Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Synthesis and Larvicidal Activity of Morpholinophenyl and Benzyl-Fluoro Pyrimidine Derivatives
Satya Kumar Avula1, Shashi Kanth Boddu2 and Prashanthi Yarasani3*2Department of Chemistry, Annie Besant PG College, Osmania University, Telangana State, India
3Department of Chemistry, Mahatma Gandhi University, Nalgonda, Telangana State, India
Prashanthi Yarasani, Department of Chemistry, Mahatma Gandhi University, Nalgonda, Telangana State, India, Email: dryprasanthi@gmail.com
Abstract
A series of 5-fluoro-N4-(3-(4-substitutedbenzylamino)phenyl)-N2-(4-morpholinophenyl) pyrimidine-2,4-diamine derivatives 7a-J were prepared and tested for their larvicidal activity against third instar larvae at 24, 48 and 72 hours and values were compared with Malathion as a positive control. Compounds 7a, 7c, 7f, 7g and 7i exhibited good larvicidal significant activity. Interestingly, compounds 7b, 7d, 7e, and 7h showed excellent activity very likely due to the presence of functional groups attached to the benzyl ring.
Keywords
Larvicidal activity, Morpholinophenyl, Pyrimidines, Pharmacophore, Synthesis
Introduction
Mosquito-borne diseases, such as dengue fever, malaria, encephalitis, yellow fever are major threats to millions of people throughout the world. The common reasons being the tropical, subtropical climate, poor drainage system especially during rainy seasons, adversely maintained fish ponds, irrigation ditches and rice fields which provide abundant mosquito breeding places. Although chemical vector control programs have been carried out for long time, these mosquito-vector diseases remain because of the refusal by householders to house spraying with synthetic insecticides [1,2]. The main vector of these diseases is the mosquito Aedes aegypti (A. aegypti) that transmits dengue fever, dengue hemorrhagic fever and yellow fever over many areas of tropics and subtropics [3]. Among them dengue fever is the most dangerous and case list is also increasing day by day. In the last five years most of the insecticides did not show any effect on insects like mosquitoes due to increased drug resistance [4,5]. These studies have emphasized for the new, more effective larvicidal agents. In the pursuit of design of new drugs, the development of hybrid molecules through the combination of different pharmacophores in one frame, could lead to compounds with interesting biological properties. Prompted by these observations, in the present study, the synthesis and larvicidal screening of new derivatives 7a-J bearing different pyrimidines and morpholinophenyl pharmacophores moieties as hybrid molecules possessing larvicidal activity were investigated.
The 4-morpholinophenyl derivatives represent an interesting class of compounds possessing a wide spectrum of biological activities. A large number of morpholinophenyl derivatives exhibited antibacterial [6], antifungal [7], anticonvulsant [8], analgesic [9], anticancer [10] and antiviral activity [11]. Several types of pyrimidines were proved to possess valuable pharmacological properties, such as antiviral [12], antioxidant [13], antimalarial [14], anticonvulsant [15], against Alzheimer’s disease [16], antidepressant [17], antimicrobial [18], antitubercular [19], anti-inflammatory [20], antiplatelet [21], antibacterial, antifungal [22], anticancer agents [23], antiproliferative agent [24] and anti-HIV agents [25]. Integration of these moieties may show synergistic effect and hence in the present study an attempt is made for the synthesis of some novel heterocyclic molecules containing the 4-morpholinophenyl and pyrimidine systems, with a hope that they may exhibit a synergistic and larvicidal activity. In the present communication we report the synthesis and larvicidal activity of new fluoro pyrimidines linked with morpholinophenyl and benzyl derivatives.
Experimental
General experimental protocols
Melting points were determined in open capillaries on a Mel-Temp apparatus and are uncorrected. All the reactions were monitored by Thin Layer Chromatography (TLC). The silica gel F254 plates were used for TLC in which the spots were examined under UV light and then developed by an iodine vapor. Column chromatography was performed with silica gel (BDH 100-200 mesh). Solvents were purified according to standard procedures. The spectra were recorded with the following instruments; NMR: Varian Gemini 400 MHz (1H), 75 MHz (13C) and 376 MHz (19F) spectrometer; ESIMS: VG-Autospec micromass, analyses of all the compounds were recorded in LCQ Fleet, Thermo Fisher Instruments Limited. Organic extracts were dried over anhydrous Na2SO4.
General procedure for the synthesis of 2-chloro-5-fluoro-N-(3-nitrophenyl)pyrimidin-4-amine (3)
3-Nitrobenzenamine 2 (20 mmol) was added to a mixture of 2,4-dichloro-5-fluoropyrimidine 1 (10 mmol) and K2CO3 ( 15 mmol) in dimethyl sulfoxide (10 mL). The reaction mixture was stirred at 90ºC and followed by TLC. After completion of the reaction (monitored by TLC), the mixture was diluted with ethyl acetate and washed with water. The ethyl acetate fraction was separated, dried over Na2SO4 and was evaporated. The obtained compound was recrystalized to get 2-chloro-5-fluoro-N-(3-nitrophenyl) pyrimidin-4-amine as a white solid (2.83 g, 88%), m.p. 167-169ºC; 1H NMR (CDCl3, 400 MHz): δ 8.24 (brs, 1H), 7.62 (d, 1H, J=8.70 Hz), 7.43 (s, 1H), 7.40 (t, 1H, J=8.2 Hz), 7.32-7.28 (m, 1H), 7.20 (d, 1H, J=7.0 Hz); 13C NMR (CDCl3, 75 MHz): δ 156.6, 155.5, 153.4, 147.6, 143.8, 141.8, 130.2, 122.8, 114.8, 111.7; 19F NMR (CDCl3, 376 MHz): δ -118.34 (s, 1F); ESIMS: m/z 291 (M+Na)+. Anal. Cald. for C10H6ClFN4O2: C, 45.82; H, 3.20; N, 21.78%; Found: C, 45.84; H, 3.22; N, 21.76%.
General procedure for the synthesis of 5-fluoro-N4-(3-(nitrophenyl)-N2-(4-morpholino-phenyl)pyrimidine-2,4-diamine (4)
To a stirred solution of compound 3 (10 mmol), 4-morpholinobenzenamine (20 mmol) and K2CO3 (15 mmol) in dimethyl sulfoxide (10 mL). The reaction mixture was stirred at 90ºC and followed by TLC. After completion of the reaction, the mixture was diluted and extracted with ethyl acetate solution. The organic layer was dried over anhydrous Na2SO4. Finally the compound was concentrated and recrystalized to get pure compound of 5-fluoro-N4-(3-(nitrophenyl)-N2-(4-morpholinophen-yl)pyrimidine-2,4-diamine as a pale yellow solid (4.26 g, 86%), m.p. 191-193ºC; 1H NMR (CDCl3, 400 MHz): δ 8.23 (brs, 1H), 8.20 (brs, 1H), 7.76-7.68 (m, 2H), 7.40 (t, 1H, J=8.2 Hz), 7.36 (s, 1H), 7.21 (dd, 1H, J=7.05, 8.0 Hz), 6.86 (d, 2H, J=7.32 Hz), 6.78 (d, 2H, J=7.34 Hz), 3.90 (t, 4H, J=4.95 Hz), 3.27 (t, 4H, J=4.83 Hz); 13C NMR (CDCl3, 75 MHz): δ 165.6, 154.4, 151.8, 148.5, 143.2, 140.4, 132.6, 132.2, 130.3, 123.2, 119.2, 115.3, 114.6, 110.8, 69.4, 51.8; 19F NMR (CDCl3, 376 MHz): δ -118.31 (s, 1F); ESIMS: m/z 411 (M+H)+. Anal. Cald. for C20H19FN6O3: C, 58.54; H, 3.65; N, 20.32%: Found: C, 58.50; H, 3.67; N, 20.36%.
General procedure for the synthesis of 5-fluoro-N4-(3-(aminophenyl)-N2-(4-morpholinophenylpyrimidine-2,4-diamine (5)
The compound 4 (10 mmol) in a mixture of methanol-ethyl acetate, (1:2, 20 mL) was treated with 10% Pd-carbon (5% w/w). The reaction was subJected to hydrogenation under 50 psi hydrogen gas pressures at room temperature and the reaction was monitored by TLC. After completion of the reaction, the mixture was filtered through a celite pad and concentrated under reduced pressure to afford pure product as a pale yellow solid (3.47 g, 78%), m.p. 205-206ºC; 1H NMR (CDCl3, 400 MHz): δ 8.26 (brs, 1H), 8.20 (brs, 1H), 7.56-7.44 (m, 4H), 7.36 (t, 1H, J=7.2 Hz), 7.30 (s, 1H), 7.10 (dd, 1H, J=7.0, 8.20 Hz), 6.89 (d, 2H, J=7.30 Hz), 6.20 (s, 2H), 3.86 (t, 4H, J=4.89 Hz), 3.15 (t, 4H, J=4.78 Hz); 13C NMR (CDCl3, 75 MHz): δ 165.8, 154.6, 151.4, 149.0, 143.2, 139.8, 132.6, 132.3, 130.4, 119.4, 115.0, 106.5, 102.4, 69.4, 51.8; 19F NMR (CDCl3, 376 MHz): δ -118.33 (s, 1F); ESIMS: m/z 403 (M+Na)+. Anal. Cald. for C20H21FN6O: C, 63.27; H, 5.38; N, 22.82%; Found: C, 63.30; H, 5.35; N, 22.85%.
General procedure for the synthesis of 5-fluoro-N4-(3-(4-substitutedbenzylaminophenyl)-N2-(4-morpholinophenyl) pyrimidine- 2,4-diamine derivatives (7a-J)
To a solution of compound 5 (10 mmol) in acetone 20 mL was added K2CO3 (25 mmol) at room temperature. This was followed by the addition of substituted benzyl halides 6 (12 mmol). The reaction mixture was heated under reflux for 3 h and the completion of reaction was checked by TLC. The reaction mixture was cooled to room temperature and poured into ice-water followed by extraction with ethyl acetate. The combined organic layer was evaporated, recrystalized to get pure compounds (7a-J) as solids.
5-Fluoro-N4-(3-(4-fluorobenzylamino)-phenyl)-N2-(4-morpho-linophenyl)pyrimidine-2,4-diamine (7a)
Pale yellow solid (0.62 g, 91%), m.p. 238-240ºC; 1H NMR (DMSO-d6, 400 MHz): δ 8.24 (brs, 1H), 8.22 (brs, 1H), 7.58-7.46 (m, 3H), 7.43 (s, 1H), 7.34 (d, 2H, J=8.54 Hz), 7.28-7.14 (m, 4H), 7.10 (t, 1H, J=10.0 Hz), 6.94 (d, 2H, J=7.84 Hz), 6.26 (brs, 1H), 4.42 (d, 2H, J=5.61 Hz), 3.82 (t, 4H, J=4.88 Hz), 3.28 (t, 4H, J=4.79 Hz); 13C NMR (DMSO-d6, 75 MHz): δ 165.5, 161.4, 154.5, 151.2, 148.4, 143.8, 140.2, 137.4, 132.6, 132.3, 130.5, 128.8, 118.8, 115.2, 104.9, 103.7, 99.4, 69.2, 51.7, 46.3; 19F NMR (DMSO─d6, 376 MHz): δ -118.30 (s, 1F); ESIMS: m/z 489 (M+H)+. Anal. Cald. for C27H26F2N6O: C, 66.42; H, 5.36; N, 18.40%; Found: C, 66.38; H, 5.33; N, 18.38%.
5-Fluoro-N4-(3-(4-fluoro-2-(trifluorometh-yl)benzylamino)-phenyl)-N2-(4-morpholinophen-yl)pyrimidine-2,4-diamine (7b)
Colorless solid (0.81 g, 86%), m.p. 255-257ºC; 1H NMR (DMSO-d6, 400 MHz): δ 8.34 (brs, 1H), 8.28 (brs, 1H), 7.62-7.56 (m, 4H), 7.42 (t, 1H, J=8.58 Hz), 7.36 (d, 2H, J=4.58 Hz), 7.28 (s, 1H), 7.20-7.12 (m, 2H), 6.98 (d, 2H, J=8.12 Hz), 6.28 (brs, 1H) 4.45 (d, 2H, J=5.58 Hz), 3.84 (t, 4H, J=4.86 Hz), 3.29 (t, 4H, J=4.82 Hz); 13C NMR (DMSO-d6, 75 MHz) δ: 165.7, 161.2, 155.0, 151.4, 148.6, 144.0, 140.3, 132.6, 132.3, 130.5, 128.9, 127.5, 121.8, 119.2, 118.8, 115.1, 112.2, 104.8, 103.5, 99.8, 69.4, 51.8, 46.4; 19F NMR (DMSO-d6, 376 MHz): δ -118.27 (s, 1F), -122.39 (m, 3F); ESIMS: m/z 579 (M+Na)+. Anal. Cald. for C28H25F5N6O: C, 58.63; H, 3.78; N, 14.98%; Found: C, 58.65; H, 3.75; N, 14.96%.
5-Fluoro-N4-(3-(3-fluorobenzylamino)-phenyl)-N2-(4-morpho-linophenyl)pyrimidine-2,4-diamine (7c)
Light brown solid (0.53 g, 80%), m.p. 223-224ºC; 1H NMR (CDCl3, 400 MHz): δ 8.22 (brs, 1H), 8.19 (brs, 1H), 7.60-7.48 (m, 4H), 7.42 (d, 2H, J=8.60 Hz), 7.38 (s, 1H), 7.34-7.28 (m, 3H), 7.12 (t, 1H, J= 8.20 Hz), 6.98 (d, 2H, J=10.0 Hz), 6.27 (brs, 1H), 4.45 (d, 2H, J=5.62 Hz), 3.79 (t, 4H, J=4.76 Hz), 3.26 (t, 4H, J=4.81 Hz); 13C NMR (CDCl3, 75 MHz): δ 165.5, 162.9, 154.4, 151.3, 148.6, 143.6, 139.8, 132.3, 132.1, 130.4, 122.7, 119.2, 115.1, 113.8, 113.5, 104.8, 103.6, 99.8, 69.3, 51.8, 46.3; 19F NMR (CDCl3, 376 MHz): δ -118.19 (s, 1F); ESIMS: m/z 511 (M+Na)+. Anal. Cald. for C27H26F2N6O: C, 66.36; H, 5.38; N, 18.38%; Found: C, 66.39; H, 5.36; N, 18.40%.
5-Fluoro-N4-(3-(4-(trifluoromethoxy)ben-zylamino)phenyl)-N2-(4-morpholinophenyl)pyri-midine-2,4-diamine (7d)
White solid (0.64 g, 83%), m.p. 260-262ºC; 1H NMR (CDCl3, 400 MHz): δ 8.45 (brs, 1H), 8.38 (brs, 1H), 7.82-7.74 (m, 3H), 7.63 (s, 1H), 7.51 (d, 2H, J=10.0 Hz), 7.44-7.32 (m, 4H), 7.20 (t, 1H, J= 8.62 Hz), 7.10 (d, 2H, J=8.86 Hz), 6.32 (brs, 1H), 4.48 (d, 2H, J=5.80 Hz), 3.92 (t, 4H, J=4.72 Hz), 3.38 (t, 4H, J=4.89 Hz); 13C NMR (CDCl3, 75 MHz): δ 165.5, 161.4, 155.2, 151.3, 148.5, 143.8, 139.8, 133.9, 132.5, 130.7, 128.0, 121.7, 119.3, 115.2, 114.0, 104.7, 103.5, 99.8, 69.6, 51.7, 46.2; 19F NMR (CDCl3, 376 MHz): δ -118.32 (s, 1F), -123.84 (m, 3F); ESIMS: m/z 577 (M+Na)+. Anal. Cald. for C28H26F4N6O2: C, 58.80; H, 3.78; N, 16.08%; Found: C, 58.78; H, 3.75; N, 16.06%.
5-Fluoro-N4-(3-(2-fluoro-3-(trifluorometh-yl)benzylamino)-phenyl)-N2-(4-morpholinophen-yl) pyrimidine-2,4-diamine (7e)
Yellow solid (0.80 g, 92%), m.p. 245-247 ºC; 1H NMR (DMSO-d6, 400 MHz): δ 8.42 (brs, 1H), 8.33 (brs, 1H), 7.90-7.82 (m, 4H), 7.71 (s, 1H), 7.63 (d, 2H, J=8.02 Hz), 7.40 (t, 1H, J=7.0 Hz), 7.36-7.28 (m, 2H), 6.97 (d, 2H, J=10.2 Hz), 6.35 (brs, 1H), 4.52 (d, 2H, J=5.84 Hz), 3.95 (t, 4H, J=4.82 Hz), 3.39 (t, 4H, J=4.90 Hz); 13C NMR (DMSO-d6, 75 MHz): δ 164.8, 159.7, 155.3, 151.2, 148.7, 143.8, 139.6, 132.4, 132.2, 131.6, 130.3, 124.6, 124.4, 118.9, 118.5, 117.7, 115.1, 104.6, 103.3, 99.8, 69.5, 51.6, 44.6; 19F NMR (DMSO-d6, 376 MHz): δ -118.29 (s, 1F), -122.86 (m, 3F); ESIMS: m/z 557 (M+H)+. Anal. Cald. for C28H25F5N6O: C, 58.60; H, 3.75; N, 14.86%; Found: C, 58.63; H, 3.78; N, 14.88%.
5-Fluoro-N4-(3-(3-nitrobenzylamino)phen-yl)-N2-(4-morpholinophenyl)pyrimidine-2,4-diamine (7f)
Pale yellow solid (0.71 g, 85%), m.p. 234-235ºC; 1H NMR (CDCl3, 400 MHz): δ 8.20 (brs, 1H), 8.17 (brs, 1H), 7.58-7.45 (m, 4H), 7.40 (d, 2H, J=10.0 Hz), 7.35 (s, 1H), 7.32-7.25 (m, 3H), 7.10 (t, 1H, J= 8.28 Hz), 6.96 (d, 2H, J=8.64 Hz), 6.25 (brs, 1H), 4.42 (d, 2H, J=5.64 Hz), 3.76 (t, 4H, J=4.52 Hz), 3.32 (t, 4H, J=4.75 Hz); 13C NMR (CDCl3, 75 MHz): δ 164.5, 155.2, 151.0, 148.6, 147.5, 143.9, 142.6, 139.8, 133.6, 132.4, 130.5, 123.4, 121.9, 119.1, 115.0, 104.5, 103.4, 99.8, 69.2, 51.5, 45.2; 19F NMR (CDCl3, 376 MHz): δ -118.27 (s, 1F); ESIMS: m/z 538 (M+Na)+. Anal. Cald. for C27H26FN7O3: C, 62.80; H, 4.65; N, 18.78%; Found: C, 62.78; H, 4.62; N, 18.80%.
