Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
Synthesis Characterization and Evaluation of Novel Substituted Azole Derivatives
Gejalakshmi S1*, Dhanalakshmi S2 and Harikrishnan N1
1Department of Pharmaceutical chemistry, Dr. M.G.R, Educational and Research Institute, Vellapanchavadi, Tamil Nadu, India
2Department of Pharmaceutical Analysis, Dr. M.G.R, Educational and Research Institute, Vellapanchavadi, Tamil Nadu, India
- *Corresponding Author:
- Gejalakshmi S
Department of Pharmaceutical chemistry
Dr. M.G.R, Educational and Research Institute
Vellapanchavadi, Tamil Nadu, India
Abstract
Benzimidazole is a very stable nucleus and the derivatives of 2-methyl benzimidazole may show different and better biological activity. The presence of the thiazolidinone ring in a benzimidazole derivative enhances its antimicrobial activity. 2-methyl benzimidazole derivative are formed by the condensation of o-phenylenediamine with acetic acid. Combination of two or more active moieties into one is a common procedure, can result in augmenting the activity and removes untoward side effects. Hence, the present work focuses on synthesis of potent antimicrobial benzimidazole derivatives by the condensation of Schiff's bases of 2-methyl benzimidazole moiety with thiomalic acid affording the thiazolidinone ring and to study their antimicrobial activity. In antibacterial activity (Staphylococcus aureus and Escherichia coli), it shows that compound TE6 is the most active compound and in antifungal activity (Candida albicans), TE3 & TE4 are found to be most active.
Keywords
2 methyl benzimidazole, Antifungal, Antibacterial, O-phenylenediamine.
Introduction
The practice of medicinal chemistry is devoted to the discovery and development of new agents for treating diseases. The process of establishing new drugs is exceedingly complex and involves the talents of people from a variety disciplines, including Chemistry, Biochemistry, Physiology, Pharmacology, Pharmaceutics and Medicine. Medicinal chemistry is concerned mainly with the organic, analytical and biologic chemical aspects of this process, but its people must interact productively with those in other disciplines. Thus it occupies a strategic position at the interface of chemistry and biology [1].
Medicinal chemistry is a science whose roots lie in all branches of chemistry and biology. It concerns essentially the understanding and explanation of the mechanism of actions of drugs. On this base it attempts to establish relationship between chemical structure and biological activity and link biodynamic behavior to the chemical reactivity and physical properties of therapeutic agents [1].
The present work deals with the characterization of some new Benzimidazoles (Figure 1) containing Thiazolidinone derivatives. Despite a numerous attempts to develop new structural prototype in the search for more effective antimicrobials, the benzimidazoles still remains as one of the most versatile class of compounds against microbes and therefore are useful substrate for further molecular exploration.
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and imidazole. The most prominent benzimidazole compound in nature is N-ribosyl-dimethylbenzimidazole, which serves as an axial ligand for cobalt in vitamin B12.
Benzimidazole, in an extension of the well-elaborated imidazole system, has been used as carbon skeletons for N-heterocyclic carbenes. The NHCs are usually used as ligands for transition metal complexes. They are often prepared by deprotonating an N, N’-disubstituted benzimidazolium salt at the 2-position with a base.
Benzimidazole is white coloured, slightly solid, slightly soluble in water and soluble in ethanol. It is a dicyclic compound having imidazole ring (containing two nitrogen atoms at nonadjacent positions) fused to benzene. Benzimidazole and its derivatives are used in organic synthesis and vermicides or fungicides as they inhibit the action of certain microorganisms. Examples of benzimidazole class fungicides include carbendazim, chlorfenazole, cypendazole, debacarb, fuberidazole, furophanate, mecarbinzid, rabenzazole, thiabendazole, thiophanate. Benzimidazole structure is the nucleus in some drugs such as proton pump inhibitors and anthelmintic agents.
IUPAC name : 1H-benzimidazole
Molecular formula : C7H6N2
Molar mass : 118.14 g mol-1
Melting point : 170-172°C
Boiling point : 360°C
Thiazolidinone [2]
The broad and potent activity of 4-thiazolidinones has established it as one of the biologically important scaffold. Thiazolidine is an important scaffold known to be associated with several biological activities. 4-Thiazolidinones are derivatives of thiazolidine with a carbonyl group at the 4-position. Substituents in the 2-, 3-, and 5-positions may be varied, but the greatest difference in structure and properties is exerted by the group attached to the carbon atom in the 2-position (R, R’ and X).
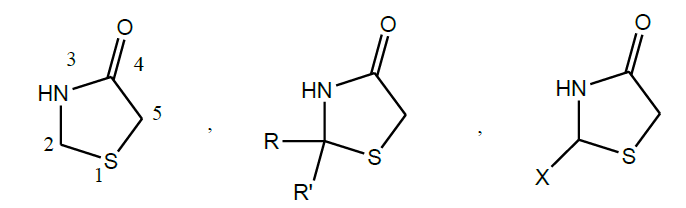
4-thiazolidinones are well known heterocyclic compounds for their spectrum of biological activities such as antibacterial, antifungal, antitubercular, anthelmintic, antithyroid, local anaesthetic, monoamine oxidase inhibition, Anticonvulsant activity, Hypnotic activity, Anticancer activity, Antihistaminic activity (H1-antagonist), Antiviral activity, Anti-inflammatory activity (COX-inhibitors), Follicle stimulating hormone (FSH) receptor agonist activity.
Materials and Methods
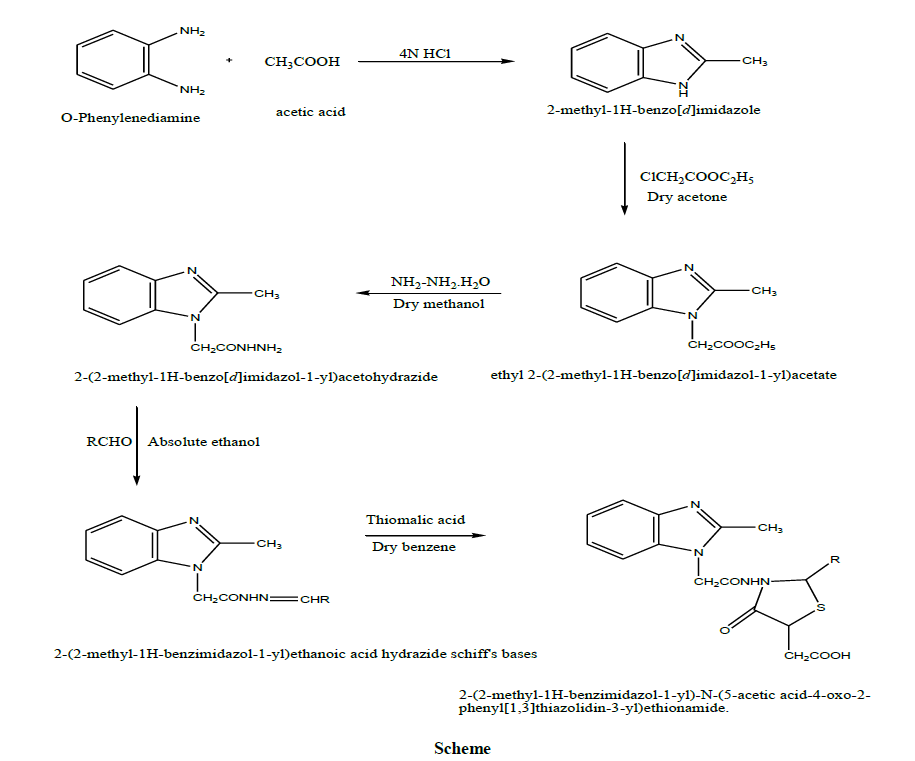
The above scheme represents the complete process of the synthesis of the compounds (Table 1).
| S. NO. | Code | IUPAC Name |
|---|---|---|
| 1. | TE1 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(4-methoxyphenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
| 2. | TE2 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(4-chlorophenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
| 3. | TE3 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(4-methylphenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
| 4. | TE4 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(2-chlorophenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
| 5. | TE5 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(4-hydroxyphenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
| 6. | TE6 | 2-(2-methyl-1H-benzimidazol-1-yl)-N-{5-aceticacid-4-oxo-2-(4-nitrophenyl) [1, 3] thiazolidin-3-yl}ethionamide. |
Table 1: IUPAC Names of the synthesized compounds TE1-TE6.
