Research - Der Pharma Chemica ( 2022) Volume 14, Issue 2
Synthesis, Characterization and in-vitro Antimicrobial activity Studies of Co (II), Ni (II) and Cu (II) Complexes with ONSO Donor Coumarin Schiff Bases
Shrishila N. Unki* and Shekappa D LamaniShrishila N. Unki, Department of Chemistry, SB Arts and KCP Science College, Vijaypura-586103. Karnataka, India, Email: shrishailnunki@gmail.com
Received: 27-Jan-2022, Manuscript No. dpc-22-52528; Accepted Date: Jan 29, 2022 ; Editor assigned: 29-Jan-2022, Pre QC No. dpc-22-52528; Reviewed: 15-Feb-2022, QC No. dpc-22-52528; Revised: 21-Feb-2022, Manuscript No. dpc-22-52528; Published: 27-Feb-2022
Abstract
A series of Co(II), Ni(II) and Cu(II) complexes have been synthesized with Schiff bases derived from 3-substituted-4-amino-5-mercapto-1,2,4-triazole and 8-acetyl-7-hydroxy-4-methylcoumarin. The chelation of the complexes has been proposed in the light of analytical, spectral (IR, UV–Vis, 1H NMR, ESR and FAB-mass), magnetic and thermal studies. The measured molar conductance values indicate that, the complexes are non-electrolytic in nature. The redox behavior of the complexes was investigated with electrochemical method by using cyclic voltammetry. The Schiff bases and their metal complexes have been screened for their in vitro antibacterial (Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Salmonella typhi) and antifungal activities (Candida albicans, Cladosporium and Aspergillus niger) by MIC method.
Keywords
Coumarin; Antimicrobial; IR Spectra; Schiff bases; Spectroscopic studies; Transition metal complexes
Introduction
Chromenes, especially 2-oxo-2H-chromenes (coumarins), have been extensively studied due to their commercial applications in several fields. These compounds have outstanding optical properties, including an extended spectral range, high quantum yield, superior photostability and good solubility in common solvents. Typical feature of chromene derivatives is that photophysical and spectroscopic properties can be readily modified by introduction of substituents in the chromene ring, giving themselves more flexibility to fit to various applications [1-3]. Coumarins display a remarkable array of biochemical and pharmacological actions, some of which suggest that, certain members of this group of compounds may significantly affect the function of various mammalian cellular systems [4].
Schiff bases are important class of ligands in coordination chemistry and their complexing ability containing different donor atom is widely reported. The chemistry of transition metal complexes containing heterocyclic donor continues to be of interest on account of their biological importance. There is a growing interest in the studies on the metal complexes of Schiff bases derived from triazoles and its derivatives which are biologically important ligands [5]. Schiff base metal complexes have been widely studied because of their industrial and biological applications; several derivatives of these have been used as drugs. The triazole Schiff bases constitute one of the most important classes of O, N, and S donor atoms. Triazoles and their derivatives have been proved effective bacteriocides [6], pesticides [7], fungicides [8-9] and insecticides [10-11].
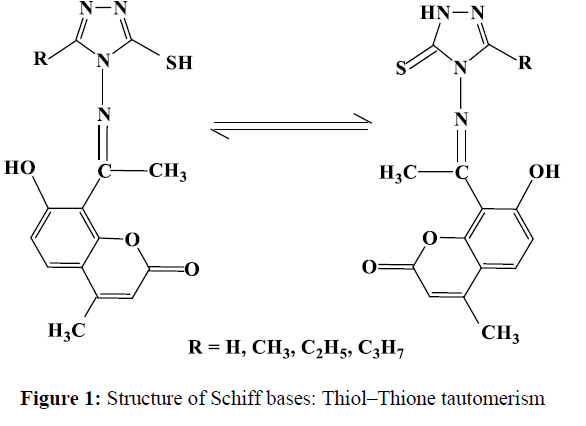
A survey of the literature reveals that, no work has been carried out on the synthesis of Co(II), Ni(II) and Cu(II) complexes with newly synthesized Schiff bases derived from 3-substituted-4-amino-5-mercapto-1,2,4-triazole and 8-acetyl-7-hydroxy-4-methylcoumarin. These Schiff bases have donor sites with the OONS sequence and varied coordination abilities. This nature of the Schiff bases (Figure-1) have attracted our attention and aroused our interest in elucidating the structure of metal complexes. The Schiff bases and their metal complexes were screened for their in-vitro antimicrobial activity.
Experimental
Analysis and Physical Measurements
Carbon, hydrogen and nitrogen were estimated by using Elemental Analyzer Truspec (Leco Corporation USA). The IR spectra of the Schiff bases and their Co(II), Ni(II) and Cu(II) complexes were recorded on a HITACHI-270 IR spectrophotometer in the 4000-250 cm-1 region in KBr disc. The electronic spectra of the complexes were recorded in HPLC grade DMF and DMSO solvent on a VARIAN CARY 50-BIO UV-spectrophotometer in the region of 200-1100 nm. The 1H-NMR spectra of ligands were recorded in DMSO-d6 on a BRUKER 300 MHz spectrometer at room temperature using TMS as an internal reference. The fluorescence studies of Schiff bases and their metal complexes were recorded on HITACHI F- 7000 Fluorescence Spectrophotometer (Made in Japan). The solutions of 10-3 M concentration were prepared in HPLC grade DMF and DMSO solvents and the experiment was carried out at room temperature. The electrochemistry of the metal complexes was recorded on CHI1110Aelectrochemical analyzer (Made in U.S.A) in dimethyl formamide (DMF) containing 0.05 M n-Bu4NClO4 as the supporting electrolyte. The ESR spectrum was recorded on Varian-E-4X-band EPR spectrometer and the field set is 3000 G at modulation frequency of 100 K Hz under liquid nitrogen temperature using TCNE as ‘g’ marker. FAB-Mass spectra were recorded on a JEOL SX 102/DA-6000 mass spectrometer/data system using Argon/Xenon (6KV, 10Am) as the FAB gas. The accelerating voltage was 10 KV and the spectra were recorded at room temperature and mnitrobenzyl alcohol was used as the matrix. Thermogravimetric analyses data were measured from room temperature to 1000ºC at a heating rate of 10ºC/min. The data were obtained by using TA Instruments Water LLC, New castel, Delware. USA. Model; DCS Q 20, 2009. Molar conductivity measurements were recorded on ELICO-CM-82 T Conductivity Bridge with a cell having cell constant 0.51 and magnetic moment of the complexes was carried out by using faraday balance.
Synthesis
All chemicals and solvents used were of AR grade. All metal (II) salts were used as their chlorides. 3-substituted-4-amino-5-mercapto-1,2,4- triazoles were synthesized by reported methods [12].
Synthesis of 7-Hydroxy-4-Methylcoumarin [13]
A mixture of dry resorcinol (0.2 mol) and ethylacetoacetate (0.2 mol) is cooled to 0-5ºC and conc. sulphuric acid (25 mL) is added gradually with constant shaking. The reaction mixture is then kept in a refrigerator for 24 h. and poured into crushed ice with stirring. The separated solid is filtered washed with water and recrystallized from ethanol as cream colored needles. Yield: 80%; MP: 182ºC.
Synthesis of 7-Acetoxy-4-Methyl Coumarin [14]
A mixture of 7-hydroxy-4-methyl coumarin (0.16 mol) and freshly distilled acetic anhydride (0.56 mol) was refluxed for 1.5 hr under anhydrous conditions. While the solution was hot, it was poured to crushed ice and the separated product was filtered and washed with water. It was crystallized from methanol as colorless needles. Purity of the compound was established by a single spot in TLC and the melting point was in agreement with the literature value (150ºC); m.p 152-154ºC.
Synthesis of 8-Acetyl-7-Hydroxy-4-Methyl Coumarin [14]
A mixture of 7-acetoxy-4-methyl coumarin (0.01 mol) and anhydrous AlCl3 (0.03 mol) was heated under anhydrous conditions in an oil bath at 125oC and the temperature was raised during 2.5 hr period to 170ºC. To the reaction mixture, crushed ice and dilute HCl were added with stirring and the mixture was left for 2-3 hr in order to decompose the complex. The separated product was filtered, washed with water and recrystallized from ethanol. A single spot in TLC indicated the purity of the compound. m.p. 159-160ºC, yield 68%.
Synthesis of Schiff Bases [I-IV]
The Schiff bases were synthesized by the condensation of 3-substituted-4-amino-5-mercapto-1,2,4-triazole (0.01 mol) and 8-Acetyl-7-Hydroxy-4- Methylcoumarin (0.01 mol), dissolved in 30 ml alcoholic medium containing few drops of concentrated HCl. The resulting mixture was refluxed for 3-4 h. The solid product separated on evaporation of the solvent was filtered, washed with alcohol and then finely recrystalized from EtOH.
Synthesis of Co (II), Ni (II) and Cu (II) Complexes
An alcoholic solution (30ml) of Schiff bases (I-IV) 1 mmol was refluxed with 1 mmol of CoCl2.6H2O/NiCl2.6H2O/CuCl2.2H2O in 30ml ethanol solution on steam bath for 1h. Then, to the reaction mixture 2 mmol of sodium acetate was added and refluxtion was continued for 3h. The separated complex was filtered, washed thoroughly with water, Ethanol, Ether and finally dried in vacuum over fused CaCl2
. Pharmacology
In vitro antibacterial and antifungal assay
The biological activities of Synthesized Schiff bases and their Co (II), Ni (II) and Cu(II) complexes have been studied for their antibacterial and antifungal activities by agar and potato dextrose agar diffusion [15-16] method respectively. The antibacterial and antifungal activities were done at 25, 50 and 100 μg/mL concentrations in DMSO solvent by using four bacteria (Escherichia coli, Staphilococcus aureus, Bascillus subtilis and Salmonella typhi) and three fungi (Aspergillus niger, Candida albicans and cladosporium) by the MIC method [32]. These bacterial strains were incubated for 24h at 37ºC and fungal strains were incubated for 48h at 37ºC. Standard antibacterial and antifungal drug (Gentamycine) and antifungal drug (Flucanazole) were used for comparison under similar conditions.
Result and Discussions
All the Co(II), Ni(II) and Cu(II) complexes were stable at room temperature, non-hygroscopic, insoluble in water and many common organic solvents but soluble in DMF and DMSO and infusible at high temperature. All the metal complexes are thought to be polymeric in nature. The elemental analysis results for the Co (II), Ni (II) and Cu (II) complexes agree with the calculated values showing that the complexes have 1:1 stoichiometry of the type ML.2H2O, where ‘L’ stands for a deprotonated ligand. The observed molar conductances of the complexes in DMF for 10-3 M solutions at room temperature were consistent with the non-electrolytic nature (Table 1).
| Comp. No. |
Empirical formula | M% | C% | N% | S% | Molar conductance Ohm-1 cm-2 mole-1 | Mag. Moments (µeff BM) | ||||
| Obsd | Calcd | Obsd. | Calcd | Obsd. | Calcd | Obsd. | Calcd | ||||
| 1 | Co(C14H10N4O3S).2H2O | 14.36 | 14.42 | 41.05 | 41.07 | 13.60 | 13.69 | 7.80 | 7.82 | 19.22 | 4.61 |
| 2 | Co(C15H12N4O3S).2H2O | 13.90 | 13.94 | 42.51 | 42.55 | 13.16 | 13.23 | 7.50 | 7.56 | 22.06 | 4.76 |
| 3 | Co(C16H14N4O3S).2H2O | 13.48 | 13.50 | 43.90 | 43.93 | 12.77 | 12.81 | 7.28 | 7.32 | 24.52 | 4.90 |
| 4 | Co(C17H16N4O3S).2H2O | 13.00 | 13.08 | 45.21 | 45.23 | 12.33 | 12.41 | 7.03 | 7.09 | 17.28 | 4.96 |
| 5 | Ni(C14H10N4O3S).2H2O | 14.11 | 14.21 | 41.15 | 41.17 | 13.70 | 13.72 | 7.79 | 7.84 | 20.01 | 3.11 |
| 6 | Ni(C15H12N4O3S).2H2O | 13.71 | 13.74 | 42.62 | 42.65 | 13.22 | 13.27 | 7.52 | 7.58 | 23.07 | 3.20 |
| 7 | Ni(C16H14N4O3S).2H2O | 13.26 | 13.30 | 44.00 | 44.03 | 12.81 | 12.84 | 7.26 | 7.33 | 25.85 | 3.09 |
| 8 | NI(C17H16N4O3S).2H2O | 12.82 | 12.88 | 45.31 | 45.33 | 12.40 | 12.44 | 7.10 | 7.11 | 18.03 | 3.29 |
| 9 | Cu(C14H10N4O3S).2H2O | 15.22 | 15.25 | 40.65 | 40.67 | 13.53 | 13.55 | 7.72 | 7.74 | 21.14 | 1.77 |
| 10 | Cu(C15H12N4O3S).2H2O | 14.70 | 14.45 | 42.13 | 42.15 | 13.10 | 13.11 | 7.45 | 7.49 | 23.43 | 1.76 |
| 11 | Cu(C16H14N4O3S).2H2O | 14.23 | 14.25 | 43.52 | 43.53 | 12.65 | 12.69 | 7.22 | 7.25 | 25.66 | 1.79 |
| 12 | Cu(C17H16N4O3S).2H2O | 13.82 | 13.84 | 44.80 | 44.83 | 12.01 | 12.03 | 7.01 | 7.03 | 18.46 | 1.73 |
IR Spectra
In order to study the binding mode of Schiff bases to metal ion in the complexes, IR spectrum of the Schiff bases were compared with the spectra of the metal complexes. The IR spectra of the Schiff bases (Table-2) display a broad band in the range 3109-3120 cm-1, which is ascribed to the stretching vibration of -NH of the pyrrolic nitrogen atom. Although Schiff bases in solution exists in two tautomeric conformations exhibiting thiol ↔ thione isomerism involving –N=C-SH and –NH=C-S groups in a thiol-thione equilibrium [17-18], the IR spectrum of this ligands provides evidence of the thione form in the solid state along with little of the thiol structure, where, in addition to the weak band observed at 2440-2482 cm-1, which is ascribed to the stretching vibration of SH [19-21]. The IR spectra of Schiff bases exhibited a broad band at 3251-3271 cm-1, strong band at 1714-1720, 1600-1610 and 1271-1296 cm-1 assigned to H-bonded -OH stretching, ν(C=O) lactonic carbonyl, ν(C=N) and phenolic ν(C-O) vibrations respectively. A medium band around 1055 cm-1 is characterized for ν (O-C-O).
| Schiff bases No. |
Empirical Formula | H-bonded –OH Stretching | ν (NH) | Lactonil ν (C=O) |
ν (C=N) | ν (SH) | ν (C=S) | Phenolic ν (C-O) |
| I | C14H12N4O3S | 3251 | 3120 | 1720 | 1600 | 2458 | 1107 | 1269 |
| II | C15H14N4O3S | 3269 | 3118 | 1715 | 1608 | 2440 | 1040 | 1282 |
| III | C16H16N4O3S | 3271 | 3113 | 1714 | 1610 | 2480 | 1075 | 1289 |
| IV | C17H18N4O3S | 3258 | 3109 | 1719 | 1605 | 2482 | 1182 | 1296 |
In comparison with the spectra of the Schiff bases, all the metal complexes exhibit downward shift 8-20 cm-1 of ν(C=N) indicating the participation of azomethine nitrogen in the coordination to the metal ion (Table-3).