5-Fluoro-N4-(3-(3-methyl-4-nitrobenzyl-amino)phenyl)-N2-(4-morpholinophenyl)-pyrimidine-2,4-diamine (7g)
Color less solid (0.75 g, 87%) m.p. 217-219ºC; 1H NMR (CDCl3, 400 MHz): δ 8.22 (brs, 1H), 8.14 (brs, 1H), 7.50-7.42 (m, 3H), 7.36 (d, 2H, J=8.0 Hz), 7.32 (s, 1H), 7.30-7.27 (m, 3H), 7.04 (t, 1H, J= 8.0 Hz), 6.90 (d, 2H, J=8.20 Hz), 6.28 (brs, 1H), 4.40 (d, 2H, J=5.60 Hz), 3.72 (t, 4H, J=4.52 Hz), 3.28 (t, 4H, J=4.80 Hz), 2.19 (s, 3H); 13C NMR (CDCl3, 75 MHz): δ 164.3, 155.0, 149.8, 148.4, 147.5, 143.7, 142.2, 139.6, 132.2, 132.0, 130.4, 129.8, 124.9, 123.6, 119.0, 114.8, 104.3, 103.2, 99.7, 69.1, 51.3, 45.0, 19.8; 19F NMR (CDCl3, 376 MHz): δ -118.28 (s, 1F); ESIMS: m/z 552 (M+Na)+. Anal. Cald. for C28H28FN7O3: C, 62.84; H, 4.68; N, 20.50%; Found: C, 62.86; H, 4.65; N, 20.48%.
5-Fluoro-N4-(3-(4-fluoro-2-nitrobenzyl-amino)phenyl)-N2-(4-morpholinophenyl)pyri-midine-2,4-diamine (7h)
White solid (0.69 g, 85%), m.p. 280-282ºC; 1H NMR (CDCl3, 400 MHz): δ 8.23 (brs, 1H), 8.19 (brs, 1H), 7.59-7.48 (m, 3H), 7.40 (s, 1H), 7.36 (d, 2H, J=8.56 Hz), 7.30-7.22 (m, 3H), 7.14 (t, 1H, J= 10.0 Hz), 6.98 (d, 2H, J=8.0 Hz), 6.28 (brs, 1H), 4.46 (d, 2H, J=5.74 Hz), 3.85 (t, 4H, J=4.80 Hz), 3.32 (t, 4H, J=4.46 Hz); 13C NMR (CDCl3, 75 MHz): δ 164.5, 162.0, 155.5, 151.3, 148.9, 143.8, 139.6, 132.5, 132.3, 130.0, 129.4, 121.7, 121.5, 119.2, 115.1, 111.2, 104.5, 103.6, 99.9, 69.4, 51.8, 45.5; 19F NMR (CDCl3, 376 MHz): δ -118.31 (s, 1F); ESIMS: m/z 556 (M+Na)+. Anal. Cald. for C27H25F2N7O3: C, 60.76; H, 3.82; N, 18.56%; Found: C, 60.78; H, 3.84; N, 18.54%.
2-((3-(5-Fluoro-2-(4-morpholinophenyl-amino)pyrimidin-4-ylamino)phenylamino)-methyl)-4-nitrophenol (7i)
Light brown solid (0.59 g, 86%), m.p. 210-212ºC; 1H NMR (CDCl3, 400 MHz): δ 10.48 (brs, 1H), 8.26 (brs, 1H), 8.20 (brs, 1H), 7.62-7.58 (m, 3H), 7.48 (d, 2H, J=10.0 Hz), 7.42 (s, 1H), 7.38-7.29 (m, 4H), 7.08 (t, 1H, J=8.0 Hz), 6.94 (d, 2H, J=8.0 Hz), 6.28 (brs, 1H), 4.46 (d, 2H, J=5.68 Hz), 3.82 (t, 4H, J=4.80 Hz), 3.38 (t, 4H, J=4.50 Hz); 13C NMR (CDCl3, 75 MHz): δ 165.2, 160.5, 155.3, 151.6, 148.5, 143.9, 140.5, 139.7, 132.6, 132.3, 130.4, 129.8, 124.7, 123.5, 119.4, 116.7, 115.3, 104.8, 103.5, 99.8, 69.5, 51.8, 45.6; 19F NMR (CDCl3, 376 MHz): δ -118.27 (s, 1F); ESIMS: m/z 554 (M+Na)+. Anal. Cald. for C27H26FN7O4: C, 60.04; H, 3.96; N, 18.84%; Found: C, 60.07; H, 3.94; N, 18.86%.
