuzvid, Eluru dist, A.P., India for providing necessary facilities and support to carry out this work.
REFERENCES
- World Health Organization-Global Report for Research on Infectious Diseases of Poverty, 2012. Accessed on 10/04/2013. 21. World Health Organization- Global Report for Research on Infectious Diseases of Poverty. 2012.
- Ikechukwuogu G. IJNDD. 2012; 4:p. 306-310.
- Ravindra GM, Shailaja GM, Anita AM. PharmBiol. 2007; 10:p. 766-76.
- Chirag Sharma, Bhawana Thadhaney, Gangotri Pemawat , Talesara GL. Indian JChem. 2008; 12: p.1892-1897.
- Kosalge SB, Fursule RA. Plant material Preparation of extract aqueous extract(Maceration method).2009; 2(2):p. 69–71.
- Chenchu Lakshmi KNV, Deepika Koti, Anupama B, Chakravarthy A et al. Der Pharma Chemica. 2015; 7(5):p. 64-71.
- Qureshi Md. Shamim, Giri IC, Panday VK, Choudhary R et al. Research J. Pharmacognosy and Photochemistry. 2009; 1(1):p. 78-79.
- Tetko IV. Computing chemistry on the web. Drug DiscovToday. 2005; 10:p. 1497-1500.
- Organic Chemistry Portal. http://www.organicchemistry. Org/prog/peo/ 2012.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref