Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 2
Synthesis, Characterization and Antimicrobial Activity of Some 4, 5- Dihydro. 3, 5- Diaryl- N- [(Arylamido/Imido)- Alkyl] Pyrazole- 1 Carbothioamides/Carboxamides
Soniya Singh1*, V.K. Pandey1 and P. K. Shukla22Division of Microbiology, Cental Drug Research Institute (CDRI), Lucknow (U.P.), India
Soniya Singh, Department of chemistry, Unversity of Lucknow, Lucknow (U.P.), India, Email: soniyasingh.chem@gmail.com
Received: 17-Sep-2020 Accepted Date: Feb 20, 2021 ; Published: 26-Feb-2021
Abstract
Synthesis of substituted pyrazoles derivatives was undertaken employing a convenient and easily accessible procedure. In this article some 4, 5- dihydro- 3, 5- diaryl- N- [(anylamidolimido) alkyl] pyrazole- 1- carbothrioamides/carboxamides were synthesized from 1, 3- diaryl- prop- 2- enes as starting material for their characterization and antimicrobial activity involving five fungal and four bacterial strain in vitro. Synthesized substituted pyrazole compounds were obtained in the yields varying 53-68%.
Keywords
Pyrazole- 1- carbothioamide/carboxamide, Chalcones, N- (Hydroxyalkyl) arylamides\imides
Introduction
Literature survey reveals that the pyrazole compounds are mainly effective as antipyretic and analgesic agents [1]. Result has confirmed that a pyrazoledicarboxamide containing a sugar moiety in its molecular architecture is specifically active against respiratory syncytial virus (RSV) invitro [2]. It is phosphorylated by adenine kinase. In addition, a pyrazole containing a sulphonamido moiety, not currently available in the United States, has been shown to displace first generation sulphonylureas from protein binding sites on human serum albumin. This increases the concentration of the free active drug and produces a more intense reaction that may cause hypoglycemia [3]. Two anti-inflammatory steroids are known which contain pyrazole ring which have 2000 times the potency of cortisol [4]. These pyrazole derivatives do not exhibit mineralocorticoid activity and were found to have high affinity for cytoplasmic glucocorticoid receptors [5]. A simplified class of pyrazoles was found in some cases to provide activity comparable to betamethasonevalerate in the human vaso constriction assay [6,7]. In pursuit of developing therapeutically more potent agents, the authors thought it worthwhile to extend the scope and validity of these observatives to synthesis of some novel pyrazole derivatives for studying their anti- microbial potentials.
Materials and Methods
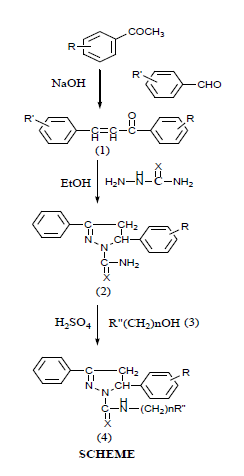
All the reagents and solvents used in the synthetic work were of laboratory grade and procured from sigma Aldrich India and 4D fine chemicals. Eight new 4, 5- dihyro- 3, 5- diaryl- N-[(arylamido/imido) alkyl] pyrazole-1-carbothioamides/carboxamides were synthesized from 1, 3- diaryl- prop- 2- en- 1- ones on reacting with various chemicals involving three synthetic sequences. The synthesized compounds were properly characterized may FTIR, 1HNMR, 13CNMR mass spectrometry and chemical anaylsis. Antibacterial activity was carried out against five fungal strains and four bacterial strains following two fold serial dilution technique as recommended by National Committee for Clinical Laboratory Standards (NCCLS). The melting points of the synthesized compounds were determined in open glass capillaries in Toshniwal electric melting point apparatus and therefore the recorded values are uncorrected IR spectra (νmaxin cm-1) were recorded in KBr on Perkin-Elemer 157 spectrophotometer in region νmax 4000-400 cm-11HNMR in CDCl3 on DRX (300 MHz) NMR spectrometer using TMS as internal standard and 13CNMR spectra in CDCl3 on a DRX (40MHz) NMR spectrometer using TMS as an internal standard (chemical shift in δ ppm). The mass spectra were recorded on Joel SX- 102 (FAB) mass spectrometer in which p-nitrobenzlylalcohol was used as matrix.
1,3- Diaryl- Prop- 2-en- 1- ones (Chalcones)
An aromatic aldehyde (0.1 mole) and an arylmethyl- ketone (0.1 mole) were dissolved in ethanol (50 ml) and a solution of sodium hydroxide (5%, 50 ml) was added to the ethanloic solution of aldehyde and ketone. The resultant solution was stirred vigorously, crystals of chalcones separated out, filtered, washed repeatedly with cold water, air dried and recrystallized from ethanol.
1, 3- Diphenyl- porp- 2- ene- 1- one:
Yellow crystalline mass, M. P. 450C [450C] [8], yield 69%
1-(2- Hydroxyphenyl), 3-phenyl-prop- 2-ene-1-one:
brown crystals M. P. 850C [860C][9], yield 64%
1-Phenyl, 3(4- methylphenyl-prop- 2-ene-1-one:
white needles M. P. 450C [450C][10], yield 70%
1-(2- Hydroxyphenyl), 3-(4-methylphenyl)-prop- 2-en-1-one:
Grey crystalalline mass M. P. 500C [510C][11], yield 61%
4,5- Dihydro- 3,5- diaryl pyrazole-1- carbothioamide/carboxamides(2)
A mixture of 1,3- diaryl- prop- 2- en- 1- one (1) (0.05 mole) and thiosemicarbazide/semicarbazide (0.1 mole) in absolute ethanol (100 ml) was heated under reflux five hours. Solvent ethanol was distilled off and the residue was washed initially with cold water and then with sodium-bi-carbonate solution. The solid thus obtained was dried in vacuum and recrtystallized from ethanol.
2a: 4,5- Dihydro- 3,5- diphenylpyrazole- 1- carbothioamide
m.p. 1120C white crystalline solid, IR (KBrpellot, cm-1) 1396 (carbothioamide, C=S), 1631 (C=N) 1HNMR (300 MHz, CDCl3δppm) δ 7.06-8.01 (m, 10 H, ArH), 9.31 (s, 2H, CSNH2), 3.88(t,1H, CH-CH2, J=8), 3.25 (d, 2H, CH-CH2, J=6), 13CNMR (40 MHz, CDCl3, δppm), δ 36.40, 62.82, 78.80, 119.50, 125.75, 131.36, 133.56, 134.20, 136.56, 137.31, 140.22, 164.22, 168.80; MS: m/z 281 (M+). Anal.calcd. For C16H15N35: C, 68.32; N; 5.37; N, 14.85 Found C, 68.29; H, 5.38; N; 14.89
2b: 4,5-Dihydro- 3,5-diphenylpyrazole- 1- carboxamide
m.p. 1750C, brown crystals, IR (KBr Pellet, cm-1); 1660 (amide C=O), 1640 (C=N), 1HNMR (300 MHz, CDCl3,, δppm) δ 6.90-7.25 (m, 10H, ArH) 9.15 (brs; 2H, CONH2) 3.30 (d, 2H, CH-CH2, J=6), 3.75 (t, 1H, CH=CH2; J=8), 13CNMR (40 MHz, , CDCl3, δppm) δ 115.50, 121.45, 125.20, 131.40, 136.25, 0.75, 169.20; MS: m/z 265 (M+)’ Anal.calcd. For C16H15N30: C, 75.45; H, 5.66; N, 15.85 Found C, 75.20; H, 5.60; N, 15.90
2c: 4,5- Dihydro- 3- phenyl- 5- (2-hydroxyphenyl)- phrazole- 1- carbothioamide
m.p. 950C, white needles, IR(KBr pellet, cm-1), 1405 (carbothioamide, C=S), 1635 (C=N), 1HNMR (300 MHz, CDCl3, δppm) δ6.85-7.15 (m,9H, ArH), 4.50 (s, 1H, ArOH), 9.25 (s, 2H, CSNH2), 3.35 (d, 2H, CH-CH2, J=6), 3.70 (t, 1H, CH-CH2, J=8), 13CNMR (40 MHz, CDCl3, δppm), 120.50, 125.62, 128.40, 135.65, 136.10, 137.50, 139.00, 142.50, 165.00, 171.20; Mass: m/z 297(M+), Anal.Calcd. For C16H15N30S: C, 64.64; H, 5.05; N,14.14 Found C, 64.40; H, 5.05; N,14.10.
2d: 4,5- Dihydro- 3-phenyl- 5- (2- hydroxyphenyl)- pyrazole- 1- Carboxamide,
M. P. 1200C, orange coloured crystalline mass, IR (KBr pellet, cm-1) 1665 (amide C=O), 1645(C=N),1HNMR (300 MHz, CDCl3, δ ppm ) δ 6.64-7.10 (m, 9H, ArH), 4.64 ( s, 1H, ArOH), 8.5 (brs, 1H, CONH2), 3.40 (d, 2H, CH-CH2. J=6), 3.80 (t, 1H, CH-CH2, J=8) 13CNMR (40 MHz, CDCl3, δ ppm), δ 116.50, 119.25, 121.45, 127.40, 131.64, 135.00, 139.50, 140.75, 142.00, 166.00, 179.24; Mass: m/z 281 (M+), Anal.Calcd. For C16H15N3O2: C, 65.54; H, 5.33; N, 14.95 Found, C, 65.44, H, 5.30; N, 14.90.
2e: 4,5Dihydro- 3- (4- methylphenyl)- 5- phenyl- pyrazole- 1-thiocarboxamide,
M.P. 900C, white crystalline solid, IR (KBr Pellet, cm-1) 1335 carbothiomide (C=S),1642 (C=N), 1HNMR (300MHz), CDCl3,δppm), 7.00-7.50 (m, 9H, ArH), 2.20 (s, 3H, ArCH3), 8.90 (s, 2H, CSNH2), 3.85 (t,1H, CH-CH2, J=8), 3.25 (d, 2H, CH-CH2, J=6),13CNMR (40MHz , CDCl3, δppm), δ 119.50, 121.24, 125.60, 129.20,135.40, 139.64, 140.90, 142.00, 143.10, 167.24, 169.00 MS; m/z 295(M+), Anal. Calcd. For C17H17N3S: C, 69.15; H, 5.76, N, 14.24, Found, C, 70.10; H, 5.82; N, 14.20.
2f: 4,5Dihydro- 3- (4- methylphenyl)- 5- phenyl- pyrazole- 1-thiocarboxamide:
m.p. 1200C, Grey coloured crystalline solid, IR (KBr Pellet, cm-1) 1664 (amide C=O), 1635 (C=N),1HNMR (300 MHz), CDCl3, δppm), 6.54-7.20 (m, 9H, ArH), 2.30 (s, 3H, ArCH3), 9.10 (trs, 2H, CONH2), 3.30 (d, 2H, CH-CH2, J=6), 3.80 (t, 1H, CH-CH2, J=6), 13 CNMR (40 MHz, CDCl3, δppm), δ115.50, 119.24, 121.20, 125.64, 129.10, 133.64, 137.20, 139.10, 141.54, 164.20, 168.54 MS; m/z 279(M+), Anal. Calcd. For C17H17N3O: C, 73.11; H, 6.09; N, 15.05; Found, C, 72.95; H, 6.10; N, 14.92.
2g: 4,5Dihydro- 3- (4- methylphenyl)- 5-(2-hydroxylphenyl)- pyrazole- 1-thiocarboxamide:
m.p. 1250C, White crystalline mass, IR (KBr Pellet, cm-1) 1330 (carbothioamide C=S), 1640 (C=N), 1HNMR (300 MHz), CDCl3, δppm), 7.20-7.45 (m, 8H, Ar-H), 2.30 (s, 3H, ArCH3), 4.65 (brs, 1H, Ar-OH), 3.34 (d, 2H, CH-CH2, J=6), 8.80 (s, 2H, CSNH2), 3.90 (t,1H, CH-CH2, J=8), 13CNMR (40 MHz , CDCl3, δppm), δ116.50, 119.45, 121.24, 127.36, 131.24, 131.24, 135.75, 139.00, 142.25, 166.44, 168.75 MS; m/z 311(M+), Anal. Calcd. For C17H17N3OS: C, 65.20; H, 5.46; N, 13.50; Found C, 65.20; H, 5.41; N, 13.39.
2h: 4,5Dihydro- 3- (4- methylphenyl)- 5-(2-hydroxy phenyl- pyrazole- 1-thiocarboxamide:
m.p. 1400C, Brown coloured crystalline solid, IR (KBr Pellet, cm-1) 1670 (amide C=O), 1632 (C=N), 1HNMR (300 MHz), CDCl3, δppm), 6.80-7.10 (m, 8H, ArH), 2.42 (s, 3H, ArCH3), 8.50 (brs, 2H, CONH2), 3.34 (d, 2H, CH-CH2, J=6), 3.84 (t, 1H, CH-CH2, J=8), 4.85 (brs,1H, Ar-OH), 13CNMR (40 MHz , CDCl3, δppm), δ 117.52, 121.20, 124.64, 129.64, 129.55, 133.40, 137.64, 141.20, 143.10, 167.66, 171.25 MS; m/z 295(M+), Anal. CalcdFor C17H17N3O2: C, 69.11; H, 5.76; N, 14.24; Found, C, 69.20; H, 5.68; N, 14.20.
General method for the preparation of 4,5-Dihydro- 3, 5- diaryl- N- [(arylamide/imide) alkyl]- pyrazole-1-carothioamides/ carboxamides (4)
A mixture of 4,5-dihydro- 3,5- diphenylprazole- 1- carbothioamide/carboxamides(2) (0.02 mole) and amido/imidoalkanol(3) (0.01 mole) was dissolved in minimum quantity of conc. H2SO4 in ice-cold condition with constant stirring. The solution thus obtained, was mechanically stirred for about one hour, cooled at room temperature and poured into crushed ice slowly with stirring. A precipitate was obtained which was allowed to settle down. It was filtered off washed with cold water, dried under vacuum and recrystallized from diluted ethanol (Table 1).
| Compound code | R | R’ | R’’ | X | m | Molecular weight | Yield (%) |
|---|---|---|---|---|---|---|---|
| 4a | H | H | S | 2 | 454 | 59 | |
| 4b | H | H | O | 1 | 424 | 62 | |
| 4c | H | 2- OH | S | 1 | 456 | 60 | |
| 4d | H | 2- OH | O | 2 | 454 | 53 | |
| 4e | 4-CH3 | H | S | 1 | 428 | 58 | |
| 4f | 4-CH3 | H | O | 1 | 412 | 68 | |
| 4g | 4-CH3 | 2- OH | S | 1 | 470 | 65 | |
| 4h | 4-CH3 | 2- OH | O | 1 | 428 | 59 |
Table 1: value for R, R’, R”, X, n, physical data for synthesized compounds
4(a):4,5- Dihydro- 3,5- diphenyl- N-[(phthalimido/ethyl)]- pyrazole- 1- carbothioamide
M. P. 700C, orange crystals, IR (KBr, Pellet, cm-1 1340 carbothioamide (C=S), 1635 (C=N), 1710 (imide, C=O), 1HNMR (300 MHz, CDCl3, δppm) δ 7.06- 8.01 (m, 14H, ArH), 9.31 (s,1H,CSNH2), 3.80 (t, 1H, CH-CH2, J=8), 3.34 (d, 2H, CH-CH2, J=6), 6.90 (s, 4H, NCH2CH2N, because of symmetrical nature, a singlet was observed at lower field), 13CNMR (40 MHz, CDCl3, δppm) δ 36.40, 62.82, 78.80, 125.75, 131.36, 133.56, 134.20, 136.56, 137.31, 140.22, 150.10, 164.22, 168.80, MS: 454 (M+), Anal. Calcd. For C26H22N4O2S:C, 68.72; H, 4.84; N, 12.33 Found C, 68.52; H, 4.75; N, 12.30.
4(b): 4,5- Dyihydro- 3-phenyl-5- 2-hydroxyphenyl- N-[(phthalimido/methyl)]- pyrazole- 1- carbothioamide
m.p. 120°C, yellow crystalline solid, 1R (KBr, Pellet, cm-1 1665 (amide C=O), 1722(imide, C=O), 1636 (imide C=N), 1HNMR (300MHz, CDCl3, δppm) δ 6.80- 7.15 (m, 14H, ArH), 4.20 (s, 2H, N-CH2-N), 5.55 (s, 1H, CONH), 3.85 (t, 1H, CH-CH2, J=8), 3.36 (d, 2H, CH-CH2, J=6), 13CNMR (40 MHz, CDCl3, δppm) δ 40.20, 61.64,74.20, 121.45, 125.62, 128.36, 132.40, 135.20, 135.75, 141.20, 143.41, 145.00, 152.24, 166.40, 168.75, MS: 424(M+) Anal. Calcd.For C25H20N4O3; C, 77.54; H, 4.45; N, 13.21 Found C, 77.25; H, 4.26; N, 13.16.
4(c):4,5- Dihydro- 3- phenyl- 5-(2-hydroxy-phenyl)-N-[(phthalimido/methyl)]- pyrazole- 1- carbothioamide
m.p. 1100C, white crystals, IR (KBr, Pellet, cm-1) 1342 (carbothioamide C=S), 1711(imide, C=O), 1640( C=N), 1HNMR (300MHz, CDCl3, δppm) δ 6.85- 7.50(m, 13H, ArH), 5.52(S,1H,Ar-OH), 14.20(S,2H,N-CH2,J=6) 3.85(t, 1H, CH-CH2, J=8), 3.35 (d, 2H, CH-CH2, J=6), 13CNMR (40 H2, CDCl3, δppm) δ 42.20, 60.54,65.25, 117.42, 119.22, 121.46, 123.35, 127.46, 131.33, 135.60, 138.42,140.30, 142.69, 151.64, 164.26, 169.10, ms;456(M+), Anal.Caled.For C25H20N4O3:C, 65.78;H, 4.35H, N, 12.28 Found C, 65.25; H, 4.26; N, 12.25
4(d): 4,5- Dihydro- 3-phenyl-5- 2-hydroxyphenyl- N-[(phthalimido/ethyl)]- pyrazole- 1- carboxamide
M. P. 80°C, yellow crystalline mass, IR (KBr, Pellet, cm-1 1682 (amide C=O), 1705 (imide, C=O), 1635 (C=N), 1HNMR (300 MHz, CDCl3, δppm) δ 6.72- 7.45 (m, 13H, ArH), 5.40 (s, 1H, ArOH), 4.15 (s, 2H, N-CH2-N), 3.85 (t, 1H, CH-CH2, J=8), 3.40 (d, 2H, CH-CH2, J=6), 5.90 (s, 4H, N-CH2-H2-N), symmetrical 13CNMR (40 Hz, CDCl3, δppm) δ 41.10, 59.45, 66.00, 118.20, 120.24, 122.43, 125.11, 129.46, 132.00, 136.44, 139.20, 141.64, 151.12, 165.46, 169.18, MS; 454 (M+), Anal.Calcd. For C26H22N4O4: C, 68.74; H, 4.84; N, 12.33, Found C, 68.50; H, 4.74; N, 12.30.
4(e): 4,5- Dihydro- 3-(4methylphenyl)-5- phenyl- N-[(benzamido/methyl)]- pyrazole- 1- carbothioamide
m.p. 78°C, yellow crystals, IR (KBr, Pellet, cm-1), 1336 (carbothioamide, C=S), 1685 (amide C=O), 1708 (imide C=O), 1639 (C=N), 1HNMR (300MHz, CDCl3, δppm) δ 7.10- 7.68 (m, 14H, ArH), 2.43 (s, 3H, ArCH3), 8.70 (brs,1H, CONH), 4.25 (s, 2H, CH-CH2), 3.90 (t, 1H, CH-CH2, J=8), 3.45(d, 2H, CH-CH2, J=6), 9.10 (s, 1H, CSNH), 13CNMR (40 MHz, CDCl3, δppm) δ 37.44, 58.62, 77.54, 124.20, 131.44, 133.65, 135.44,137.60, 139.21, 141.36 152.00, 166.42, 168.75, MS 412 (M+), Anal.Calcd. For C25H24N4OS: C, 70.05; H, 5.60, N, 13.08, Found C, 69.75; H, 5.70; N, 12.90.
4(f): 4,5- Dihydro- 3-(4-methylphenyl)-5- phenyl-N-[(benzamido/methyl)]- pyrazole- 1- carboxamide
m.p. 85°C, Red coloured crystalline solid, IR (KBr, Pellet, cm-1), 1665 (amide C=O), 1705 (imide, C=O), 1636 (C=N), 1HNMR (300MHz, CDCl3, δppm) δ 6.90- 7.25 (m, 14H, ArH), 2.45 (s, 3H, ArCH3), 8.75 (brs,1H, CONH), 4.20 (s, 2H, N-CH2) 3.85 (t, 1H, CH-CH2, J=8), 3.40 (d, 2H, CH-CH2, J=6), 13CNMR (40 MHz, CDCl3, δppm) δ. 38.66, 59.90, 78.20, 119.54, 121.26 123.30, 125.44, 128.36, 131.40, 134.66, 137.25, 140.25, 142.25, 151.20, 164.54, 169.66, MS: C25H24N4O2 Anal. Calcd.For C, 72.64; H, 5.82, N, 13.59 Found C, 72.52; H, 5.90; N, 13.57.
4(g): 4,5- Dihydro- 3-(4-methylphenyl)-5- (2- hydroxyphenyl- N-[(phthalimido)methyl)]- pyrazole- 1- carbothioamide
m.p. 95°C, Brown crystals, IR (KBr, Pellet, cm-1) 1341 (thioamide C=S), 1705 (imido, C=O), 1636 (imide C=N), 1636 (C=N), 1HNMR (300MHz, CDCl3, δppm) δ 6.85-7.26 (m, 12H, ArH), 5.40 (s, 1H, ArOH), 4.25 (s, 2H, N-CH2-N), 2.42 (s, 3H, ArCH3), 3.90 (t, 1H, CH-CH2, J=8), 3.54 (d, 2H, CH-CH2, J=6), 8.87 (s, 1H, S=C-NH), 13CNMR (40 MHz, CDCl3, δppm) δ 41.64, 69.26, 77.00, 114.36, 117.54, 119.21, 122.84, 125.60, 129.64, 132.54, 135.64, 138.70, 139.21, 141.40, 152.62, 167.32, 170.50; MS; 470 (M+). Anal calcd.For C26H22N4O3S; C, 66.64; H, 4.6; N, 19.19 Found C, 66.56; H, 4.70; N, 11.85.
4(h): 4,5- Dihydro- 3-(4-methylphenyl-5- (2- hydroxyphenyl- N-[benzamido)methyl)]- pyrazole- 1- carboxamide
m.p. 90°C, Red crystalline mass, IR (KBr, Pellet, cm-1) 1680 (amide C=O), 1712(imide, C=O), 1638 (C=N), 1HNMR (300MHz, CDCl3, δppm) δ 7.00- 7.45 (m, 12H, ArH), 5.15 (s, 1H, CONH), 4.25 (s, 2H, N-CH2-N,), 3.80 (t, 1H, CH-CH2 J=8), 3.35 (d, 2H, CH-CH2); 13CNMR (40 MHz, CDCl3, δppm) δ 41.50, 60.25, 73.46, 119.50, 121.34,, 125.25, 129.36, 132.64, 135.68, 137.10, 139.44, 141.62, 151.46, 164.86, 169.25, MS; 428(M+) Anal.calcd. For C25H24N4O3; C, 70.09; H, 5.60; N, 13.08 Found C, 69.55; H, 5.65; N, 12.97.
N- (Hydrozyalkyl)-arylamides/ imides(3) N-(Hydroxymethyl)- phthalimide (3a)
A mixture consisting of phthalimide (0.01 mole), formaldehyde (40%, 0.25 mole) and water (100 ml) was boiled under reflux until a clear solution resulted. After cooling overnight in the refrigerator the solid thus obtained, was filtered off washed with cold water, dried in air and recrtystallized from ethanol, yield 90%, m.p. 1390C [137-1410C]. In order to further purify, 2.15g of N (hydroxymethyl)-phthalimide obtained by the earlier method was dissolved in pyridine (5ml) by stirring, cooled and filtered. It was left to crtystallize at room temperature. Pyridine complex crystallized in long lustrous needles and collected after cooling. On drying over concentrated sulphuric acid, the crystals lost their lustre and came to constant weight afer 24 hours. Dried product melted at 148.50C. Recrystallization from acetone resulted in a white crtytalline mass and melted at 1490C [1490C][12,13].
N- (Hydroxyethyl)-phthalimide (3b)
Finely powdered phthalic anhydride (0.1 mole) and β-aminoehtanol (0.12 mole) wre heated under reflux at a constant temperature of 2100C (±10C) for one hour. During this period, water which formed, eliminated from the reaction mixture and the resultant hot solution on cooling solidified in white crystalline form. The solid thus obtained was washed with diluted HCl in order to remove any unreacted β-aminoethanol. It was further washed with cold water, dried in air and recrstallized from ethanol in form white crystalline needles, yield 77%, m.p. 1480C [148-1490C][14].
N- (Hydroxymethyl)-benzamide (3c)
A mixture of benzamide (0.1 mole), formaldehyde (40%, 0.25 ml), potassium carbonate (0.1g) in water (100 ml) was heated under reflux at 1000C for 2 hours, with constant stirring. The resultant hot solution was cooled to room temperature and refigerated overnight. The white solid thus obtained was filtered off, washed with cold water dried and recrystallized from ethanol, yield 77%, m.p. 1480C [148-1490C][15].
Antimicrobial Activity
All the eight target compounds viz, 4,5- dihydro- 3,5- diaryl- N; [(arylamido/imido) alkyl] pyrazole-1- carbothioamides/ carboxamides (4) were evaluated for their antimicrobial activity against four bacterial strains viz, Escherichia coli (ATCC- 9637) (Ec), Psuedomonasaeruginosa (ATCCBAA- 427) (Pa), Staphylococcus aureus (ATCC- 25923) (Sa) and Klebsiella pneumonia (ATCC-27736) (Kp). Gentamycin was taken as the standard drug having minimum inhibitory concentration (MIC) values of 0.18, 25, 6.25 and 0.18 μg/ml against Ec, Pa, Sa and Kprestpectively and five fungal strains viz; Candida albicans[(Ca), Cryptococcus neoformans (Cn), Trichophyteamentagrophyte(Tm), Aspergillusfumigatus(Af) and Candida parapsilasis (Cp). Fluconazole was taken as the standard drug having MIC values of 0.5, 1.0, 1,0, 2.0 and 1.0 μg/ml against Ca, Sn, Tm, Af and Cprestpectively.
Two fold serial broth dilution technique as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) was employed for antibacterial and antifungal screening of the test compounds. MIC was determined in μg/ml against each test sample upto a maximum concentration of 50μg/ml.
Antibacterial activity
Materials and Method
Bacteria were maintained on nutrient against slants. Testing was done on peptone broth. After innoculaton with a loopful of culture from the slant, the seeded broths were incubated at 37 ± 10C for 24 hours. The compound under investigation was in dimethylsulphoxide (DMSO) to get a 1.0 mg/ml solution. This solution (0.2 ml) was added to 1.8 ml. of seeded broth and from the first dilution, one ml of this was diluted with a further 1.0 ml of the second broth to produce the second dilution and the process was repeated till six such dilutions were obtained. A set of tubes containing only inoculated broth was kept as control. After incubation for 24 hours, the last tube with no growth of micro-organism was taken to represent the minimum inhibitory concentration(MIC) expressed in μg/ml [16,17].
Antifungal activity
Material and Method
The investigational compounds were dissolved in dimethylsuphoxide (DMSO) to obtain a 1.0 mg/ml stock solution. Appropriate seeded broths were prepared. Solution of the test material (0.1 ml) was added to 1.8 ml of the seeded broth and this formed the first dilution. Subsequently, 1.0 ml of this was diluted with a further 1.0 ml of the seed broth to give second dilution and so on till 10 to 12 such dilutions were obtained. A set of tubes containing only seeded broths and suitable solvent controls wre also maintained under identical condition. The tubes were incubated at 280C and the minimum inhibitory concentrations (MICs) of the products (based upon visual appearance of growth) was noted after 72/ 96 hours. The last tube with no apparent growth of the microorganism was taken to represent the MIC of the test compound and is expressed in μg/ml [18,19].
Antimicrobial activity data are recorded in Table 2
| Compound no. | Antibactirial MIC in µg/ml | Antifungal activity, MIC in µg/ml | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ec | Pa | Sa | Kp | Ca | Cn | Tm | Af | Cp | |||
| 3a | 50 | >50 | 25 | 12.5 | >50 | >50 | 50 | >50 | 50 | ||
| 3b | 25 | 25 | 6.25 | 12.5 | >50 | >50 | 50 | 50 | >50 | ||
| 3c | 25 | 50 | 6.25 | 12.5 | >50 | >50 | 50 | >50 | >50 | ||
| 3d | >50 | 50 | 50 | 25 | >50 | >50 | >50 | >50 | >50 | ||
| 3e | 50 | 25 | 12.5 | 6.25 | >50 | >50 | 50 | >50 | >50 | ||
| 3f | >50 | 50 | 50 | >50 | 25 | 25 | 6.25 | 50 | 50 | ||
| 3g | 50 | 50 | 25 | 12.5 | >50 | >50 | 50 | >50 | >50 | ||
| 3h | >50 | 50 | 25 | 25 | 25 | 25 | 3.12 | 25 | 50 | ||
Table 2: Antimicrobial activity
Results and Discussions
It is seen form the antibacterial activity data incorporated in Table-II that all the compounds except only one compound (4f) exhibited measurable degree of antibacterial activity against KlebsisllaPneumoniae (Kp) Two compounds viz, (4b) and (4c) showed equivalent magnitude if antibacterial activity against Staphylococcus aureus (Sa) MIC value 6.25 for each compound if compared with the standard drug gentamycin (MIC of 6.25). Compound (4e) also displayed the same order of antibacterial activity against Kp as MIC value determined was found to be 6.25 compounds 4a, 4b, 4c and 4g were found to be moderately antibacterialy active against Kp and Sa; however these compounds could not provoke better activity against Escheria Coli (Ec) and Pseudomonas aeruginosa(Pa). In contrast, these compounds showed less significant antifungal activity. Thus, compounds (4f) and (4h) showed promising antifungal activity against only one fungus i.e. against Trichophytonmentagrophyte(Tm). Thus, the compound 4f having R=4-methyl, R’=H and a benzamido substituents had a MIC value of 6.25 while the compound (4h) containing R=4-methyl, R’=2-OH and a benzamido substituent showed comparatively a better antifungal activity (MIC 3.12) against (Tm), other six compounds of this series could not display any measureable level of antifungal activity against all the five fungal strains.
Conclusion
It is apparent from the biological activity data incorporated in Table 2 that only three compounds have shown excellent antibacterial and antifungal activity in vitro, therefore more studies are needed in order to obtain better antimicrobial activity by incorporating other pharmacophoric groups in the molecular architecture of these compounds.
Acknowledgment
The authors express their sincere thanks to the Head, Department of Chemistry, Lucknow University, Lucknow for providing necessary laboratory facilities and to the Director, Central Drug Research Institute (CDRI), Lucknow for providing elemental, spectral and biological activity data reported in this manuscript.
References
- A Kar, Medicinal chemistry, New Age International(P) Ltd. Publishers. 2007, p. 287- 294.
- JM Woods, CLP Marrand CR Penn, Antiviral chemistry chemother.1994, 5: p. 340.
- EL Hengesh, Medicinal chemistry “Drugs Affecting Sugar Metabolism, 4th Edition, 1995, p. 598.
- K Erlenmeyer and E Willi. Chim Acta. 1935, 18: p. 740.
- R Hirschmann, MG Steingberg, JH Berchschacher, et al., J Amer Chem Soc. 1963, 85: p. 120.
- SS Simmons, EB Thompsonand DF Johnson., Beochem Biophys Res Commun. 1979, p. 793- 800.
- S Sugar, Y Jajiwar, Y Kaubara et al., Chem Pharm Bull. 1986, 34: p. 1618.
- AI Vogel, “Practical Organic chemistry”, English Language Book Society, Longman Group Ltd.,1971, p. 709.
- FG Mann and BC Saunders, “Practical Organic Chemistry”, 4th Edition, Longman Group Ltd., 1960, p. 231.
- M Upadhyay, Lucknow University, Lucknow, 2005, p. 146-147.
- J Tscherniac. Chem Abstr. 1902, 2: p. 1084.
- SRJ Buc. J Am Chem Soc. 1947, 69: p. 254.
- E Sakellarios, J Am Chem Soc. 1937, 59: p. 422.
- NW Hirwe and KN Rana. Ber. 1939, 72: p. 1346.
- GB Singh, BB Dixit and VK Vijjain. J Ind Chem Soc. 1968, 45:p. 262.
- VD Tripathi, SK Agrawal, OP Srivastava et al., Ind J Pharm Sci. 1978, 40: p. 129-131.
- ML Dhar, MN Ghar, BN Dhawan et al., Ind J Exp Biol. 1968, 6: p. 232-247.
- S Wahab, RN Tandon. Z Jacob et al., Ind Bot Sci. 1981, 60: p. 278-298.
- RS Vermaand SA Imam, Ind J Microbial. 1973, 13: p. 45.