Procedure for synthesis of 2-methyl-1H-benzimidazole [MB] [3]
O-phenylenediamine (4 g, 0.04 mol) was condensed with acetic acid (1.7 ml, 0.03 mol) in 50 ml 4 N HCl. The reaction mixture was stirred for about 4 h. With magnetic stirrer at 80ºC. The compound was precipitated by adding concentrated ammonia solution, filtered and washed with Compound was recrystallized from water and ethanol. The percentage yield was 80% and melting point was found to be 192ºC-194ºC.
Procedure for synthesis of ethyl 2(2-methyl-1H-benzimidazol-1-yl) acetate [BA] [4]
Ethylchloroacetate (1.06 ml, 0.01 mol) was added to a solution of 2-methyl-1H-benzimidazole (1.32 g, 0.01 mol) in dry acetone (20 ml). To that mixture, anhydrous K2CO3 was added and the reaction mixture was refluxed for 1 h. Acetone was removed after completion of reaction and the residue crystallized from ethanol. The percentage yield was 85% and melting point was found to be 186ºC-188ºC.
Procedure for synthesis of 2-(2-methyl-1H-benzimidazol-1 yl) acetohydrazide [4]
To a solution of ethyl 2-(2-methyl-1H-benzimidazole-2-yl) acetate (2.18 g, 0.01 mol) dissolved in dry methanol (50 ml), 99% hydrazine hydrate (1 ml) was added and the mixture was refluxed for 4-5 h. The reaction mixture was cooled and the solid obtained was filtered, washed with small quantity of cold methanol. The percentage yield was 37% and melting point was found to be 248ºC-250ºC.
Procedure for synthesis of 2-(2-methyl-1H-benzimidazol-1-yl) ethanoic acid hydrazide schiff’s bases [5]
A mixture of 2-(2-methyl-1H-benzimidazol-1-yl) acetohydrazide (2.04 g, 0.01 mol) and the appropriate aromatic aldehyde, namely o-chlorobenzaldehyde, p-chlorobenzaldehyde, p-nitrobenzaldehyde, p-hydroxybenzaldehyde, anisaldehyde, p-methyl benzaldehyde (0.012 mol), was refluxed in absolute ethanol (20 ml) for 5 h. The reaction mixture was concentrated, cooled and the formed precipitate was filtered off, dried and then recrystallized from ethanol and water. The percentage yield and melting points are given in Table 2.