5-Fluoro-N4-(3-(4-methoxybenzylamino)-phenyl) -N2-(4-morpholinophenyl)pyrimidine-2,4-diamine (7J)
Pale yellow solid (0.74 g, 78%), m.p. 218-220ºC; 1H NMR (CDCl3, 400 MHz): δ 8.21 (brs, 1H), 8.18 (brs, 1H), 7.50-7.48 (m, 3H), 7.45 (s, 1H), 7.38 (d, 2H, J=8.0 Hz), 7.34-7.28 (m, 4H), 7.06 (t, 1H, J= 10.0 Hz), 6.85 (d, 2H, J=8.0 Hz), 6.25 (brs, 1H), 4.38 (d, 2H, J=5.80 Hz), 3.90 (s, 3H), 3.70 (t, 4H, J=4.80 Hz), 3.20 (t, 4H, J=4.88 Hz); 13C NMR (CDCl3, 75 MHz): δ 164.5, 158.3, 155.2, 151.4, 148.6, 143.5, 139.7, 134.1, 132.5, 132.3, 130.6, 128.0, 118.8, 114.9, 114.3, 104.5, 103.3, 99.9, 69.2, 58.8, 46.2; 19F NMR (CDCl3, 376 MHz): δ -118.34 (s, 1F); ESIMS: m/z 523 (M+Na)+. Anal. Cald. for C28H29FN6O2: C, 68.42; H, 4.98; N, 16.86%; Found: C, 68.40; H, 4.96; N, 16.88%.
Biological Results
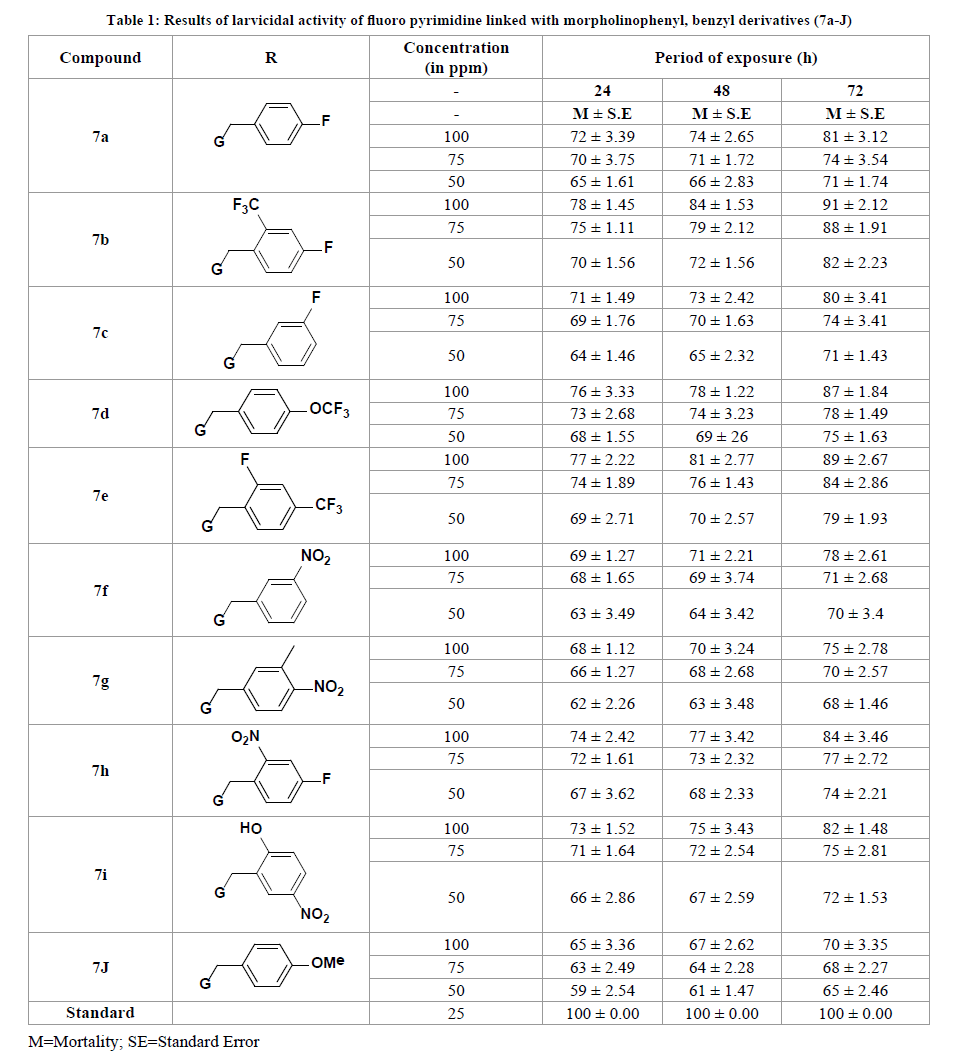
The primary means of controlling mosquito-borne diseases is by spraying insecticides. This led to insecticidal resistance and thus urged for the synthesis of new compounds having high potential activity. The compounds 7a-J synthesised in this protocol have proved to be promising agents showing impressive larvicidal activity. Mortality of larvae varied with different concentrations of compounds. The mortality of third instar larvae is indicated in Table 1. Among all the newly synthesized compounds, 7b showed highest mortality (91 ± 2.12) and reached to standard value at 100 ppm concentration at 72 hrs exposures, followed by 7d, 7e and 7h compounds. Impressive mortality was observed for, 7a, 7c, 7f, 7g and 7i compounds. The values 65 ± 1.12, 67 ± 3.24, 70 ± 2.78 mortality rates were considered as a least at 100 ppm concentration in 24, 48 and 72 hrs exposure of compound 7g respectively. The Structure-Activity Relationship (SAR) of compounds 7a-J was determined using the data presented in Table 1. SAR studies revealed that the presence of a strong electron-withdrawing group on the benzyl ring increases the anti-larvicidal activity. In particular, compounds with fluoro groups in either position 2 or position 4 of the benzyl ring demonstrated an enhanced potency against different panels of microbial strains.
The mortality results were identified as mortality in % ± Standard error and p value less than 0.05 was considered as significant. Corrected mortality was calculated with the Abbott’s formula [26].
% of mortality=[Number of dead larvae/ Number of larva introduced] × 100 Corrected mortality=Observed mortality in treatment– observed mortality in control × 100/100–control mortality.
Biological Assay
Preparation of different concentrations of compound
One gram of each newly synthesized series of compounds 7a-J were taken in different tubes and mixed with 100 ml of distilled water to each tube and these were considered as the blank. Then different concentrations ranging from 50, 75 and 100 ppm were prepared from the above stock. In contrast, 25 ppm of Malathion was prepared and it was considered as standard. All chemicals and reagents used were of analytical grade and obtained from Sigma.
Squito rearing
The larva were collected from Godavari area of Rajahmundry and confirmed by entomologist, Hyderabad. These larval forms were placed in plastic and enamel tray having 500 mL tap water and these larvae were feed with diet composed of brewer’s yeast, dog biscuits and algal products in the ratio 3:1:1 respectively. Then the collected pupae were transferred into plastic bowl (12 × 12 cm) filled with water by taking the help of dipper. Later the plastic Jar was in mosquito cage (90 × 90 × 90 cm diameter) for adult’s emergence. All these larval forms were maintained certain conditions such as 27 ± 2ºC temperature, 75-85% relative humidity and 14:10 light and dark cycle. Before blood feeding, sugar solution (10% sucrose) was provided for 3 days. Adult female mosquitos were moved to feed on rabbit for blood (normally exposed site is dorsal side and 1 rabbit for day) continued up to 5 days for sufficient blood intake for development and growth. After completion of blood meal, that enamel water tray were moved to cage for oviposition. By following the method of Kamaraj et al. [27], all were maintained and reared in the lab.
Larvicidal bioassay
The larvicidal assay was done by the standard protocol of WHO with slight modifications [28]. Different concentrations (50, 75 and 100 ppm) of newly synthesized compounds (7a-J) were transferred in to glass petridish. In every batch 25 larvae of 3rd instar were transferred to the petri dish and 25 ppm concentration of Malathion was also transferred to petridish and exposed with larvae forms. Results were observed in every 24, 48, 72 hrs. The dead larvae were identified when they are failed to move after probing with needle in the siphon or cervical region. In this same way the experiment was carried out for 4 replicates simultaneously to all the concentrations. During the entire experimental period the larvae were unfed.
Conclusion
In conclusion, we have presented an efficient and benign synthesis of 5-fluoro benzyl amino pyrimidine systems containing morpholino-phenyl rings with excellent yields. All the synthesized compounds have been investigated for their larvicidal activity. From our newly synthesized compounds, it is seen that compounds 7b 7d, 7e and 7h have shown excellent larvicidal activity. The activity of compounds 7a, 7c, 7f, 7g and 7i was very impressive. Accordingly, this novel class of new 5-fluoro-(4- substitutedbenzylamino)-(4-morpho-linophenyl) pyrimidine derivatives emerged as a valuable lead series with great potential as larvicidal agents.
Acknowledgements
The authors are thankful to the authorities of the UoN and CSIR-UGC New Delhi for providing facilities.
References
[1] C.F. Curtis, N. Pasteur, Geneva: World Health Organization Mineographed Document Series. 1980, 782.
[2] E. Antonio, C. Alssio, B. Alexander, A. Anna, M. Andera, J. Nat. Prod., 2008, 71, 1897.
[3] T. Yang, L. Liang, F. Guiming, S. Zhong, G. Ding, R. Xu, G. Zhu, N. Shi, F. Fan, Q. Liq, J. Vector. Ecol., 2009, 34, 148.
[4] N. JirakanJanakit, P. Rongnoparut, S. Saengtharatip, T. Chareonviriyaphap, S. Duchon, C. Bellec, S. Yoksan, J. Econ. Ento., 2007, 100, 545.
[5] M. Sarwar, N. Ahmad, M. Toufiq, Pak. J. Bot., 2009, 41, 3047.
[6] A.P. Piccionello, R. Musumeci, C. Cocuzza, C.G. Fortuna, A. Guarcello, P. Pierro, A. Pace, Eur. J. Med. Chem., 2012, 50, 441.
