Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Synthesis and Biological Activity Studies of Novel Arylazo Pyrazole Derivatives
Revanasiddappa BC* and Jose N
Department of Pharmaceutical Chemistry, NGSM Institute of Pharmaceutical Sciences of Nitte (Deemed to be University), Paneer, Deralakatte, Mangalore-575 018, Karnataka, India
- *Corresponding Author:
- Revanasiddappa BC
Department of Pharmaceutical Chemistry
NGSM Institute of Pharmaceutical Sciences of Nitte (Deemed to be University)
Paneer, Deralakatte, Mangalore-575 018, Karnataka, India
Abstract
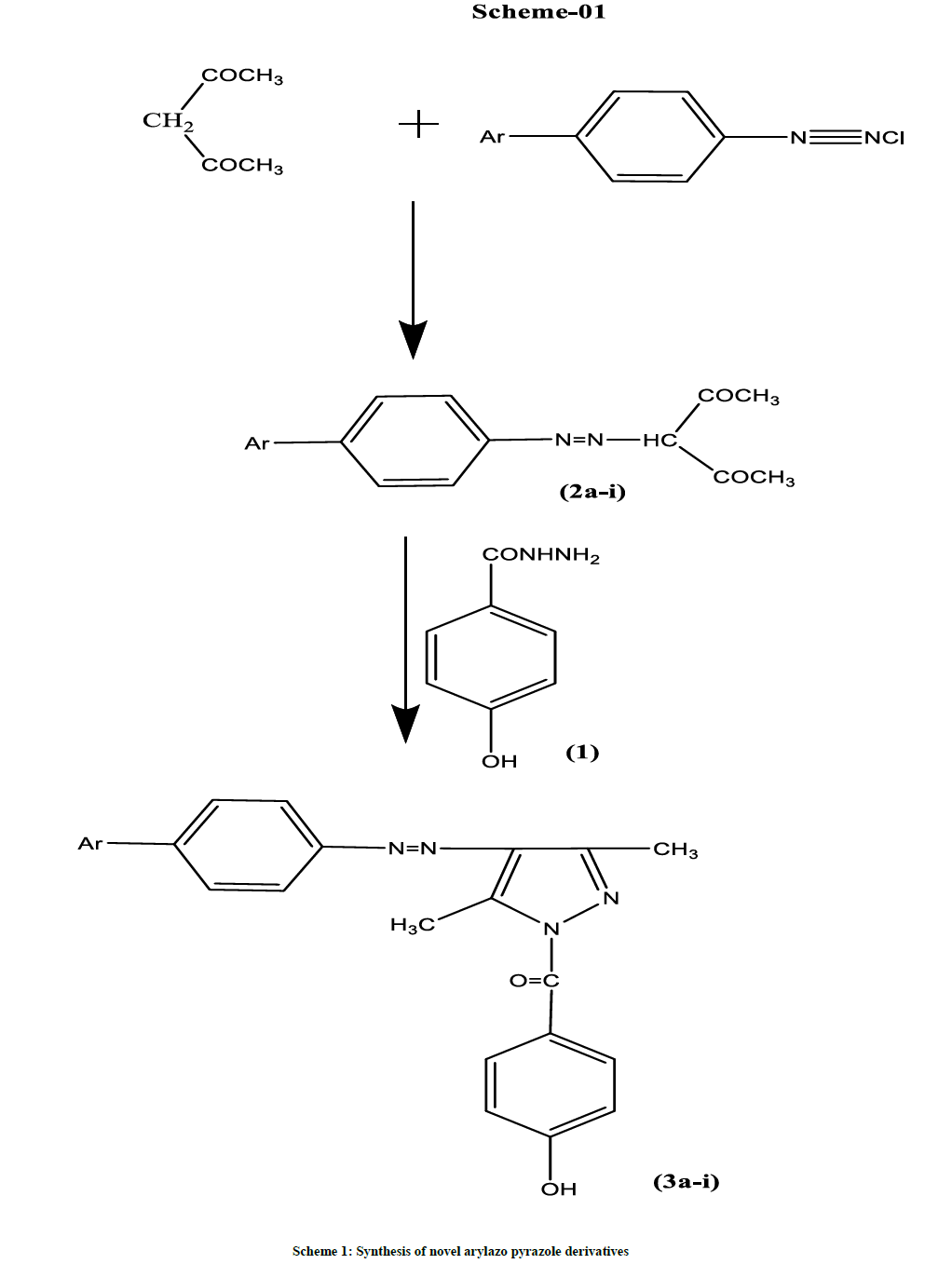
A series of novel 3,5-dimethyl arylazo pyrazoles (3a-i) were synthesized by reacting various oxybutyrates with methyl paraben (1) in glacial acetic acid medium. The key intermediate oxybutyrates (2a-i) were prepared from substituted phenyl diazonium salts and acetyl acetone in alcohol medium. The structures of the synthesized compounds have been established on the basis of spectral data (IR, 1H-NMR, Mass). The antibacterial and antifungal activity of the compounds has also been evaluated. Few of them have shown significant antifungal activity when compared to standard Flucanazole.