| Compound name | R | MPºC | Yield% | Molecular formula | Spectral data | ||
|---|---|---|---|---|---|---|---|
| IR KBr (g,cm-1) | 1H-NMR (CDCl3,d,ppm) | Mass spectra (m/z) | |||||
| TE1 | - OCH3C6H4 | 160-162 | 87 | C22H22N4O5 | 3423.08 N-H, O-H(stretching) 2926.05, 2850.67 Aliphatic O-H stretching 1739.73 C=O stretching (Thiazolidinone) 1603.14 Bending (Amide) 1460.21 CH3 Bending | 7.520 Aromatic C-H (4H) 6.096 C-2 proton of thiazolidinone N-CH2 (2H) proton | 357 |
| TE2 | - ClC6H4 | 223-225 | 92 | C21H19N4O4Cl | 3403.46 N-H stretching and O-H stretching Aliphatic O-H stretching 1716 1636.42 Bending (Amide) | 7.585 Aromatic C-H (4H) of phenyl group 7.082 Aromatic C-H | 358 |
| TE3 | - CH3C6H4 | 193-195 | 76 | C22H22N4O4 | C=O stretching (Thiazolidinone) 2.915 CH2 of CH2COOH | 6.045 C-2 proton of thiazolidinone 4.345 N-CH2 (2H) proton3.185 C-5 proton of thiazolidinone | |
| TE4 | - ClC6H4 | 172-174 | 89 | C21H19N4O4Cl | 1636.52 Bending (Amide) | 7.532 Aromatic C-H (4H) 7.089 Aromatic C-H6.039 C-2 proton of thiazolidinone 4.3514.351 N-CH2 (2H) proton | |
| TE5 | - OHC6H4 | 205-207 | 67 | C21H20N4O5 | 1383.14 CH3 Bending (alkane) | 4.332 C-5 proton of thiazolidinone 2.905 CH2 of CH2COOH2.2653H (S) CH3 OF C2 of benzimidazole N-CH2 (2H) proton | |
| TE6 | - NO2 C6H4 | 212-215 | 73 | C21H19N5O6 | 3403.98 N-H stretching and O-H stretching 2925.89, 2852.16 Aliphatic O-H stretching 1708.42 C=O stretching (Thiazolidinone) 1618.13 Bending (Amide) | 6.053C-2 proton of thiazolidinone 4.352 N-CH2 (2H) proton | |
Table 2: Yield % and melting points of the synthesized compounds TE1-TE6
Procedure for synthesis of 2-(2-methyl-1H-benzimidazol-1-yl)-N-(5-acetic acid-4-oxo-2-phenyl [1, 3] thiazolidin-3-yl) ethionamide [TE] [5]
A mixture of 2-(2-methyl-1-H-benzimidazole-1-yl) ethanoic acid hydrazide Schiff’s base (0.005 mol) and thiomalic acid (0.9 g, 0.006 mol) was refluxed in dry benzene (50 ml) for 6 h. The solvent was evaporated and the reaction mixture was neutralized with cold dilute sodium bicarbonate solution. The formed product was filtered off and recrystallized from ethanol and water [6-8].
Microbiological screening
The antimicrobial activity of the various synthesized compounds TE1, TE2, TE3, TE4, TE5, and TE6 were studied in the following manner (Table 3).
| S. No. | Compound | Zone of inhibition against | |
|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | ||
| 1 | TE1 | 13 mm | 15 mm |
| 2 | TE2 | 15 mm | 14 mm |
| 3 | TE3 | 17 mm | 19 mm |
| 4 | TE4 | 13 mm | 18 mm |
| 5 | TE5 | 14 mm | 14 mm |
| 6 | TE6 | 16 mm | 11 mm |
| 7 | Amikacin (std) | 16 mm | 17 mm |
| 8 | DMSO (control) | Resistant | Resistant |
Table 3: Antimicrobial activity of the various synthesized compounds TE1- TE6
Antibacterial activity [9]
Organism used: Staphylococcus aureus (MTCC 96), Escherchia. Coli (MTCC 722)
Standard used: Amikacin (30 μg/disc)
Control: Dimethyl sulfoxide (DMSO)
Test Concentration: 50 μg/ml
Incubation temperature: 37°C
Incubation time: 18 h.
Method: Filter paper disc method
Principle: Gel diffusion
Composition of media [10]
Muller-Hinton agar media
Beef extract: 30 g
Casein hydrolysate: 17.5 g
Soluble starch: 1.5 g
Agar: 20 g
Sodium hydroxide: 5 g
Distilled water: 1000 ml
Final pH at 25°C: 7.4 ± 0.2
Muller Hinton agar media of 100 ml was prepared as per the composition and sterilized in the autoclave at 15 Lbs/in2 for 20 min. When the media was in warm molten state, 15 ml of the medium was transferred (using sterile boiling tube marked already for measuring) into the sterile petri plates in a laminar air flow bench aseptically. The medium was allowed to solidify and incubated at 37ºC to check free from contamination.