[7] G. Zhou, P.C. Ting, R. Aslanian, J. Cao, D.W. Kim, R. Kuang, J.F. Lee, J. Schwerdt, H. Wu, R.J. Herr, A.J. Zych, J. Yang, S. Lam, S. Wainhaus, T.A. Black, P.M. McNicholas, Y. Xu, S.S. Walker, Bioorg. Med. Chem. Lett., 2011, 21, 2890.
[8] C.R. Prakash, S. RaJa, G. Saravanan, Chem. Biol. Drug. Design., 2012, 80, 524.
[9] R.S. Joshi, P.G. Mandhane, S.D. Diwakar, S.K. Dabhade, C.H. Gill, Bioorg. Med. chem. Lett., 2010, 20, 3721.
[10] N. Bouloc, J.M. Large, M. Kosmopoulou, C. Sun, A. Faisal, M. Matteucci, J. Reynisson, N. Brown, B. Atrash, J. Blagg, E. McDonald, S. Linardopoulos, R. Bayliss, V. Bavetsias, Bioorg. Med. Chem. Lett., 2010, 20, 5988.
[11] A.M. Boguszewska, M. Krawczyk, A. NaJda, K. Kopanska, A. Stankiewicz-Drogon, W. Zagorski-OstoJa, M. Bretner, Biochem. Biophy. Res. Comm., 2006, 341, 641.
[12] K.R. Babu, K.V. Rao, Y.N. Kumar, K. Polireddy, K.V. Subbaiah, M. Bhaskar, V. Lokanatha, C.N. RaJu, Antivir. Res., 2012, 95, 118.
[13] S. Maddila, A.S. Kumar, S. Gorle, M. Singh, P. Lavanya, S.B. Jonnalagadda, Lett. Drug. Design. Discov., 2013, 10, 186.
[14] X. Deng, A. Nagle, T. Wuc, T. Sakata, K. Henson, Z. Chen, K. Kuhen, D. Plouffe, E. Winzeler, F. Adrain, T. Tuntland, J. Chang, S. Simerson, S. Howard, J. Ek, J. Isbell, D.C. Tully, A.K. ChatterJee, N.S. Gray, Bioorg. Med. Chem. Lett., 2010, 20, 4027.
[15] L.P. Guan, X. Sui, Y. Chang, Z.A. Yan, G.Z. Tong, Y.L. Qu, Med. Chem., 2012, 8, 1076.
[16] T. Mohamed, J.C.K. Yeung, M.S. Vasefi, M.A. Beazely, P.P.N. Rao, Bioorg. Med. Chem. Lett., 2012, 22, 4707.
[17] J.Y. Kim, D. Kim, S.Y. Kang, W.K. Park, H.J. Kim, M.E. Jung, E.J. Son, A.N. Pae, J. Kim, J. Lee, J. Bioorg. Med. Chem. Lett., 2010, 20, 6439.
[18] S. Maddila, P. Lavanya, S.B. Jonnalagadda, C.V. Chunduri, Chem. J., 2012, 23, 124.
[19] D. Rai, M. Johar, T. Manning, B. Agrawal, D.Y. Kunimoto, R. Kumar, J. Med. Chem., 2005, 48, 7012.
[20] M.S. Mohamed, R. Kamel, S.S. Fatahala, Eur. J. Med. Chem., 2010, 45, 2994.
[21] O. Bruno, C. Brullo, S. Schenone, A. Ranise, F. Bondavalli, E. Barocelli, M. Tognolini, F. Magnanini, V. Ballabeni, II Farmaco., 2002, 57, 753.
[22] S. Maddila, P. Lavanya, S.B. Jonnalagadda, C.V. Rao, Asian. J. Chem., 2013, 25, 385.
[23] A. Kamal, D. Dastagiti, M.J. Ramaiah, S. Reddy, E.V. Bharathi, M.K. Reddy, M.V.P. Sagar, T.L. Reddy, S.N.C.V.L. Pushpavalli, M. Pal-Bhadra, Eur. J. Med. Chem., 2011, 46, 5817.
[24] V. Guagnano, P. Furet, C. Spanka, V. Bordas, M. Le Douget, C. Stamm, J. Bruggen, M.R. Jensen, C. Schnell, H. Schmid, M. Wartmann, J. Berghausen, P. Druckes, A. Zimmerlin, D. Bussiere, J. Murray, D.J. Porta, J. Med. Chem., 2011, 54, 7066.
[25] M.S. Novikov, R.W. Buckheit Jr, K. Temburnikar, A.L. Khandazhinskaya, A.V. Ivanov, K.L. Seley-Radtke, Bioorg. Med. Chem., 2010, 18, 8310.
[26] World Health Organization, WHO/VBC., 1981, 81, 807.
[27] C. Kamaraj, A.A. Rahuman, A. Bagavan, A. Paras. Res., 2008, 103, 325.
[28] W.S. Abbott, J. Econ. Entom., 1925, 18, 265-267.



