Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Synthesis, Characterization and Antioxidant Activity of Novel 2-Aryl Benzothiazole Derivatives
Amrita Parle1 and Shaista Amin2*
1Department of Pharmaceutical Chemistry, Delhi Institute of Pharmaceutical Science and Research, New Delhi- 110017, India
2Centre for Medicinal Chemistry, Delhi Pharmaceutical Sciences and Research University, New Delhi-110017, India
- Corresponding Author:
- Shaista Amin
Centre for Medicinal Chemistry
Delhi Pharmaceutical Sciences and Research University, New Delhi-110017, India
Abstract
In the present study, a series of benzothiazole derivatives were synthesized. Reactions were monitored using thin layer chromatography technique and the newly synthesized derivatives were characterized by ATIR and Proton Nuclear Magnetic Resonance (1H-NMR) techniques. Antioxidant assay was performed using 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) method and 2,2-Diphenyl-1- Picrylhydrazyl (DPPH) method. The antioxidant activity was found to be better in ABTS assay than DPPH assay. It was found that the synthesized benzothiazole derivatives showed significant radical scavenging potential.
Keywords
Antioxidant, Benzothiazoles, 2-Chloronitrobenzene, Polyphosphoric acid
Introduction
Free radicals play an important role in cell's life and death. These are unstable/unpaired electrons in their outermost shell and may become highly reactive. Reactive Oxygen Species (ROS) are generated from molecular oxygen/nitrogen through Electron Transport Chain (ETC), cytochrome P450 and other cellular, sub-cellular functions. They affect beneficial metabolic and cellular processes adversely and play key role in development of pathological conditions of the body. In healthy individuals, it is normally balanced by endogenous antioxidant system. If the endogenous antioxidants fail to overcome the reactive metabolites production, then exogenous antioxidants would be necessary to balance redox status. Antioxidants are the molecules that help to protect cells from oxidative stress [1]. Antioxidants are either present naturally in various types of food (Fruits and vegetables) or taken as dietary supplements. They play defensive role against oxygen free radical toxicity in our body [2]. Thus antioxidants are considered as scavengers of free radicals.
Free radicals are constantly being generated in the body through various mechanisms, and are also being removed by endogenous antioxidant defensive mechanisms that act either by scavenging free radicals, decomposing peroxides, and/or binding with pro-oxidant metal ions. Free radicals lead to cell damage and homeostatic disruption causing diseases including diabetes, cirrhosis, cancer and cardiovascular diseases [3,4]. Imbalance between antioxidant defenses and free radical production generates oxidative stress which is responsible to damage essential biological entities like nucleic acids, lipids, proteins, producing excess ROS [5]. Thus balance between the pro-oxidant and antioxidant is very important for the cell survival. All antioxidants generally influence the redox status, thereby protecting cells against Reactive Oxygen Species (ROS).
The cell damage through free radical mediated reactions is usually protected by enzymatic and non-enzymatic defense mechanisms of the body. Enzymes like catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase directly/indirectly contributes to defense against the generated ROS. The non-enzymatic antioxidants like glutathione, vitamin E and C, uric acid, albumin, bilirubin, N-Acetyl cysteine and melatonin are actually the scavengers of ROS and RNS [6].
Classification of antioxidants
Antioxidants are classified in two ways
1. The first way of classification is described by Gutteridge and Halliwell [7].
• Primary antioxidants: Involved in the prevention of oxidant formation
• Secondary antioxidants: Are the scavengers of ROS.
• Tertiary antioxidants: Repair the oxidized molecules through sources like dietary or consecutive antioxidants.
2. The second way of classification is:
• Exogenous antioxidants (dietary sources) and
• Endogenous antioxidants.
Endogenous antioxidants
It can be categorized into primary antioxidants and secondary antioxidants. Super Oxide Dismutase (SOD), catalase and glutathione peroxidase are the primary antioxidant enzymes which inactivate the ROS into intermediates [8]. Secondary antioxidant enzymes are glutathione reductase, glucose-6-phosphate dehydrogenase, glutathione-S-transferase and ubiquinone. They work directly to detoxify ROS by decreasing the peroxides level and continuously supplying the Nicotinamide Adenine Dinucleotide Phosphate (NADPH) and glutathione for primary antioxidant enzymes to maintain their proper functioning. Copper, iron, manganese, zinc, selenium enhance the activity of antioxidant enzymes [9,10].
Exogenous antioxidants
These are mainly derived from food and other dietary sources. Several herbs, spices, vitamins, foods, vegetables, etc. exhibit antioxidant activities. Various phytochemicals like flavonoids, isoflavones, flavones, anthocyanins, coumarins, lignans, catechins, isocatechins, epicatechin, etc. found in natural foods also show antioxidant properties.
Materials and Methods
Experimental
All chemicals and solvents were supplied by Sigma Aldrich, Merck, and CDH under certificate of purity. The melting range of the synthesized compounds was measured by Scientech-2211 digital auto melting/boiling point apparatus. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded on Bruker 400 MHz NMR spectrometer using Deuterated Chloroform (CDCl3) as solvent. Chemical shifts were reported in Parts Per Million (PPM) relative to internal standard Tetramethylsilane (TMS). IR spectra were recorded on Bruker- Alpha 1005151/06 ATIR spectrophotometer.
Reaction progress was checked by TLC using Merck Silica gel 60 F-254 coated glass plates. The solvent system used was n-Hexane: Ethyl acetate in the ratio of 2:3.
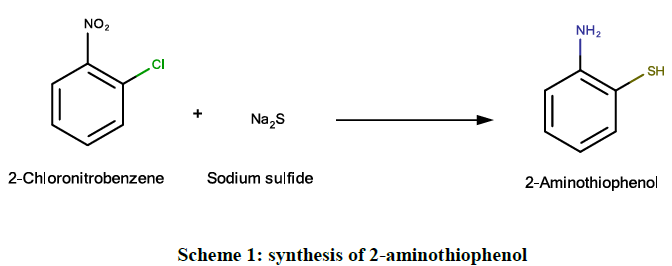
Step I: For synthesis of 2-aminothiophenol
A clear solution of sodium sulphide non-anhydrate (4.8 g, 0.02 M) in water (20 ml) was prepared. 2-chloronitrobenzene (1.28 g, 0.008 M) was added to it in one single portion and the mixture was refluxed for 8 hrs. After 4 h, small amount of yellow colored oil appeared in the reaction mixture due to the formation of 2-chloroaniline as the by-product. The reaction mixture was cooled after 8 h and then extracted with ether to remove 2-chloroaniline. The aqueous layer containing sodium salt of 2-aminothiophenol was saturated with sodium chloride and then acidified with glacial acetic acid. Addition of acetic acid should be done carefully to get the maximum yield of 2-aminothiophenol (Scheme 1).
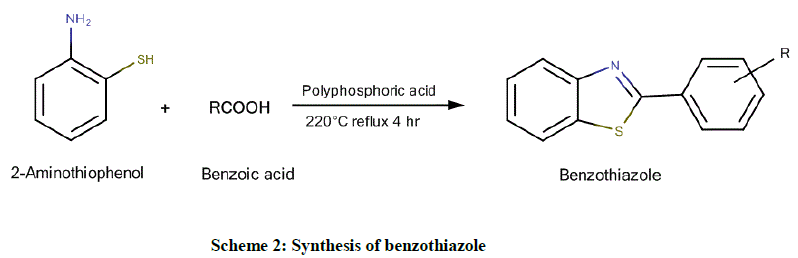
Step II: For synthesis of benzothiazoles
Using 2-aminothiophenol and benzoic acid (Scheme 2): Equimolar quantities of 2-aminothiophenol and substituted benzoic acid were added to 15 g of polyphosphoric acid and refluxed for 4 h at 220°C. The reaction mixture was cooled and poured into a large volume of rapidly stirred ice cold water. The slurry was made alkaline with 50% sodium hydroxide solution. The progress of the reaction was monitored by TLC, using n- Hexane: Ethyl acetate in the ratio of 2:3 as the mobile phase. During the basification, ice was added to prevent an excessive rise in temperature. The crude product was obtained by extracting the reaction mixture with toluene and subsequent evaporation of the solvent in rotary vacuum evaporator followed by recrystallization from ethanol.
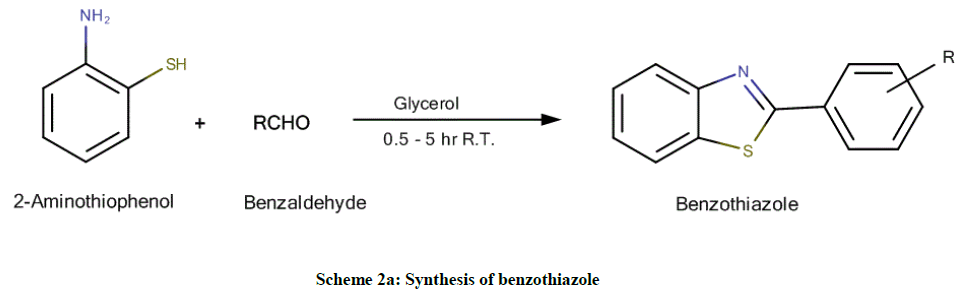
Using 2-aminothiophenol and benzaldehyde (Scheme 2a): Equimolar quantities of 2-aminothiophenol (1.25 g, 10 mmol) and the appropriate aldehyde (10 mmol) in glycerol (10 ml) were heated until a clear solution was obtained and then left at room temperature for 0.5-5 h (TLC control). The reaction mixture was quenched with water and the resulting solid product was collected by filtration, dried and recrystallized from ethanol to afford final compounds (Tables 1 and 2).
| Name | Structure | IUPAC name |
|---|---|---|
| BTA-1 | 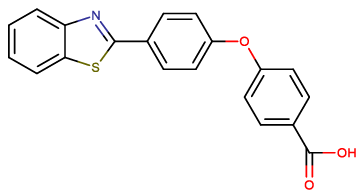 |
4-[4-(1,3-benzothiazol-2-yl) phenoxy] benzoic acid |
| BTA-2 | 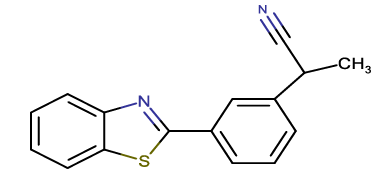 |
2-[3-(1,3-benzothiazol-2-yl) phenyl] propanenitrile |
| BTA-3 | 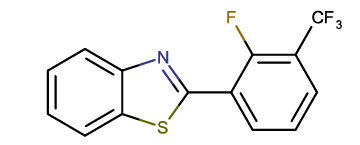 |
2-[2-fluoro-3-(trifluoromethyl) phenyl]-1,3-benzothiazole |
| BTA-4 | 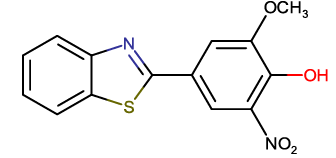 |
4-(benzothiazol-2-yl)-2-methoxy-6-nitrophenol |
| BTA-5 | 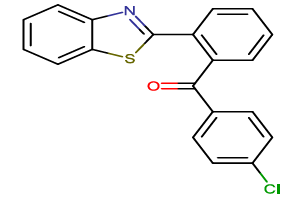 |
2-[2-(4-chlorobenzoyl) phenyl]-1,3-benzothiazole |
| BTA-6 | 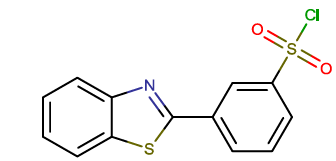 |
3-(1,3-benzothiazol-2-yl)benzene-1-sulfonyl chloride |
| BTA-7 | 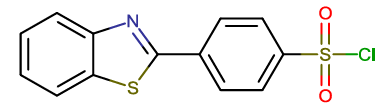 |
4-(1,3-benzothiazol-2-yl)benzene-1-sulfonyl chloride |
| BTA-8 | 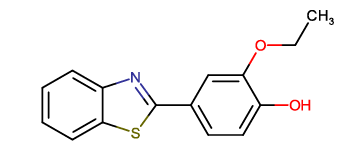 |
4-(1,3-benzothiazol-2-yl)-2-ethoxyphenol |
| BTA-9 | 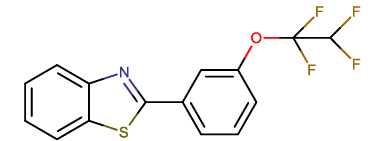 |
2-[3-(1,1,2,2-tetrafluoroethoxy) phenyl]-1,3-benzothiazole |
| BTA-10 | 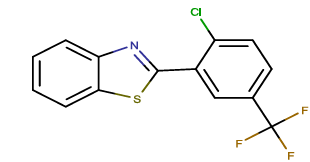 |
2-[2-chloro-5-(trifluoromethyl) phenyl]-1,3-benzothiazole |
| BTA-11 | 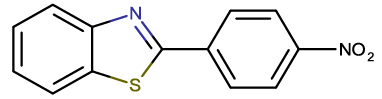 |
2-(4-nitrophenyl) benzothiazole |
| BTA-12 | 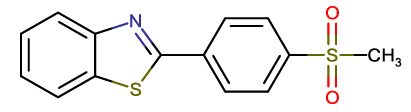 |
2-(4-methanesulfonyl phenyl)-1,3-benzothiazole |
Table 1: List of synthesized compounds
| Name | Molecular formula | Molecular weight | Melting Point (˚C) | Yield (%) | Solubility |
|---|---|---|---|---|---|
| BTA-1 | C20H13NO3S | 347.39 | 121-123 | 62 | Chloroform, DMSO, ethanol, methanol |
| BTA-2 | C16H12N2S | 264.34 | 114-120 | 71 | Chloroform, DMSO, ethanol |
| BTA-3 | C14H7F4NS | 298 | 115-120 | 74 | Chloroform, DMSO, ethanol, methanol |
| BTA-4 | C14H10N2O4S | 303.31 | 118-121 | 56 | Chloroform, DMSO, ethanol, methanol |
| BTA-5 | C20H12ClNOS | 349.83 | 115-117 | 82 | Chloroform, DMSO, ethanol, methanol |
| BTA-6 | C13H18ClNO2S2 | 309.79 | 116-121 | 65 | Chloroform, DMSO, ethanol |
| BTA-7 | C13H18ClNO2S2 | 309.79 | 117-119 | 83 | Chloroform, DMSO, ethanol, methanol |
| BTA-8 | C15H13NO2S | 271.22 | 125-127 | 70 | Chloroform, DMSO, ethanol, methanol |
| BTA-9 | C15H8F4NOS | 327.3 | 112-116 | 56 | Chloroform, DMSO, ethanol, methanol |
| BTA-10 | C14H7ClF3NS | 313.73 | 115-119 | 70 | Chloroform, DMSO, ethanol, methanol |
| BTA-11 | C13H8N2O2S | 256.28 | 116-118 | 54 | Chloroform, DMSO, ethanol, methanol |
| BTA-12 | C14H11NO2S2 | 289.37 | 120-125 | 69 | Chloroform, DMSO, ethanol, methanol |
Table 2: Physical data of synthesized compounds
Antioxidant activity
The antioxidant assay was performed by the method of Mensor et al. [12,13]
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) assay
The DPPH radical is one of the few stable organic nitrogen radicals, which bears a deep purple color. This free radical, stable at room temperature, is reduced in the presence of an antioxidant molecule. It is commercially available. Because of a strong absorption band centered at about 520 nm, the DPPH radical has a deep violet color in solution, and it becomes colorless or pale yellow when reduced. The ability can be evaluated by Electron Spin Resonance (EPR) or by measuring the decrease in its absorbance. Antioxidant assays are based on the loss of the DPPH color at 517 nm after reaction with the test compounds; the reaction is monitored by UV-Visible spectrophotometer.
Procedure: 0.01 m solution of DPPH was prepared in methanol. Methanolic solutions of all the compounds in the following concentration ranges were prepared (40, 60, 80 and 100 μg/ml). Similarly solutions of ascorbic acid in the same concentration range were prepared. 1 ml of DPPH solution was added to 1 ml of sample solution and to ascorbic acid as well. The volume was finally made up to 3 ml using methanol. The test tubes containing the assay mixture were kept at a dark and cool place for 30 min. Immediately after incubation the absorbance of the DPPH solution, blank (methanol), samples and ascorbic acid containing DPPH reagent were recorded at 517 nm on a UV-Visible spectrophotometer. The anti-oxidant activity was measured using the formula:
% Scavenging or Inhibition = [(Ao-As)/Ao] × 100
Where, Ao=Absorbance of the DPPH solution without sample, As=Absorbance of DPPH solution with sample.
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
The assay is based on the ability of different compounds to scavenge 2,2-azino-bis(ethylbenzthiazoline-6-sulfonic acid) radical cation. ABTS radicals have characteristic absorbance at 734 nm. This absorbance decreases when the radical is reduced by any antioxidant compound. The decrease in the absorbance can be measured using a UV-Visible spectrophotometer at 734 nm.
Procedure: 7 mM ABTS stock solution in water was prepared. To it 2.45 mM solution of potassium persulfate was added in 1:1 ratio (v/v). This reaction mixture was left undisturbed in a dark place for about 16 h for generation of the radicals. This solution was further diluted using methanol so that it has a stable absorbance of 0.700 ± 0.05 at 734 nm. Methanolic solutions of all compounds including ascorbic acid were prepared in the concentration ranges of 40, 60, 80 and 100 μg/ml. To 0.1 ml of the test solutions 1 ml of distilled ABTS solutions was added. Absorbance of the test and the standard solutions were taken immediately at 734 nm. The anti-oxidant activity of the samples were determined using the equation
% E = [(Ac-At)/Ac] × 100
Where, E=Antioxidant activity, Ac=Absorbance of the ABTS stock solution, At=Absorbance of the test compounds
Results and Discussion
The ATIR of the compounds was found to be in accordance with the data reported in literature [11]. The major peaks were recorded at 1728- 1605 cm-1 for C=N group, at 1365-1305 cm-1 and 722-640 cm-1 for C-S group, Absorption peaks for other functional groups were also observed in the respective derivatives. 1H-NMR: NMR peak for CH3 group was found at δ=1.53-3.99 ppm. The NMR peak for aromatic hydrogens Ar-H was found to be in the range δ=6.99-8.32 ppm (Table 3).
| Name | IR spectra data | 1H-NMR spectra data (CDCl3) |
|---|---|---|
| BTA-1 | 1710.98 ν (C=O), 1690.08 ν (C=N), 1515.41 ν (C-C), 1411.45 ν (C=C), 1058.96 ν (C-O-C), 754.78 ν (Ar C-H), 686.54 ν (C-S) | d=11.0 (s, 1H, COOH), 8.32-8.00 (m, 4H, Ar-H), 7.55 (t, 2H, Ar-H), 7.45 (d, 2H, Ar-H), 7.13 (d, 2H, Ar-H), 6.72 (d, 2H, Ar-H) |
| BTA-2 | 2303.91 ν (C≡N), 1665.11 ν (C=N), 1585.15 ν (C-C), 1431.68 ν (C=C), 759.19 ν (Ar C-H), 645.11 ν (C-S) | d=8.20-8.05 (d, 2H, Ar-H), 7.52 (t, 2H, Ar-H), 7.33-6.99 (m, 4H, Ar-H), 3.46 (m, 1H, CH), 1.57 (d, 3H, CH3) |
| BTA-3 | 1607.75 ν (C=N), 1515.50 ν (C-C), 1465.28 ν (C=C), 1050.58 ν (C-F), 745.14 ν (Ar C-H), 692.81 ν (C-S) | d=8.25-8.12 (d, 2H, Ar-H), 7.56 (t, 2H, Ar-H), 7.46 (d, 1H, Ar-H), 7.34 (d, 1H, Ar-H), 7.02 (t, 1H, Ar-H) |
| BTA-4 | 3376.99 ν (OH), 1615.35 ν (C=N), 1549.28 ν (C-C), 1468.13 ν (C=C), 1318.19 ν (C-N), 1041.48 ν (C-O-C), 799.98 ν (Ar C-H), 672.07 ν (C-S) | d=8.32-8.00 (d, 2H, Ar-H), 7.88 (s, 1H, Ar-H), 7.52 (t, 2H, Ar-H), 7.25 (s, 1H, Ar-H), 5.08 (s, 1H, OH), 3.99 (s, 3H, CH3) |
| BTA-5 | 1801.85 ν (C=O), 1650.31 ν (C=N), 1515.40 ν (C-C), 1435.28 ν (C=C), 756.63 ν (Ar C-H), 722.64 ν (C-Cl), 692.40 ν (C-S) | d=8.03-8.00 (d, 2H, Ar-H), 7.85 (d, 1H, Ar-H), 7.70 (d, 2H, Ar-H), 7.59-7.32 (m, 7H, Ar-H) |
| BTA-6 | 1610.16 ν (C=N), 1544.62 ν (C-C), 1409.12 ν (C=C), 1199.10 ν (SO2Cl), 796.71 ν (Ar C-H), 679.41 ν (C-S) | d=8.32-8.05 (m, 3H, Ar-H), 7.72 (d, 1H, Ar-H), 7.69 (d, 1H, Ar-H), 7.55 (m, 3H, Ar-H) |
| BTA-7 | 1605.61ν (C=N), 1506.82 ν (C-C), 1439.52 ν (C=C), 1204.16 ν (SO2Cl), 754.50 ν (Ar C-H), 670.11 ν (C-S) | d=8.15 (d, 1H, Ar-H), 8.12 (d, 1H, Ar-H), 7.99 (d, 2H, Ar-H), 7.72 (d, 2H, Ar-H), 7.54 (t, 2H, Ar-H) |
| BTA-8 | 3367.45 ν (OH), 1601.01 ν (C=N), 1508.41 ν (C-C), 1437.38 ν (C=C), 1039.98 ν (C-O-C), 750.17 ν (Ar C-H), 655 ν (C-S) | d=8.59-8.05 (d, 2H, Ar-H), 7.49 (d, 2H, Ar-H), 6.97-6.68 (m, 3H, Ar-H), 4.65 (s, 1H, OH), 3.97 (m, 2H, CH2), 1.53 (t, 3H, CH3) |
| BTA-9 | 1728.86 ν (C=N), 1596.70 ν (C-C), 1439.12 ν (C=C), 1192.42 ν (C-F), 832.32 ν (Ar C-H), 722.53 ν (C-S) | d=8.25 (d, 1H, Ar-H), 8.12 (d, 1H, Ar-H), 7.56 (t, 2H, Ar-H), 7.21 (t, 1H, Ar-H), 7.04- 6.73 (m, 3H, Ar-H) |
| BTA-10 | 1698.40 ν (C=N), 1515.09 ν (C-C), 1404.36 ν (C=C), 1077.57 ν (C-F), 789.28 ν (C-Cl), 742.04 ν (Ar C-H), 641.72 ν (C-S) | d=8.32- 8.00 (d, 2H, Ar-H), 7.71 (s, 1H, Ar-H), 7.55 (t, 2H, Ar-H), 7.40 (d, 1H, Ar-H), 6.72 (d, 1H, Ar-H) |
| BTA-11 | 1669.26 ν (C=N), 1586.48 ν (NO2), 1516.91 ν (C-C), 1417.09 ν (C=C), 752.05 ν (Ar C-H), 657.15 ν (C-S) | d=8.28- 8.04 (m, 4H, Ar-H), 7.73 (d, 2H, Ar-H), 7.51 (t, 2H, Ar-H) |