Keywords
Pyrazole, Arylazo, Antimicrobial, Antifungal.
Introduction
Antipyrine is one of the first drug which is composed of Pyrazole moiety and later it attracted many researchers. Pyrazoles are more commonly known as 1, 2-diazole has become a popular topic due to its diversified activities. Pyrazoles are basically 5-membered heterocyclic class of compounds containing nitrogen as one of the hetero atom.
At present so many Pyrazole drugs are available in the market such as Celecoxib is potent COX-2 inhibitor, Pyrazofurin is potential of antiviral activity, Sulfinpyrazone is used for chronic gout, Metamizole/Dipyrone is used as analgesic and antipyretic. Other examples of pyrazole derivatives as NSAID are Ramifenazone, Lonazolac, Rimonabant. Aminopyrine or Aminophenazone are still used as anti-inflammatory, antipyretic, and analgesic agents. The Pyrazole ring is found to present in leading available drugs such as Celebrex, Sildenafil (Viagra), and Difenamizole.
Pyrazole are associated with various biological activities like, antimicrobail [1], anticancer [2], antiviral [3], anti-inflammatory [4], antitubercular [5], antagonists of the CB1 receptor [6], enzyme inhibitor agents [7]. Diazonium salts are of great interest since a long time because they play a important role in the organic synthesis and are commercially important coloring agents [8,9]. Many of the azo compounds found importance in medicinal and pharmaceutical as dyes. The incorporation of azo groups/ dyes has been reported to enhance the biological activity of various heterocyclic compounds. The azo compounds are reported with antibacterial [10], optical activity [11], antifungal [12], and antioxidant activities [13]. In light of the above biological profile exhibited by the Pyrazoles and azo compounds, prompted us to synthesize a novel series of arylazo pyrazole derivatives and also for their biological activities.
Materials and Methods
Chemistry
IR spectra (in KBr pellets) were recorded on a Alpha Brucker IR Spectrometer. 1H-NMR spectra were recorded on Bruker Avance II 400 MHz NMR spectrometer using DMSO-d6 as solvent and TMS as internal standard. Mass spectra of the compounds were recorded with ESI. The purity of the compounds was checked by Thin Layer Chromatography (TLC) on precoated 0.2 mm Merck silica gel 60 plates.
Procedure for the synthesis of 3,5-dimethyl-arylazo pyrazoles (3a-i)
A mixture of oxybutyrates (2a-i) (0.01 mol) and methyl paraben (1) (0.01 mol) in glacial acetic acid (30 ml) was refluxed for 24-32 h. After cooling, the reaction mixture was poured over crushed ice. The resulting solid was filtered, washed with water and recrystallized from ethanol. Physico-chemical data of compounds (3a-i) are given in Table 1.
| Comp | Ar-NH2 | Molecular formula | Molecular weight | MP (°C) | Yield (%) |
|---|---|---|---|---|---|
| 3a | C6H5 | C18H16N4O2 | 320 | 110-112 | 75 |
| 3b | 4-NO2 | C18H15N5O4 | 365 | 130-132 | 72 |
| 3c | 4-Br | C18H15BrN4O2 | 399 | 90-92 | 68 |
| 3d | 2-F | C18H15FN4O2 | 338 | 80-82 | 68 |
| 3e | 2-Cl | C18H15ClN4O2 | 354 | 120-122 | 72 |
| 3f | 2-NO2 | C18H15N5O4 | 365 | 105-107 | 73 |
| 3g | 2-CH3 | C19H18N4O2 | 334 | 137-139 | 78 |
| 3h | 4-(CH3)2 | C20H21N5O2 | 363 | 142-144 | 62 |
| 3i | 2,4-(CH3)2 | C20H20N4O2 | 348 | 167-169 | 70 |
Table 1: Physical data of substituted arylazo pyrazole derivatives (3a-i)
3b: IR (KBr, cm-1): 1333, 1512 (NO2), 1598 (C=N), 1674 (C=O), 3074 (C-H), 3466 (OH).1H-NMR (CDCL3): 2.52 (s, CH3, 3H), 2.63 (s, CH3, 3H),), 7.69 - 8.27 (m, Ar-H, 8H), 13.96 (s, OH, 1H). MS (m/z):366 (M+1).
3c: IR (KBr, cm-1): 645 (C-Br), 1516 (C=C), 1622 (C=N), 1670 (C=O), 3052 (C-H), 3471 (OH).1H-NMR (CDCL3): 2.48 (s, 2xCH3, 6H), 7.44 - 8.02 (m, Ar-H, 8H), 14.31 (s, OH, 1H).
3d: IR (KBr, cm-1): 693 (C-F), 1518 (C=C), 1627 (C=N), 1677 (C=O), 2984 (C-H), 3470 (OH).1H-NMR (CDCL3): 2.48 (s, 2xCH3, 6H), 7.18 - 7.98 (m, Ar-H, 8H. MS (m/z):338 (M+).
3e: IR (KBr, cm-1): 747 (C-Cl), 1515 (C=C), 1585 (C=N), 1678 (C=O), 3008 (C-H), 3471 (OH). 1H-NMR (CDCL3): 2.49 (s, 2xCH3, 6H), 7.34 - 7.97 (m, Ar-H, 8H), 10.60 (s, OH, 1H). MS (m/z):355 (M+1).
Results and Discussion
Chemistry
The key intermediate oxybutyrates (2a-i) [14] were prepared from substituted phenyl diazonium salts and acetyl acetone in alcohol medium. The title compounds arylazo pyrazole derivatives (3a-i) were synthesized by reacting methyl paraben (1) with oxybutyrates in glacial acetic acid medium. The structures of the compounds (3a-i) have been confirmed by Infrared radiation (IR), Proton Nuclear Magnetic Resonance (1H-NMR), and Mass spectroscopy. The synthetic approach to the title compounds is outlined in Scheme 1. All the physical characteristics of the synthesized compounds are given in Table 1.
Antimicrobial activity
All the synthesized compounds (3a-i) were tested for their antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphyloccus aureus by Cup-Plate agar diffusion method [15] at 100 μg/ml. The fungicidal activity of the compounds was evaluated against Asperigillus flavus and Asperigillus fumigatus by using Cup-Plate agar diffusion method [15] at 100 μg/ml. Ciprofloxacin and Flucanazole were used as standard drugs for the comparison of antibacterial and antifungal activities respectively. Inhibitory activity was measured in mm as the diameter of the observed inhibition zones. The results are tabulated in Table 2.
| Compound | Diameter of zone of inhibition(mm) | |||||
|---|---|---|---|---|---|---|
| Bacillus subtilis | Escherichia coli | Pseudomonas aeruginosa | Staphyloccus aureus | Asperigillus flavus | Asperigillus fumigatus | |
| 3a | 8 | 8 | 8 | 8 | 18 | 13 |
| 3b | 8 | 8 | 8 | 8 | 12 | 24 |
| 3c | 8 | 8 | 8 | 8 | 19 | 24 |
| 3d | 12 | 8 | 13 | 12 | 19 | 13 |
| 3e | 12 | 8 | 8 | 12 | 12 | 24 |
| 3f | 12 | 10 | 10 | 11 | 13 | 22 |
| 3g | 12 | 13 | 13 | 12 | 14 | 18 |
| 3h | 12 | 13 | 13 | 13 | 15 | 17 |
| 3i | 11 | 13 | 13 | 13 | 13 | 18 |
| Ciprofloxacin | 24 | 23 | 24 | 23 | - | - |
| Fluconazole | - | - | - | - | 24 | 23 |
Table 2: Data of antimicrobial activity of substituted arylazo pyrazole derivatives (3a-i)
Conclusion
Antibacterial screening revealed that the compounds showed weak to moderate activity against all the pathogenic organisms. Compounds 3a, 3b, 3c, 3d, 3e showed weak activity against E. coli. Compound 3g, 3h, 3i displayed moderate activity against all the four organisms. Among the tested compounds, none of the compounds was found to be potent when compared to standard drug Ciprofloxacin.
Antifungal screening revealed that, some of the tested compounds showed equal potent activity against A. fumigatus, when compared to standard Fluconazole. Compounds 3b, 3c, 3e showed good activity against A. fumigatus and found to be potent antifungal agents when compared to standard. Compounds 3a, 3c, 3d showed good activity against A. flavus. Remaining compounds have shown moderate activity against A. flavus
Acknowledgement
Authors are thankful to NGSM Institute of Pharmaceutical Sciences, Nitte University, Mangalore for providing research facilities. Dr. Avtar Singh, Sophisticated Instrument Analytical Facility, Punjab University, Chandigarh for providing NMR spectral data. The authors also thank Oxygen Health Care Research Private Ltd, Ahmedabad for providing mass spectral data.
References
- F. Abrigach, B. Bouchal, O. Riant, Y. Mace, A. Takfaoui, Med. Chem., 2016, 12, 83-89.
- A.R. Ali, E.R. El-Bendary, M.A. Ghaly, I.A. Shehata, Eur. J. Med. Chem., 2014, 75, 492-500.
- A.E. Rashad, M.I. Hegab, R.E. Abdel-Megeid, J.A. Micky, F.M. Abdel-Megeid, Bioorg. Med. Chem., 2008, 16, 7102-7106.
- A.K. Tewari, V.P. Singh, P. Yadav, G. Gupta, A. Singh, R.K. Goel, Bioorg. Chem., 2014, 56, 8-15.
- V. Pathak, H.K. Maurya, S. Sharma, K.K. Srivastava, A. Gupta, Bioorg. Med. Chem. Lett., 24, 2014, 2892-2896.
- A. Fulp, Y. Zhang, K. Bortoff, H. Seltzman, R. Snyder, R. Wiethe, G. Amato, R. Maitra, Bioorg. Med. Chem., 24, 2016, 1063-1070.
- T. Harit, F. Malek, B.E. Bali, A. Khan, K. Dalvandi, Med. Chem. Res., 21, 2011, 2772- 2778.
- R. Kasımoğulları, M. Bülbül, B.S. Arslan, B. Gökçe, Eur. J. Med. Chem., 45, 2010, 4769-4773.
- R. Kumar, Y.C. Joshi, J. Serb. Chem. Soc., 73, 2008, 937-943.
- I.M. Awad, A.A. Aly, A.M. Abdel Alim, R.A. Abde, S. H. Ahmed, J. Inorg. Biochem., 33(2), 1988, 77-89.
- S. Wang, S. Shen, H. Xu, Dyes Pigments., 44(3), 2000, 195-198.
- K.R. Raghavendra, K. Ajay Kumar, Int. J. ChemTech Res., 5(2), 2013, 1756-1760.
- A.A.H. Kadham, A.A. Al-Amiery, A.Y. Musa, A.B. Mohamad, Int. J. Mol. Sci., 12, 2011, 5747-5761.
- B.C. Revanasiddappa, S. Susan Varghese, J. Kalsi, M.S. Jisha, N. Jose, Ind. J. Het. Chem., 23,2013, 135-138.
- R. Cruichshank, J.P. Duguid, B.P. Marmoin, H.A. Swan, The Practice of Medical Microbiology, 12th (Edn.), Churchill Livingstone, London, 1975, 2, 190.