| Complex No. |
ν(OH) | ν(C=O) | ν(C=N) | Phenolic ν(C-O) |
ν(M-N) | ν(M-O) |
| 1 | 3432 | 1705 | 1592 | 1325 | 468 | 489 |
| 2 | 3440 | 1700 | 1600 | 1333 | 473 | 467 |
| 3 | 3429 | 1704 | 1599 | 1342 | 463 | 471 |
| 4 | 3412 | 1708 | 1590 | 1346 | 468 | 484 |
| 5 | 3422 | 1706 | 1595 | 1344 | 474 | 487 |
| 6 | 3435 | 1699 | 1589 | 1358 | 465 | 481 |
| 7 | 3436 | 1695 | 1593 | 1346 | 463 | 484 |
| 8 | 3408 | 1702 | 1602 | 1348 | 484 | 456 |
| 9 | 3425 | 1706 | 1587 | 1352 | 470 | 487 |
| 10 | 3408 | 1703 | 1590 | 1345 | 481 | 459 |
| 11 | 3417 | 1700 | 1600 | 1349 | 446 | 486 |
| 12 | 3432 | 1709 | 1590 | 1358 | 443 | 467 |
The absorption bands associated with the ν(OH) of the phenolic groups (observed at 3251-3271 cm-1 in the free ligands) disappeared in the IR spectra of the metal complexes, indicating that, the loss of phenolic proton on complexation and formation of metal-oxygen bonds. The high intensity band due to phenolic ν(C-O) appeared in the region at 1269-1296 cm-1 in the Schiff bases appeared as a medium to high intensity band in the 1325-1358 cm-1 region in the complexes and supports the suggestion that the ligands coordinate through their deprotonated form.
The most notable change in the Schiff bases spectral features when coordinated to metal ion is the lower frequencies about 10-20 cm-1 in lactone ν(C=O), suggesting that, the metal is coordinated to the lactone oxygen [22]. This further supported by downward shift in ν (O-C-O) of the coumarin ring [23]. The presence of coordinated water was suggested by the very broad absorption band around 3408-3440 cm-1 in the IR spectra of complexes. Bands at the region 760-800 and 705-720 cm-1 may be attributed to rocking and wagging modes of coordinated water [24-26]. New bands at 500-400 cm-1 are tentatively assigned to ν(M-O), ν(M-N) and ν(M-S) (metal–ligand) stretching bands. On the basis of IR data, it is concluded that, all the metal ions are coordinated to the azomethine nitrogen, phenolic oxygen, sulphur atom and lactone oxygen.
Thus, the IR spectral data results provide strong evidences for the complexation of the tetradentate Schiff bases and also suggests that, the complexes are exist in the solid state as polymeric structure with bonding of metal likely to both the deprotonated phenolic oxygen and lactose carbonyl oxygen.
1H NMR Spectral Studies of Schiff Bases
The spectral data of 1H NMR of all the Schiff bases are given in Table-4. In the 1H NMR spectra of Schiff bases II exhibit the singlet at 11.97 ppm (s, 1H) is ascribed to NH and a sharp signal at 10.09 ppm (s, 1H) is attributed to OH protons respectively. As multiplet, the aromatic ring protons are observed in the range 6.9-7.3 ppm (m, 4H). In addition to these signals, a sharp singlet at 3.45 ppm (s, 1H) is due to SH protons. The singlet observed at 2.48 ppm (s, 3H) and 2.85 ppm (s, 3H) are attributed to the coumarin methyl protons and the triplet observed at 2.70(t, 3H) are due to triazole methyl protons (Table-4).
| Schiff base | 1H NMR (d6-DMSO) (ppm) |
| I | 12.09 (s, 1H, NH), 10.01 (s, 1H, OH), 6.7-7.2 (m, 4H, Ar-H), 3.42 (s, 1H, SH), 2.46 (s, 3H, CH3), 2.83 (s, 3H, CH3). |
| II | 11.97 (s, 1H, NH), 10.09 (s, 1H, OH), 6.9-7.3 (m, 4H, Ar-H), 3.45 (s, 1H, SH), 2.48 (s, 3H, CH3), 2.85 (s, 3H, CH3), 2.70 (s, 3H, CH3 triazole). |
| III | 12.02 (s, 1H, NH), 10.10 (s, 1H, OH), 6.5-7.1 (m, 4H, Ar-H), 3.43 (s, 1H, SH), 2.49 (s, 3H, CH3), 2.81 (s, 3H, CH3), 2.10 (q, 2H, CH2), 2.72 (s, 3H, CH3 triazole). |
| IV | 12.96 (s, 1H, NH), 10.12 (s, 1H, OH), 6.6-7.0 (m, 4H, Ar-H), 3.41 (s, 1H, SH), 2.48 (s, 3H, CH3), 2.82 (s, 3H, CH3), 2.12 (t, 2H, CH2), 1.90 (m, 2H, CH2), 2.70 (t, 3H, CH3 triazole). |
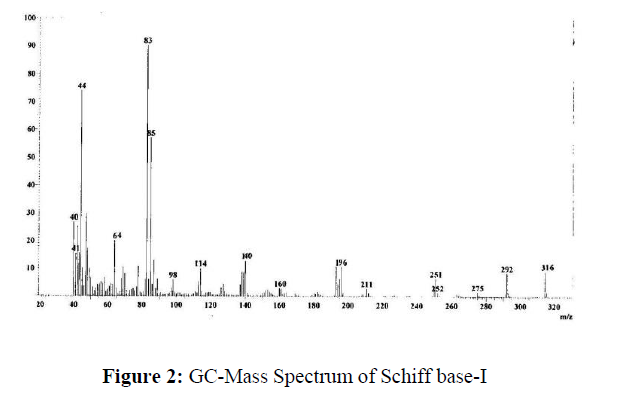
GC-Mass spectral studies of Schiff base
The GC-mass spectrum of the Schiff base I (Figure-2) shows a molecular ion (M+) peak at m/z 316 which is equivalent to its molecular weight, which confirms the proposed formula.