| BTA-12 | 1647.80 ν (C=N), 1523.76 ν (C-C), 1440.49 ν (C=C), 1022.14 (S=O), 802.37 ν (Ar C-H), 690.53 ν (C-S) | d=8.31- 8.09 (d, 2H, Ar-H), 7.97 (d, 2H, Ar-H), 7.68(d, 2H, Ar-H) 7.55 (t, 2H, Ar-H), 2.41 (s, 3H, CH3) |
Table 3: Spectral study of synthesized compounds
Antioxidant assay
The two common spectrophotometric methods to figure out the intensity of the antioxidant activity of organic compounds are based on the ability of the compound to oxidize the DPPH and ABTS. It has been reported in the literature the ABTS assay is more sensitive in identifying the antioxidant activity since it has faster reaction kinetics and a heightened response to antioxidants [14]. To assess the free radical scavenging activity of synthesized benzothiazole compounds, DPPH and ABTS assays were conducted and the results are indicated in Tables 4 and 5 respectively.
| Compounds | Dilutions (µg/ml) | |||
|---|---|---|---|---|
| 40 µg/ml | 60 µg/ml | 80 µg/ml | 100 µg/ml | |
| BTA-1 | 51.48% | 68.70% | 74.20% | 82.19% |
| BTA-2 | 42.69% | 56.89% | 69.34% | 73.53% |
| BTA-3 | 32.85% | 44.40% | 52.65% | 61.40% |
| BTA-4 | 44.50% | 54.60% | 66.93% | 81.62% |
| BTA-5 | 51.12% | 63.28% | 74.0% | 83.30% |
| BTA-6 | 32.65% | 43.48% | 55.90% | 68.14% |
| BTA-7 | 10.80% | 20.67% | 37.37% | 45.70% |
| BTA-8 | 35.87% | 55.30% | 70.34% | 86.01% |
| BTA-9 | 16.45% | 28.79% | 52.18% | 63.95% |
| BTA-10 | 12.43% | 20.70% | 27.26% | 36.76% |
| BTA-11 | 35.14% | 51.29% | 63.16% | 73.33% |
| BTA-12 | 31.75% | 43.42% | 51.33% | 63.47% |
| Ascorbic acid | 65.28% | 72.14% | 86.52% | 92.38% |
Table 4: Percentage scavenging of DPPH radical by compounds and ascorbic acid
| Compounds | Dilutions | |||
|---|---|---|---|---|
| 40 µg/ml | 60 µg/ml | 80 µg/ml | 100 µg/ml | |
| BTA-1 | 36.90% | 47.20% | 62.80% | 76.50% |
| BTA-2 | 28.12% | 37.17% | 48.60% | 59.80% |
| BTA-3 | 22.40% | 36.41% | 43.50% | 55.20% |
| BTA-4 | 30.70% | 44.43% | 61.70% | 74.50% |
| BTA-5 | 36.50% | 50.20% | 67.30% | 71.70% |
| BTA-6 | 28.60% | 34.66% | 46.17% | 54.02% |
| BTA-7 | 12.71% | 18.30% | 32.52% | 43.64% |
| BTA-8 | 33.38% | 45.50% | 62.7% | 83.16% |
| BTA-9 | 18.57% | 29.10% | 41.67% | 51.80% |
| BTA-10 | 14.60% | 25.14% | 32.70% | 43.60% |
| BTA-11 | 25.70% | 43.12% | 60.42% | 74.50% |
| BTA-12 | 31.38% | 43.50% | 60.71% | 72% |
| Ascorbic acid | 41.80% | 53.27% | 70.20% | 89.47% |
Table 5: Percentage scavenging of ABTS radical by compounds and ascorbic acid
DPPH assay: BTA-1 at 60 μg/ml showed better antioxidant activity than the standard ascorbic acid at 40 μg/ml. BTA-1 and BTA-5 at 80 μg/ml showed better antioxidant activity than the standard ascorbic acid at 60 μg/ml. BTA-8 at 100 μg/ml showed comparable antioxidant activity with the standard ascorbic acid at 80 μg/ml.