Assay was carried out by Filter paper disc method. The method followed was spread plate technique. The agar plates free from contamination were spreaded with 50 μl of 48 h old culture of bacterial test organism using sterile buds. The standard disc of Amikacin (sterile) of 8 mm diameter was placed on the petri plates. Then the discs (sterile) of 8 mm were soaked in 1 ml of test solution and in solvent DMSO. After evaporating the solvent in a sterile atmosphere, the drug impregnated discs were placed in petriplates. After 15 min the plates were incubated at 37ºC for 18 h in an inverted position. The clear zones of inhibition were measured using Hi media zone reader scale. The zones of test solutions were compared with standard Amikacin.
Antibacterial activity
Organism Used: Candida albicans (MTCC 227)
Standard Used: Ketoconazole (30 μg/disc)
Control used: DMSO
Test concentration: 50 μg/ml
Incubation temperature: 28°C
Incubation time: 24 h
Method: Filter paper disc method
Principle: Gel diffusion
Composition of media
Saboured dextrose agar media
Glucose: 40 g
Peptone: 10 g
Agar: 15 g
Distilled water: 1000 ml
Final PH: 5.4
Protocol
Assay was carried out by diffusion plate method. The method followed was spread plate technique. The agar plates free from contamination were spread with 50 μl of 48 h old culture of fungal test organism using sterile buds. The standard disc of Ketoconazole (sterile) of 8 mm diameter was in the petriplates. Then the discs (sterile) of 5 mm were soaked in 1 ml (1 mg/ml) of the test solution and in solvent control DMSO. After evaporating the solvent in a sterile atmosphere, the drug impregnated discs were placed in petriplates. After 15 min the plates were incubated for 24 h at 28oC in an inverted position. The clear zones of inhibition were measured using Hi media zone reader scale. The zones of test solutions were compared with standard Ketoconazole.
Antifungal activity
| S. No. | Compound | Zone of inhibition against Candida albicans |
|---|---|---|
| 1 | TE1 | 14 mm |
| 2 | TE2 | 12 mm |
| 3 | TE3 | 11 mm |
| 4 | TE4 | 11 mm |
| 5 | TE5 | 14 mm |
| 6 | TE6 | 17 mm |
| 7 | Ketoconazole (std) | Resistant |
| 8 | DMSO (control) | Resistant |
Results and Discussion
The KBr Spectrum of all synthesized compounds
The synthesized compounds show a band at 3400-3425 cm-1 indicates the presence of N-H stretching and O-H stretching. The band at 2926 cm-1 and 2850 cm-1 confirms the presence of aliphatic C-H stretching in the synthesized compounds. The band at 1739-1625 cm-1 is due to the C=O stretching of thiazolidinone ring. The presence of amide group in the synthesized compounds is confirmed by the band at 1603 cm-1. The band at 1460-1382 cm-1 is due to the presence of alkane CH3 bending in the synthesized compounds. From this data we confirmed the functional groups of the synthesized compounds and extra bond at 777 cm-1 in the compound TE6 and TE4 confirms the C-Cl stretching. The compounds TE6 contain a band at the 1522 cm-1 which indicates the presence of aromatic C-NO2 stretching.
NMR Spectrum of all synthesized compounds
The 300 MHZ 1H-NMR spectrum shows a signal at δ 7.5 ppm assigned for the aromatic protons of the phenyl substitution. A signal at δ 7 ppm (m) indicates the aromatic C-H protons of the benzimidazole ring. A signal at the δ 6 ppm (S) assigned for the 1H proton at C-2 position of thiazolidine ring. A singlet signal at δ 4.3 ppm assigned to the 2H proton of N-CH2A signal at 3.7 ppm is assigned for the 1H proton of the C-5 position of thiazolidinone ring. A signal at 2.9 ppm is assigned for the 2H proton of the CH2COOH of the compounds. A signal at 2.55 assigned for the 3H protons of the methyl substitution C-2 position of benzimidazole.
Antimicrobial activity
All the synthesized compounds were screened for the antimicrobial activity. In antibacterial activity (S. aureus and E. coli), it shows that compound TE3 is the most active compound and in antifungal activity (C. albicans), TE6 is found to be most active. In the antibacterial activity of the synthesized compounds TE1, TE2, TE3, TE4, TE5, and TE6, E. coli shows comparatively good sensitivity to the compound TE6 as that of standard used. S. aureus shows comparatively good sensitivity to the compound TE1 andTE5 as that of standard used. In the antifungal activity of the synthesized compounds TE1, TE2, TE3, TE4, TE5, and TE6, C. albicans shows comparatively good sensitivity to the compound TE3 and TE4 as that of standard used. The compound shows significant activity than the standard used.
Conclusion
The approach of the present study was to synthesize2-(2-methyl-1H-benzimidazol-1-yl)-N-(5-acetic acid-4-oxo-2-phenyl (substituted) [1, 3] thiazolidin-3-yl) ethionamideand hence forth evaluate the antibacterial and antifungal activity. It was established that introduction of thiazolidinone nucleus in the 2-methyl benzimidazole enhanced the antimicrobial activity. We conclude that there is vast scope for 2-methyl benzimidazole as an effective antimicrobial agent. From the Rf, melting point, solubility, IR, NMR, and mass pattern were given the evidence for the proposed structure of the synthesized novel derivatives of benzimidazole. In antibacterial activity (S. aureus and E. coli), it shows that compound TE6 is the most active compound and in antifungal activity (C. albicans), TE3 & TE4 are found to be most active. In the antibacterial activity of the synthesized compounds TE1, TE2, TE3, TE4, TE5, and TE6, E. coli shows comparatively good sensitivity to the compound TE6 as that of standard used. S. aureus shows comparatively good sensitivity to the compound TE1 andTE5 as that of standard used. In the antifungal activity of the synthesized compounds TE1, TE2, TE3, TE4, TE5, and TE6, C. albicans shows comparatively good sensitivity to the compound TE3 and TE4 as that of standard used.
References
- D.J. Abraham, Chemotherapeutic agents as sulfonamide drug-Burger Medicinal Chemistry Drug Discovery (chemotherapeutic agents), A John Wiley and sons, Inc 6th publication, 2001, 5, 557-566.
- V. Amit, K.S. Shailendra, Eur. J. Med. Chem., 2008, 43, 897-905.
- T.A. Kumar, M. Anil, Ind. J. Chem., 2006, 45B, 489-493.
- K.F. Ansari, C. Lal, Eur. J. Med. Chem., 2009, 44, 4028-4033.
- H.EI-masry Afaf, H.H. Fahmy, S.H. Abdelwahed Ali, Molecules., 2000, 5, 1429-1438.
- R.M. Silverstein, B. Morril, C. Terence, Interpretation of Infrared spectra, Nuclear Magnetic Resonance Spectroscopy, Spcetromagnetic identification oforganic compounds, John willey and sons.Inc, 5th (Edn.), 1991, 100-131, 202-225.
- Y.R.Sharma, Application of infrared spectroscopy, Elementary organicspectroscopy, principle and chemical application, S.Chand and company Ltd., 2005, 90-135.
- D.A. Skoog, F.J. Holker, A. Timothy, Nieman, Interpretation of Proton Nuclear Magnetic Resonance Spectroscopy, Principle of Instrumental Analysis, Thomson Brooks, 5th (Edn.), 2005, 457-476.
- A. Mori, C. Nishino, Phytochemistry., 1987, 26, 2231-2234.
- R.C. Dubey, Culture media-A text book of biotechnology, S. Chand, Publication, 1st (Edn.), 1993, 224-228.
- A. Chakravarti, A. Ghosh, A. Kanta, P. Kumar, Ind. J. Med. Res., 1995, 102, 13-19.
- S. Saisivam, V.B. Kishan, Ind. J. Microbiol., 2006, 46, 197.