Electronic Absorption Spectral Studies
The electronic absorption spectra of the complexes in DMF were recorded at room. The cobalt(II) complexes exhibited two distinct absorption in the region 9880-9997 cm-1 and 17760-20660 cm-1 corresponding to 4T1g(F) → 4T2g(F) (ν1) and 4T1g (F) → 4T1g (P) (ν3) transitions respectively which suggests an high spin octahedral geometry around the cobalt(II) ion [27]. The ν2 band that involves a two-electron transition is not observed in spectra because of its proximity to strong ν3 transition.
The Ni(II) complex (6), exhibited three bands at 10215, 15620 and 26112 cm-1 attributed to the 3A2g → 3T2g (ν1); 3A2g → 3T1g (F) (ν2) and 3A2g → 3T1g (P) (ν3) transitions respectively, which indicate octahedral geometry around Ni(II) ion. The value of ν2/ ν1 is found to be around 1.529 and the μeff value is around 3.179 which is within the range of 2.8-3.5 BM, suggesting the octahedral environment. The values of the nephelauxetic parameters, β, indicate low covalent character of the metal-ligand σ bonds [28]. Hence the ligand field parameters correlate the electronic spectral and magnetic properties. The ligand field parameters calculated for the Co (II) complexes are given in Table-5.
| Complex No. |
Transitions (cm-1) | ν2 Cald. cm-1 |
Dq cm-1 | B1 cm-1 | % Distortion | ν1/ ν2 | LSFE | µeff Cald. BM |
β | βº % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ν1 | ν2 | ν3 | ||||||||||
| 5 6 7 8 |
10239 10215 10231 10226 |
15615 15620 15606 15631 |
26108 26112 26123 26110 |
16245.00 16225.78 16242.80 16234.79 |
1023.9 1021.5 1023.1 1022.6 |
775.76 779.51 778.18 777.78 |
3.881 3.733 3.921 3.719 |
1.525 1.529 1.525 1.529 |
35.105 35.023 35.078 35.061 |
3.178 3.179 3.179 3.179 |
0.735 0.738 0.737 0.737 |
26.537 26.182 26.308 26.346 |
The electronic spectra of Cu (II) complexes showed low intensity broad band around 14380-14423 cm-1 is assignable to 2T2g ← 2Eg transition. Another high intensity band at 25477-25513 cm-1 is due to symmetry forbidden ligand → metal charge transfer. On the basis of electronic spectra distorted octahedral geometry around Cu (II) ion is suggested [29]. On the basis of electronic spectra, distorted octahedral geometry around Cu(II) ion is suggested.
Magnetic Studies
The magnetic moments obtained at room temperature are listed in Table-1. The magnetic measurement for Co (II) complexes showed magnetic moment value 4.6-5.0 which is well within the range of 4.3-5.2 BM and Ni(II) complexes showed the magnetic moment value 3.0-3.3 within the range of 2.8-3.5 BM suggesting consistency with their octahedral environment [30, 31]. The Cu (II) complexes showed magnetic moment 1.74-1.79 BM, is slightly higher than the spin-only value 1.73 BM expected for one unpaired electron, which offers possibility of an octahedral geometry [32].
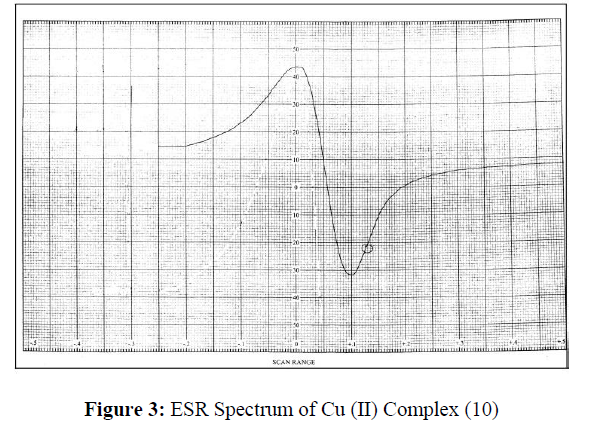
ESR Studies
The ESR spectral studies of Cu (II) complex provide information of the metal ion environment. The ESR spectrum of the Cu (II) complex was recorded in DMSO at room temperature (RT) and depicted in Figure-3. The Cu (II) complex (10) exhibits the g║ value of 2.0513 and g┴ value of 2.0248. These values g║>g┴ indicate that, the unpaired electron lies predominantly in the dx2 –y2 orbital [33]. The trend g║>g┴>2.0023 observed for the complexes indicate that, the unpaired electron is localized in the dx2-y2 orbital of the Cu(II) ion and are characteristic of the axial symmetry. Thus, the results suggested that, the Cu (II) complex (10) possess distorted octahedral geometry.