ABTS assay: BTA-1, BTA-4, BTA-5, BTA-8, BTA-11 and BTA-12 at 60 μg/ml showed better antioxidant activity than the standard ascorbic acid at 40 μg/ml. BTA-1, BTA-4, BTA-5, BTA-8, BTA-11 and BTA-12 at 80 μg/ml showed better antioxidant activity than the standard ascorbic acid at 60 μg/ml. BTA-1, BTA-4, BTA-5, BTA-8, BTA-11 and BTA-12 at 100 μg/ml showed better antioxidant activity than the standard ascorbic acid at 80 μg/ml.
From the results it was observed that the compounds showed a generalized trend of being equally active or more active at higher concentration than the standard ascorbic acid at lower concentration. Out of 12 compounds synthesized, 3 compounds (BTA-1, BTA-5 and BTA-8) showed better or equal antioxidant activity than the standard ascorbic acid according to DPPH assay and 6 compounds (BTA-1, BTA-4, BTA-5, BTA-8, BTA-11 and BTA-12) showed better or equal antioxidant activity than the standard ascorbic acid according to ABTS assay. It is already reported in the literature that ABTS assay for determining antioxidant activity is more sensitive than DPPH assay. Our findings also are coherent with the reported fact because more compounds showed consistent good antioxidant potential with ABTS assay as compared to DPPH assay.
Conclusion
Twelve novel benzothiazole derivatives were synthesized as per the established synthetic protocols. The synthesized compounds were characterized using ATIR and 1H-NMR techniques and were screened for their antioxidant potential. Six compounds namely BTA-1, BTA-4, BTA-5, BTA-8, BTA-11 and BTA-12 showed good antioxidant action. Thus Benzothiazole derivatives can be further explored as prospective antioxidant agents.
References
- B. Poljsak, D. Šuput, I. Milisav, Oxidative Medicine and Cellular Longevity., 2013, 11.
- M. MOEIN, S. MOEIN, S. Ahmadizadeh, Molecules., 2008, 13, 2804-2813.
- A.B.A. El-Gazzar, M.M. Youssef, A.M.S. Youssef, A.A. Abu Hashem, F.A. Badria, Eur. J. Med. Chem, 2009, 44, 609-624.
- M.K. Kashif, I. Ahmad, S. Hameed, Arkivoc., 2008, 16, 311-317.
- V. Lobo, A. Patil, A. Phatak, N. Chandra, Pharmacog. Rev., 2010, 4, 118-126.
- G.R. Buettner, Arch. Biochem. Biophys., 1993, 300, 535-543.
- J.M.C. Gutteridge, B. Halliwell, Antioxidants in Nutrition, Health and Disease, Oxford University Press, Oxford, UK, 1994.
- Y.Z. Shu, J. Nat. Prod., 1998, 61, 1053-1071.
- S. Vertuani, A. Angusti, S. Manfredini, Curr. Pharm. Des., 2004, 10, 1677-1694.
- C.R. Gale, Int. Psychogeriatr., 2001, 13, 259-262.
- L. Rupali, P. Perumal, K. Nitin, V.H. Bhaskar, D. Pratibha, Int. J. Curr. Pharm. Res., 2015, 7, 34-37.
- K.U. Sadek, R.A. Mekheimer, A.M.A. Hameed, F. Elnahas, M.H. Elnagdi, Molecules., 2012, 17, 6011-6019.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice Evans, Free Rad Biol. Med., 1999, 26, 1231-1237.
- K.J. Lee, Y. Chang Oh, W.K. Cho, J.Y. Ma, Evid. Based Complement. Alternat. Med., 2015, 2015, 165457.