Thermal Studies
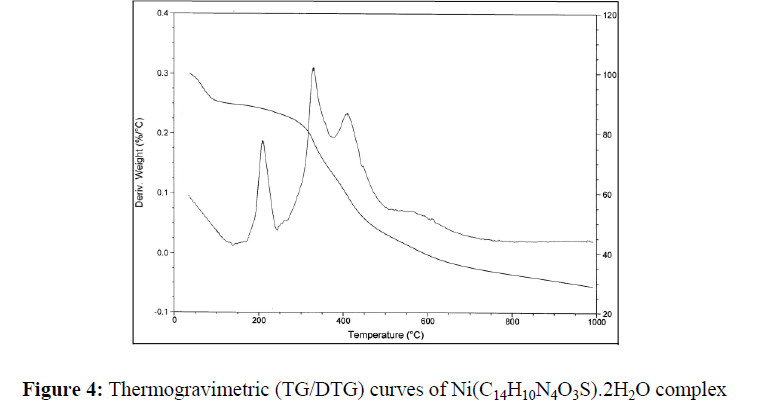
The thermal behavior of Co (II), Ni(II) and Cu(II) complexes has been studied as a function of temperature. The thermal behavior of all the complexes is almost same. Hence, only the representative TG/DTG of Co (II) (2), Ni (II) (6) and Cu (II) (10) complexes have been discussed here. Their stepwise thermal degradation data are given in Table-6.
| Empirical Formula | Decomposition temperature |
%Weight loss | Metal Oxide % | Inference | ||
| oC | Obsd. | Calcd. | Obsd. | Calcd. | ||
| Co(C14H10N4O3S).2H2O | 190-230 305-330 350-410 |
8.51 30.26 45.75 |
8.60 30.36 45.79 |
17.73 | 17.80 | Loss of coordinated water molecules Loss of 1,2,4-triazole moieties Loss of acetyl coumarin moieties |
| Ni(C14H10N4O3S).2H2O | 195-236 325-335 400–430 |
8.53 30.08 47.86 |
8.72 30.19 47.95 |
17.53 | 17.59 | Loss of coordinated water molecules Loss of 1,2,4-triazole moieties Loss of acetyl coumarin moieties |
| Cu(C14H10N4O3S).2H2O | 200-235 290-330 410–450 |
8.43 29.74 47.30 |
8.46 29.71 47.35 |
17.79 | 17.80 | Loss of coordinated water molecules Loss of 1,2,4-triazole moieties Loss of acetyl coumarin moieties |
The TG and DTG curves of Ni (C14H10N4O3S).2H2O are shown in Figure-4. The DTG curve of this complex shows three stages of decomposition within the temperature range (180-650ºC). The first step of decomposition within the temperature range (180-250ºC) corresponds to the loss of coordinated water molecule with mass loss of 8.53% (calcd. 8.72%). The second step (250-350ºC) corresponds to the loss of 1,2,4-triazole moieties with mass loss of 30.08% (calcd. 30.19%). The third step (400-560ºC) corresponds to the loss of acetylcoumarin (mass loss 47.86%; calcd. 47.95%). Finally the metal complexes decompose gradually with the formation of metal oxide above 560ºC (mass loss 17.53%).
Thus, the TG and DTG provide the useful information about the coordination of water molecules to the metal ion and the stability of the complexes.
Electrochemistry
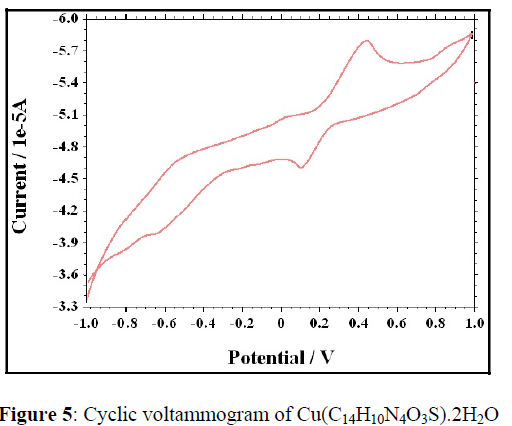
Electrochemical properties of the complexes were studied on a CHI1110A-Electrochemical analyzer in N,N-dimethyl formamide (DMF) containing 0.05 M n-Bu4NClO4 as the supporting electrolyte. A cyclic voltammogram of Cu (C14H10N4O3S).2H2O radical displays a reduction peak at Epc= 0.447V with a corresponding oxidation peak (Cu (I) radical) at Epa= 0.1. The peak separation of this couple (ΔEp) is 0.347V at 0.05V and increases with scan rate. The most significant feature of the Cu (II) complex is the Cu(II)/Cu(I) couple. The difference between forward and backward peak potentials can provide a rough evaluation of the degree of the reversibility of one electron transfer reaction. The analyses of cyclic voltametric responses with the scan rate varying 50 to 250 mV/s gives the evidence for quasi-reversible one electron oxidation state. The ratio of cathodic to anodic peak height was less than one. However, the peak current increases with the increase of the square root of the scan rates. This establishes the electrode process as diffusion controlled [33,34] (Figure 5,6).
Pharmacology Results
The Schiff bases and their metal complexes were evaluated for antimicrobial activity. The obtained results are systematized in Table 7 & 8. The antibacterial studies inferred that, Both the Schiff bases and their Co(II), Ni(II) and Cu(II) complexes showed high antibacterial and antifungal activity against all the bacterial strains. All the metal complexes possess higher antifungal activity than the Schiff bases. This higher activity of the metal complexes compared to Schiff bases is may be due to the change in structure due to coordination, which make the metal complexes act as more powerful and potent bactereostatic agents, thus inhibiting the growth of the microorganisms.
The minimum inhibitory concentration (MIC) of some selected compounds, which showed significant activity against selected bacterial and fungi strains were studied. The results indicated that these compounds were most active in inhibiting the growth of the tested organisms at 10 mgmL-1 (Table 9).
| Compd. | Conc .(μg ml-1) |
Antibacterial activity (Zone of inhibition in %) | Antifungal activity (Zone of inhibition in %) | |||||
| E. coli | S. aureus | B. subtilis | S. typhi | C. albicans | Cladosporium | A Niger | ||
| I | 25 50 100 |
37 47 55 |
- 20 31 |
39 41 53 |
29 49 58 |
52 58 67 |
- 33 44 |
52 67 69 |
| II | 25 50 100 |
38 52 59 |
- 24 30 |
37 47 55 |
31 47 52 |
53 58 66 |
- 31 42 |
57 62 69 |
| III | 25 50 100 |
33 58 60 |
- 23 33 |
34 57 59 |
33 46 59 |
55 65 68 |
- 39 41 |
55 66 68 |
| IV | 25 50 100 |
37 44 58 |
- 22 31 |
33 46 57 |
31 43 58 |
53 61 63 |
- 37 47 |
66 67 67 |
| Gentamycine | 25 50 100 |
81 85 86 |
62 73 75 |
77 79 81 |
73 78 80 |
- | - | - |
| Flucanazole | 25 50 100 |
- | - | - | - | 85 93 95 |
78 83 88 |
88 89 89 |
| Compd | Conc. (μg ml-1) |
Antibacterial activity (Zone of inhibition in %) | Antifungal activity (Zone of inhibition in %) | |||||
| E. coli | S. aureus | B. subtilis | S. typhi | C. albicans | Cladosporium | A Niger | ||
| 1 | 25 50 100 |
58 67 77 |
44 63 65 |
47 50 61 |
58 66 72 |
57 61 69 |
66 66 69 |
62 64 74 |
| 2 | 25 50 100 |
62 69 80 |
41 62 67 |
52 57 60 |
63 67 74 |
53 66 70 |
50 52 57 |
69 71 71 |
| 3 | 25 50 100 |
72 77 81 |
43 66 69 |
63 69 72 |
62 68 70 |
71 78 82 |
77 78 80 |
65 67 71 |
| 4 | 25 50 100 |
68 78 82 |
40 62 65 |
64 67 74 |
69 72 78 |
47 62 68 |
67 71 78 |
67 69 71 |
| 5 | 25 50 100 |
64 71 89 |
44 64 68 |
35 46 51 |
45 56 59 |
34 45 67 |
- 35 51 |
57 69 75 |
| 6 | 25 50 100 |
67 71 80 |
46 61 68 |
66 68 71 |
66 69 71 |
66 71 78 |
58 60 65 |
60 73 79 |
| 7 | 25 50 100 |
71 80 82 |
39 68 65 |
64 71 75 |
46 52 53 |
63 71 76 |
66 71 78 |
55 69 71 |
| 8 | 25 50 100 |
74 70 79 |
45 62 69 |
35 44 59 |
44 52 59 |
59 66 69 |
66 62 67 |
57 68 74 |
| 9 | 25 50 100 |
68 77 79 |
41 63 69 |
39 48 57 |
- 36 52 |
55 67 78 |
45 55 58 |
69 73 78 |
| 10 | 25 50 100 |
64 66 73 |
47 60 65 |
65 69 71 |
66 71 77 |
71 77 79 |
47 59 61 |
60 69 71 |
| 11 | 25 50 100 |
72 77 81 |
47 63 71 |
61 65 69 |
62 66 68 |
68 69 73 |
48 58 51 |
56 69 71 |
| 12 | 25 50 100 |
67 71 75 |
49 65 70 |
65 68 73 |
47 52 55 |
45 56 68 |
35 41 57 |
65 76 79 |
| Gentamycine | 25 50 100 |
81 85 86 |
62 73 75 |
77 79 81 |
73 78 80 |
- | - | - |
| Flucanazole | 25 50 100 |
- | - | - | - | 85 93 95 |
78 83 88 |
88 89 89 |
| Compound | E. coli | S. aureus | B. subtilis | S. typhi | C. albicans | Cladosporium | A Niger |
| I | 15 | - | 10 | 25 | 10 | 10 | - |
| II | 25 | 10 | 10 | - | - | 25 | 25 |
| III | 10 | - | 10 | - | 25 | - | - |
| IV | 10 | 10 | 25 | 25 | 25 | 15 | 25 |
| 1 | 25 | 10 | 10 | 25 | - | 25 | - |
| 2 | 25 | 10 | - | 10 | 15 | 25 | - |
| 3 | 10 | - | 25 | 10 | 10 | - | 25 |
| 4 | 10 | 10 | 10 | 25 | 10 | 10 | 10 |
| 5 | 10 | 15 | 10 | 25 | - | 25 | 25 |
| 6 | - | 25 | 25 | 25 | - | - | 15 |
| 7 | 25 | - | - | 10 | 25 | - | - |
| 8 | - | - | - | 15 | 25 | 25 | 25 |
| 9 | 10 | 15 | 10 | 25 | - | 25 | 25 |
| 10 | 15 | 10 | 10 | 25 | - | - | - |
| 11 | 10 | 10 | 10 | 25 | - | 10 | 25 |
| 12 | 25 | 10 | 25 | 10 | 25 | 10 | 25 |
Conclusions
The Co(II), Ni(II) and Cu(II) complexes of Schiff bases with 3-substituted-4-amino-5-mercapto-1,2,4-triazole and 8-acetyl-7-hydroxy-4-methylcoumarin were prepared and characterized using different analytical techniques. The synthesized Schiff bases act as tetradentate Schiff bases. The metals are coordinated to azomethine nitrogen, lactonyl oxygen, Phenolic oxygen and sulphur atom. Suggest that, the complexes were polymeric in nature. The electronic spectral data and magnetic measurements suggest that Co (II) and Ni(II) complexes are Octahedral, while Cu(II) complex has distorted octahedral geometry. ESR spectrum of Cu (II) complex also reveals that it is distorted. TG/DTG analysis indicates the presence of coordinated water molecule in the complexes. The antimicrobial studies reveal that the complexes show higher activity than the Schiff bases.
Acknowledgement
The authors are grateful to the Research Center in Chemistry, Affiliated to Rani Channamma University, Belagavi, Principal, Department of Chemistry, SB Arts and KCP Science College and Management BLDE Associon’s, Vijaypura-586103 for the facilities.
References
- Novak I and Kovac B. Electron Spectrosc Relat Phenom. 2000, 113: p. 9-13.
- Gao F, Li HR and Zang YY. Dyes Pigments. 2000, 47: p. 231.
- Bagihalli GB, Avaji PG, Patil SA et al., Eur J Med Chem. 2008, 43: p. 2639.
- Kostova, Curr Med Chem Anticancer Agents. 2005, 5: p. 29-46
- Vidyavati R, Nirodish P, Tukaram R et al., J of Chem. 2008, 5(3): p. 529.
- Chohan ZH and Praveen M. Appl Organomet Chem. 2001, 15(7): p. 617.
- Cowan JA. Curr Opin Chem Biol. 2001, 5(6):634.
- Singh H, Yadav LDS and Bhattacharya BK. J Indian Chem Soc. 1979, 56: p. 1013.
- Giri S, Singh H, Yadav LDS et al., J Indian Chem Soc. 1978, 55: p. 168.
- Rao S and Mittra AS. Indian J Chem Sect B. 1977, 15: p. 1062.
- Tanara G and Japan kokai. 1974, 973: p. 7495.
- Heng-Shan D, Bin Q, Kun W et al., Mag Res Chem. 2000, 38: p. 210.
- Ahluwalia VK, Bhagat P, Aggarwal R et al., Intermediates for organic synthesis. 2005.
- Sharan P, Giri S and Nizamuddin, J Indian Chem Soc. 1989, 66: p. 39330.
- Patil SA, Naik VH, Kulkarni AD. Sperctrochem Acta Part A. 2010, 75: p. 347.
- Sadana AK, Miraza Y, Aneja KR et al., Eur J Med Chem. 2003, 38: p. 533.
- Baraldi M, Malavasi W and Grandi R. J Chem Crystallogr. 1996, 26(1): p. 63.
- Panda S, Mishra R, Panda AK et al., J. Indian Chem Soc. 1989, 66: p. 472.
- Sengupta SK. Indian J Chem. 1981, 20A: p. 515.
- Sengupta SK, Sahni SK and Kapoor RN. Indian J Chem. 1981, 20A: p. 692.
- Goel S, Pandey OP and Sengupta SK. Thermochim Acta. 1988, 133: p.359.
- Tyaga Raju VJ, Vilas Ranbaore, Vasudha Atre et al., J Indian Chem Soc LIX. 1982, 199.
- Lewis FD and Barancyk SV. J Am Chem Soc. 1989, 111: p. 8653.
- Rai RA. J Inorg Nucl Chem. 1980, 42: p. 450-453.
- Agarwal RK, Goel N and Sharma AK. J Indian Chem Soc. 2001, 78: p.39.
- Rana VB, Jain PC, Swami MP et al., J Inorg Nucl Chem. 1975, 37: p.1826.
- Georgrieva I, Trendafilova N and Bauer G. Spectrochim Acta A. 2006, 63: p. 403.
- El-Sawaf KA, West XD, El-Saied AF et al., Trans Met Chem. 1998, 23: p. 649.
- Liu H, Wang H, Gao F et al., J Coord Chem. 2007, 60: p.2671.
- Hankare PP, Naravane SR, Bhuse VM et al., Indian J Chem. 2004, 43A: p.1464.
- Rao TR and Archan P. Synth React Inorg Met.-Org Chem. 2005, 35: p. 299.
- Singh DP, Kumar R, Malik V et al., Trans Met Chem. 2007, 32: p.1051.
- Dutta RL and Syamal A. In Elements of Magnetochemistry. 1992, p. 206.
- Bard AJ and Izatt LR. Electrochemical Methods: Fundamentals and Applications. 2001.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref