Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 10
Synthesis and Biological Activity of Cycloocta[b]pyridine Derivatives
Mahmoud NM Yousif* and Nabil M. YousifMahmoud NM Yousif, Photochemistry Department, Chemical Industries Research Division, National Research Centre, Cairo, Egypt, Email: mahmoud_nabil18@yahoo.com
Abstract
Cyclooctanone reacts with arylidene malononitrile to afford cycloocta[b]pyridine-3-carbonitrile derivatives 2a-b. 2-Amino-4-(4-chlorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine-3-carbonitrile 2a reacts with benzoyl chloride and acetic anhydride to afford compounds 3 and 4 respectively. N-(4-(4-Chlorophenyl)-3-cyano-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-yl)benzamide 3 and N-(4-(4-Chlorophenyl)-3-cyano-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-yl)acetamide 4 reacts with hydrazine hydrate to afford compounds 5a,b. 5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5a and 5-(4-Chlorophenyl)-4-imino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5b reacts with D-glucose and D-ribose to produce compounds 6a-d. Compounds 6a,b react with acetic anhydride to afford acetylated derivative 7a,b. Anticancer profile of the prepared compounds were tested against three cell lines namely A-549, CaCo-2, and HT-29.
Keywords
Cycloacta[b]pyridine; cycloocta[5,6]pyrido[2,3-d]pyrimidine; synthesis
Introduction
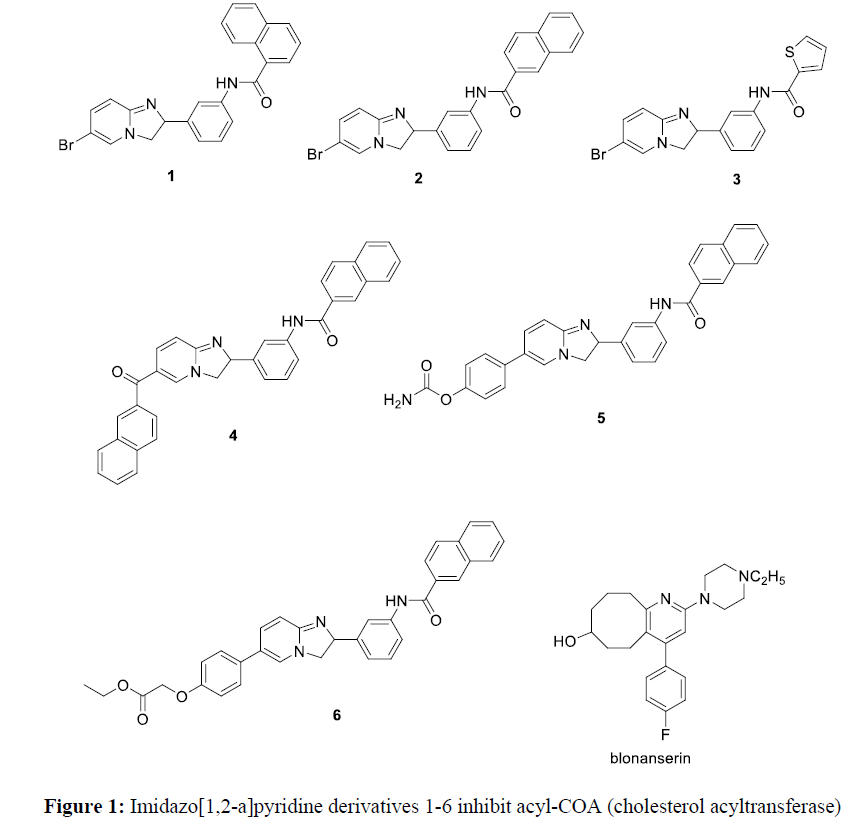
Pyridine derivatives have attracted many researchers due to its biological importance. They have antimicrobial profileagainst gram negative bacteria, gram positive bacteria, and Escherichia Coli [1]. Pyridine derivatives have herbicidal activity against against T. procumbens, E. indica, C. argentia, E. hirta, E. crusgalli, C. rotundus, and C. dactylon [1]. Also, pyridine derivatives has antifungal activity, antiviral activity, antioxidant activity, antidiabetic and anticancer activity. In addition, pyridine derivatives have antimalarial, analgesic activity, antiamoebic activity. Pyridine derivatives containing benzimidazole moiety have gastric H+/K+-ATPase inhibitory activity [1]. Imidazo[1,2-a]pyridine derivatives 1-6 inhibit acyl-COA (cholesterol acyltransferase) [1] (Figure 1).
Cycloocta[b]pyridine derivatives have many applications. The 4-arylcycloocta [b]pyridine is the main skeleton of antipsychotic drug blonanserin which is used in the treatment of schizophrenia [2].
All the above mentioned information and as a continuation of our previous work [3-19] directed us to synthesize novel cycloocta [b]pyridine derivatives for biological evaluation.
Results and Discussion
Chemistry
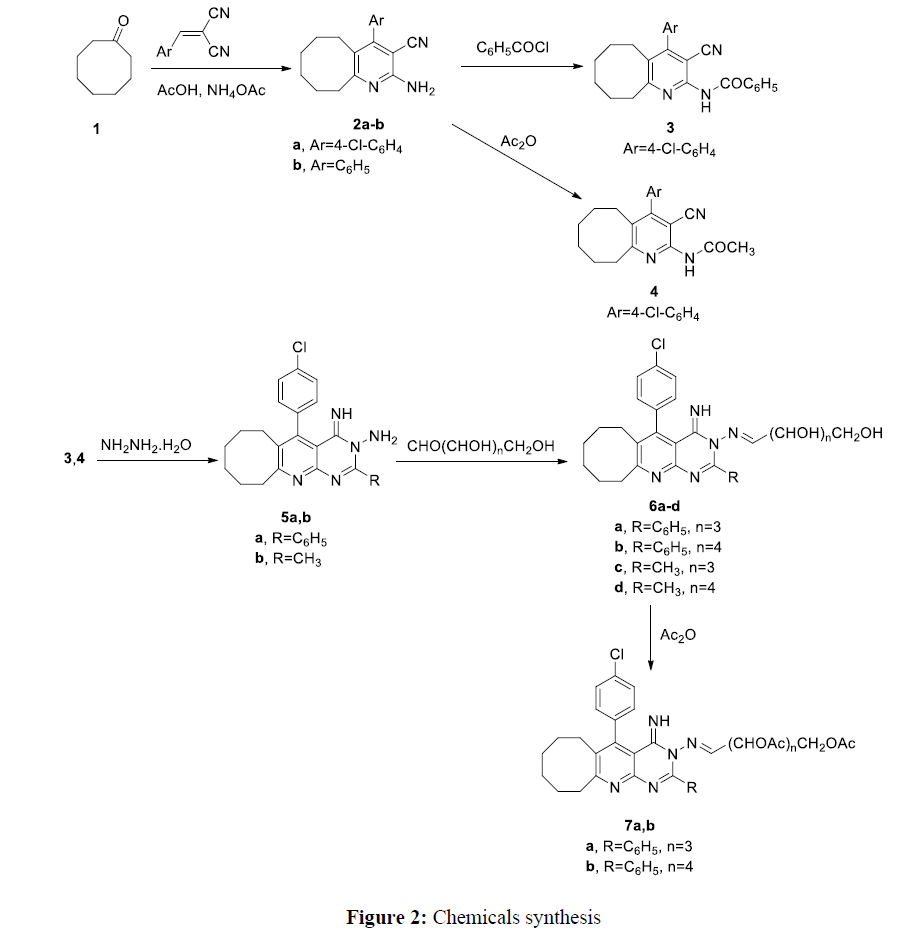
Cyclooctanone reacts with arylidene malononitrile to afford cycloocta[b]pyridine-3-carbonitrile derivatives 2a-b. Compound 2b was prepared according to different method than reported [20, 21]. Spectral data (mass, IR, 1H NMR) are in agreement with the proposed structures. Compound 2a shows appearance of absorption band for CN and NH2 group at 2235, and 3320 respectively in the IR spectrum. Compound 2a shows disappearance of absorption band for carbonyl group in the IR spectrum. The 1H NMR of compound 2a show chemical shifts at 7.40 and 7.60. corresponding to aromatic protons.
Compound 2a reacts with benzoyl chloride and acetic anhydride to afford compounds 3 and 4 respectively. The structures of compounds 3, and 4 were elucidated from IR, mass, 1H NMR spectral data. The IR of compounds 3, and 4 show absorption band of carbonyl group at 1710 and 1720 cm-1 respectively. The 1H NMR of compound 4 shows chemical shift at 2.10 (singlet) corresponding to CH3. The 13C NMR of compounds 3, and 4 show chemical shift (δ) at 165.4 and 171.2 corresponding to carbonyl group. Compounds 3 and 4 reacts with hydrazine hydrate to afford compounds 5a,b. Spectral data (IR, mass spectra, 1H NMR) of compounds 5a,b are in agreement with the suggested structures. The IR of compounds 5a,b show disappearance of absorption band for cyano group and carbonyl group. Mass spectra of compounds 5a,b show molecular ion peak at m/z 429.9 and 367.8. 5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5a and 5-(4-Chlorophenyl)-4-imino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5b reacts with D-glucose and D-ribose to produce compounds 6a-d. Compounds 6a,b react with acetic anhydride to afford acetylated derivative 7a,b. The structures of compounds 6a-d and 7a,b were confirmed by spectral data (mass spectra, IR, 1H NMR). Compounds 6a-d show absorption band for hydroxyl group in the IR spectra. The 1H NMR of compound 6a show chemical shift at 7.80 ppm corresponding to function group CH=N. Compound 7a shows absorption band for carbonyl group in the IR spectrum. The IR of compound 7a shows disappearance of absorption band for hydroxyl group. The 1H NMR of compound 7a shows chemical shift at 2.16 (singlet) corresponding to methyl group of acetyl function group (Figure 2)
Anticancer evaluation
Anticancer activity of prepared compounds was done against three tumor cell lines (adenocarcinomic human alveolar basal epithelial cells A-549, human epithelial colorectal adenocarcinoma cells CaCo-2, and human colorectal adenocarcinoma cell line HT-29) using (3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide assay [22]. The results are shown in Table 1 as anticancer activity of prepared compounds at 100 μM on three cell lines. The results exhibits that compound 7a have highest activity toward A-549 cell lines. Compounds 5a, 6d, 7a have medium activity towards A-549 cell lines as compared with doxorubicin. Compounds 2a, b, 3, 4, 6a, b have weak activity toward A-549 cell lines as compared with doxorubicin. Compound 2a has highest activity toward CaCo-2 cell lines as compared with doxorubicin. Compounds 5a, 7a have medium activity toward CaCo-2 cell lines as compared with doxorubicin. Compounds 5a, 6c, d, 7a show weak activity toward CaCo-2 cell lines as compared with doxorubicin. Compound 2a shows highest activity towards HT-29 cell lines as compared with doxorubicin. Compounds 2b, 4, 5a, b, 6a-d, 7a,b have weak activity towards HT-29 cell lines against doxorubicin. (Table 1)
| Compound No. | A-549 | CaCo-2 | HT-29 |
|---|---|---|---|
| 2a | 3.3 | 92.3 | 92.4 |
| 2b | 15.6 | 0 | 35.1 |
| 3 | 19.3 | 0 | 0 |
| 4 | 29.4 | 0.9 | 12.0 |
| 5a | 50.4 | 25.6 | 25.4 |
| 5b | 0 | 0 | 15.1 |
| 6a | 25.0 | 0 | 13.3 |
| 6b | 17.2 | 0 | 18.3 |
| 6c | 25.3 | 5.2 | 26.9 |
| 6d | 45.9 | 17.5 | 31.5 |
| 7a | 47.8 | 7.9 | 32.9 |
| 7b | 88.5 | 71 | 8.7 |
| Doxorubicin | 1oo | 100 | 100 |
p ≤ 0.01, n = 3
From the results, we can conclude structure activity relationship. Acetylated sugar in compound 7a enhances greatly the anticancer activity towards adenocarcinomic human alveolar basal epithelial cells A-549. Presence of sugar moiety linked to pyrimidine ring in compound 6d and presence of pyrimidine ring in compound 5a give medium activity toward A-549 cell lines. Presence of amino cyano function group in compounds 2a,b and presence of benzoyl and acetyl group linked to amino cyano function group in compounds 3, and 4 give weak activity towards A-549 cell lines. Presecne of sugar moiety linked to pyrimidine ring in compound 6a, b give weak activity towards A-549 cell lines. Amino cyano function group in compounds 2a, b give high activity towards CaCo-2 cell lines. Presence of 2-phenyl-diaminopyrimidine ring in compound 5a and acetylated sugar linked to 2-phenyl-pyrimidine ring in compound 7a make medium activity towards CaCo-2 cell lines. Sugar moiety linked to diamino-pyrimidine ring in compounds 6c, d make weak activity toward CaCo-2 cell lines. Amino cyano function group linked to pyridine ring in compound 2a give high activity toward HT-29 cell lines.
Experimental
The instruments used were as previously reported paper [22].
General method for preparation of compounds 2a,b
A mixture of arylidene malononitrile (0.01 mole), cyclooctanone (0.01 mole), 4 gm anhydrous ammonium acetate in 20 mL acetic acid are heated under reflux for 3 hours. After cooling the reaction mixture to room temperature, the reaction mixture is poured into cold water. The formed solid collected and crystallized from ethanol to give compounds 2a,b.
2-Amino-4-(4-chlorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine-3-carbonitrile 2a
Yield: 65%; m.p. 240-242 °C; IR (KBr) cm-1, ν: 3320 (NH2), 2235 (CN); 1H NMR (DMSO) δ/ppm: 1.20 (t, 2H, J =7.1 Hz, CH2), 1.25 (m, 2H, CH2), 1.60 (m, 2H, CH2), 2.30 (m, 2H, CH2), 2.70 (m, 2H, CH2), 3.40 (t, 2H, J =7.1 Hz, CH2), 6.80 (brs, 2H, NH2), 7.40 (d, 2 H, J =7.5 Hz, Ar), 7.60 (d, 2H, J =7.5 Hz, Ar). 13C NMR (DMSO) δ/ppm: 22.3, 24.2, 26.3, 28.5, 29.2, 29.6 (6 CH2), 115.1 (CN), 120.2, 121.4, 123.1, 125.2, 125.9, 130.7, 132.6, 135.2, 137.9, 152.3, 155.3 (11 C=). MS (m/z): 311.8 (M+, 31%). Anal. Calcd. for C18H18ClN3: C, 69.34; H, 5.82; N, 13.48; Found: C, 69.39; H, 5.90; N, 13.53.
2-Amino-4-(phenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine-3-carbonitrile 2b
Yield: 70%; m.p. 225-227 °C; IR (KBr) cm-1, ν: 3330 (NH2), 2250 (CN); 1H NMR (DMSO) δ/ppm: 1.25 (t, 2H, J =7.1 Hz, CH2), 1.29 (m, 2H, CH2), 1.71 (m, 2H, CH2), 2.42 (m, 2H, CH2), 2.76 (m, 2H, CH2), 3.21 (t, 2H, J =7.1 Hz, CH2), 6.72 (brs, 2H, NH2), 7.30 (d, 2 H, J =7.5 Hz, Ar), 7.51 (d, 2H, J =7.5 Hz, Ar). 13C NMR (DMSO) δ/ppm: 21.10, 25.12, 27.15, 28.10, 28.90, 29.10 (6 CH2), 112.28 (CN), 121.1, 122.7, 123.2, 123.6, 125.8, 128.3, 129.2, 130.7, 132.7, 135.1, 136.8 (11 C=). MS (m/z): 277.3 (M+, 41%). Anal. Calcd. for C18H19N3: C, 77.95; H, 6.90; N, 15.15; Found: C, 78.04; H, 6.98; N, 15.19.
N-(4-(4-Chlorophenyl)-3-cyano-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-yl)benzamide 3
A mixture of compound 2a (0.01 mole) and benzoyl chloride (0.01 mole) in 20 mL pyridine was refluxed for 3 hours. Then, the reaction mixture is acidified with 10 % HCl. The precipitate formed was collected, dried a recrystallized from ethanol to give compound 3.
Yield: 65%; m.p. 105-107 °C; IR (KBr) cm-1, ν: 3350 (NH), 2230 (CN), 1710 (C=O); 1H NMR (DMSO) δ/ppm: 1.20 (t, 2H, J =7.1 Hz, CH2), 1.30 (m, 2H, CH2), 1.40 (brs, 1H, NH), 1.70 (m, 2H, CH2), 2.20 (m, 2H, CH2), 2.60 (m, 2H, CH2), 3.00 (t, 2H, J =7.1 Hz, CH2), 7.40-7.60 (m, 9H, Ar). 13C NMR (DMSO) δ/ppm: 21.1, 23.2, 24.1, 25.4, 26.7, 27.9 (6 CH2), 118.5 (CN), 125.1, 125.9, 127.1, 128.2, 128.9, 129.3, 129.9, 130.1, 130.8, 132.4, 135.1, 136.8, 137.4, 138.2, 140.2, 143.7, 145.9 (17 C=), 165.4 (C=O). MS (m/z): 415.9.7 (M+, 51%). Anal. Calcd. for C25H22ClN3O: C, 72.20; H, 5.33; N, 10.10; Found: C, 72.28; H, 5.39; N, 10.18.
N-(4-(4-Chlorophenyl)-3-cyano-5,6,7,8,9,10-hexahydrocycloocta[b]pyridin-2-yl)acetamide 4
A mixture of compound 2a (0.01 mole) and 20 mL acetic anhydride is heated under reflux for 2 hours. The reaction mixture is poured into cold water. The formed solid collected and crystallized from ethanol to give compound 4.
Yield: 55%; m.p. 150-152 °C; IR (KBr) cm-1, ν: 3410 (NH), 2230 (CN), 1720 (C=O); 1H NMR (DMSO) δ/ppm: 1.25 (t, 2H, J =7.1 Hz, CH2), 1.34 (m, 2H, CH2), 1.52 (brs, 1H, NH), 1.78 (m, 2H, CH2), 2.10 (s, 3H, CH3), 2.60 (m, 2H, CH2), 3.05 (m, 2H, CH2), 3.20 (t, 2H, J =7.1 Hz, CH2), 7.34-7.53 (m, 4H, Ar). 13C NMR (DMSO) δ/ppm: 19.2 (CH3), 22.3, 23.9, 24.2, 26.1, 26.9, 29.1 (6 CH2), 116.1 (CN), 121.2, 122.3, 124.5, 125.1, 127.2, 129.1, 129.5, 131.2, 133.1, 151.2, 153.4 (11 C=), 171.2 (C=O). MS (m/z): 353.8 (M+, 33%). Anal. Calcd. for C20H20ClN3O: C, 67.89; H, 5.70; N, 11.88; Found: C, 67.93; H, 5.78; N, 11.93.
General method for preparation of compounds 5a,b
A mixture of compounds 3 and 4 (0.01 mole), and 50 mL ethanol containing 3 mL hydrazine hydrate was refluxed for 3 hours. The reaction mixture was cooled to room temperature. Then, the reaction mixture is poured to cold water. The formed solid is recrystallized from ethanol to give compounds 5a,b.
5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5a
Yield: 45%; m.p. 251-253 °C; IR (KBr) cm-1, ν: 3340, 3410 (NH, NH2); 1H NMR (DMSO) δ/ppm: 1.30 (t, 2H, J =7.1 Hz, CH2), 1.34 (m, 2H, CH2), 1.60 (m, 2H, CH2), 2.30 (m, 2H, CH2), 2.90 (m, 2H, CH2), 3.20 (t, 2H, J =7.1 Hz, CH2), 6.80 (brs, 3H, NH2, NH), 7.31-7.60 (m, 9H, Ar). 13C NMR (DMSO) δ/ppm: 22.5, 22.8, 24.5, 25.1, 25.9, 26.1 (6 CH2), 121.7, 122.7, 122.9, 124.1, 126.1, 126.8, 128.1, 128.9, 129.1, 130.3, 134.5, 137.5, 138.4, 138.9, 139.1, 142.4, 145.9, 147.3 (18 C=), 155.2 (C=N). MS (m/z): 429.9 (M+, 51%). Anal. Calcd. for C25H24ClN5: C, 69.84; H, 5.63; N, 16.29; Found: C, 69.89; H, 5.69; N, 16.34.
5-(4-Chlorophenyl)-4-imino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-amine 5b
Yield: 42%; m.p. 240-242 °C; IR (KBr) cm-1, ν: 3350, 3426 (NH, NH2); 1H NMR (DMSO) δ/ppm: 1.34 (t, 2H, J =7.1 Hz, CH2), 1.37 (m, 2H, CH2), 1.65 (m, 2H, CH2), 2.40 (m, 2H, CH2), 2.98 (m, 2H, CH2), 3.12 (s, 3H, CH3), 3.28 (t, 2H, J =7.1 Hz, CH2), 6.73 (brs, 3H, NH2, NH), 7.30 (d, 2H, J=7.5 Hz, Ar), 7.50 (d, 2H, J=7.5 Hz, Ar). 13C NMR (DMSO) δ/ppm: 20.3 (CH3), 21.3, 22.9, 24.6, 26.2, 26.1, 26.9 (6 CH2), 124.1, 126.2, 127.1, 129.3, 130.2, 133.1, 135.4, 137.9, 141.3, 145.4 (10 C=), 149.6, 153.7, 155.2 (3 C=N). MS (m/z): 367.8 (M+, 43%). Anal. Calcd. for C20H22ClN5: C, 65.30; H, 6.03; N, 19.04; Found: C, 65.38; H, 6.09; N, 19.09.
General method for preparation of compounds 6a-d
To a well stirred solution of compounds 5a,b (0.01 mole) in 50 mL ethanol containing 3 drops of glacial acetic acid, D-glucose and D-ribose (0.015 mole) dissolved in distilled water was added. The reaction mixture was headed under reflux for 5 hours, and then the half of the solvent was evaporated under reduced pressure. The precipitated solid was filtered, washed with water and recrystalized from ethanol to give compounds 6a-d.
5-((5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)pentane-1,2,3,4-tetraol 6a
Yield: 60%; m.p. 261-263 °C; IR (KBr) cm-1, ν: 3360 (NH), 3420 (OH); 1H NMR (DMSO) δ/ppm: 1.10 (t, 2H, J =7.1 Hz, CH2), 1.27 (m, 2H, CH2), 1.39 (m, 2H, CH2),1.60 (brs, 4H, OH), 1.61 (m, 2H, CH2), 1.80 (m, 2H, CH2), 2.12 (t, 2H, J =7.1 Hz, CH2), 3.50 (m, 5H, 3CHOH, CH2OH), 7.30 (brs, 1H, NH), 7.34-7.65 (m, 9 H, Ar), 7.80 (d, 1H, J=6.2 Hz, CH=N). 13C NMR (DMSO) δ/ppm: 21.2, 22.2, 23.1, 23.9, 24.1, 25.6 (6 CH2), 61.2, 63.2, 65.4, 70.1 (4 COH), 121.3 121.9, 122.1, 123.4, 124.1, 124.9, 126.1, 128.8, 129.1, 131.0, 132.9, 134.2, 135.8, 137.3, 138.2, 139.0 (16 C=), 153.1, 154.4, 156.1, 157.1 (4 C=N). MS (m/z): 562.07 (M+, 47%). Anal. Calcd. for C30H32ClN5O4: C, 64.11; H, 5.74; N, 12.46; Found: C, 64.18; H, 5.79; N, 12.52.
6-((5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)hexane-1,2,3,4,5-pentaol 6b
Yield: 55%; m.p. 256-258 °C; IR (KBr) cm-1, ν: 3410 (NH), 3530 (OH); 1H NMR (DMSO) δ/ppm: 1.24 (t, 2H, J =7.1 Hz, CH2), 1.40 (m, 2H, CH2), 1.61 (m, 2H, CH2), 1.75 (m, 2H, CH2), 1.90 (m, 2H, CH2), 2.10 (t, 2H, J =7.1 Hz, CH2), 2.50 (brs, 6H, 5OH, NH), 3.64 (m, 6 H, 4CHOH, CH2OH), 7.41-7.56 (m, 9H, Ar), 8.10 (d, 1H, J=6.2 Hz, CH=N). MS (m/z): 592.09 (M+, 51%). Anal. Calcd. for C31H34ClN5O5: C, 62.89; H, 5.79; N, 11.83; Found: C, 62.93; H, 5.84; N, 11.89.
5-((5-(4-Chlorophenyl)-4-imino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)pentane-1,2,3,4-tetraol 6c
Yield: 50%; m.p. 265-267 °C; IR (KBr) cm-1, ν: 3430 (NH), 3510 (OH); 1H NMR (DMSO) δ/ppm: 1.13 (t, 2H, J =7.1 Hz, CH2), 1.35 (m, 2H, CH2), 1.50 (m, 2H, CH2), 1.71 (m, 2H, CH2), 1.90 (m, 2H, CH2), 2.05 (t, 2H, J =7.1 Hz, CH2), 2.40 (s, 3H, CH3), 3.52 (brs, 5H, 4OH, NH), 3.79 (m, 5H, 3CHOH, CH2OH), 7.43 (d, 2H, J=7.5 Hz, Ar), 7.59 (d, 2H, J=7.5Hz, Ar), 8.25 (d, 1H, J=6.2 Hz, CH=N). MS (m/z): 500.0 (M+, 61%). Anal. Calcd. for C25H30ClN5O4: C, 60.06; H, 6.05; N, 14.01; Found: C, 60.13; H, 6.14; N, 14.09.
6-((5-(4-Chlorophenyl)-4-imino-2-methyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)hexane-1,2,3,4,5-pentaol 6d
Yield: 40 %; m.p. 270-272 °C; IR (KBr) cm-1, ν: 3390 (NH), 3510 (OH); 1H NMR (DMSO) δ/ppm: 1.23 (t, 2H, J =7.1 Hz, CH2), 1.40 (m, 2H, CH2), 1.62 (m, 2H, CH2), 1.81 (m, 2H, CH2), 1.92 (m, 2H, CH2), 2.03 (brs, 6H, 5OH, NH), 2.17 (t, 2H, J =7.1 Hz, CH2), 3.42 (m, 6H, 4CHOH, CH2OH), 7.42 (d, 2H, J =7.5 Hz, Ar), 7.57 (d, 2H, J=7.5 Hz, Ar), 8.31 (d, 1H, J=6.2 Hz, CH=N). MS (m/z): 530.02 (M+, 71%). Anal. Calcd. for C26H32ClN5O5: C, 58.92; H, 6.09; N, 13.21; Found: C, 59.01; H, 6.15; N, 13.28.
General method for preparation of compounds 7a,b
A solution of compound 6a,b (0.01 mole) in 15 ml acetic anhydride was heated under reflux for 4 hours. The reaction mixture was then cooled to room temperature and was poured into cold water. The formed solid was collected and crystallized from ethanol to give compounds 7a,b.
5-((5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)pentane-1,2,3,4-tetrayl tetraacetate 7a
Yield: 40 %; m.p. 150-152 °C; IR (KBr) cm-1, ν: 3410 (NH), 1740 (C=O); 1H NMR (DMSO) δ/ppm: 1.15 (t, 2H, J =7.1 Hz, CH2), 1.31 (m, 2H, CH2), 1.40 (m, 2H, CH2), 1.61 (m, 2H, CH2), 1.72 (m, 2H, CH2), 1.90 (t, 2H, J =7.1 Hz, CH2), 2.16 (s, 12H, 4CH3), 3.42 (m, 5H, 3CHOAc, CH2OAc), 4.21 (brs, 1H, NH), 7.32-7.51 (m, 9H, Ar), 8.21 (d, 1H, J=6.2 Hz, CH=N). 13C NMR (DMSO) δ/ppm: 20.1, 21.2, 21.9, 22.2 (4 CH3), 23.2, 24.1, 24.8, 26.2, 27.2, 28.6 (6 CH2), 120.1, 120.9, 122.3, 123.1, 124.6, 126.3, 128.1, 130.8, 132.2, 136.1, 137.0, 139.1, 141.4, 142.5, 143.1, 145.6 (16 C=), 153.1, 154.3, 155.9, 158.1 (4 C=N), 170.1 (4 C=O). MS (m/z): 730.22 (M+, 52%). Anal. Calcd. for C38H40ClN5O8: C, 62.50; H, 5.52; N, 9.59; Found: C, 62.58; H, 5.59; N, 9.64.
6-((5-(4-Chlorophenyl)-4-imino-2-phenyl-6,7,8,9,10,11-hexahydrocycloocta[5,6]pyrido[2,3-d]pyrimidin-3(4H)-yl)imino)hexane-1,2,3,4,5-pentayl pentaacetate 7b
Yield: 45%; m.p. 145-147 °C; IR (KBr) cm-1, ν: 3350 (NH), 1745 (C=O); 1H NMR (DMSO) δ/ppm: 1.23 (t, 2H, J =7.1 Hz, CH2), 1.41 (m, 2H, CH2), 1.53 (m, 2H, CH2), 1.73 (m, 2H, CH2), 1.91 (m, 2H, CH2), 2.16 (t, 2H, J=7.1 Hz, CH2), 2.34 (s, 15H, 5CH3), 3.72 (m, 6H, 4 CHOAc, CH2OAc), 5.14 (brs, 1H, NH), 7.32-7.56 (m, 9 H, Ar), 8.21 (d, 1H, J=6.2 Hz, CH=N). MS (m/z): 802.28 (M+, 33%). Anal. Calcd. for C41H44ClN5O10: C, 61.38; H, 5.53; N, 8.73; Found: C, 61.43; H, 5.61; N, 8.79.
Conclusion
Novel cycloocta[b]pyridine derivatives have been prepared and characterized. The anticancer activity of the prepared compounds was done in comparison of doxorubicin as reference drug. Several prepared compounds show good anticancer activity.
Conflict of interest
The authors confirm that no conflict of interest.
Funding
This paper received no funding.
Acknowledgement
The authors acknowledge National Research Centre.
References
- Ali Altaf A, Shahzad A, Gul Z et al., J drug des med chem. 2015, 1(1): p. 1-11.
- Maharani S and Kumar RR. Tetrahedron Letters. 2015, 56(1): p. 179-181.
- Mahmoud NM Yousif, Abdel-Rahman BA El-Gazzar, Hend N Hafez et al., Mini Rev Org Chem. 2021.
- Mahmoud NM Yousif. Mini Rev Org Chem. 2021.
- Mahmoud NM Yousif, Abdel-Rahman BA El-Gazzar and Mervat M El-Enany. Mini Rev Org Chem. 2021, 18(1): p. 43-54.
- Yousif MNM, Abdel-Rahman BA El-Gazzar, Fayed AA et al., J Appl Pharm Sci. 2020, 10(12): p. 35-43.
- Mahmoud NM Yousif, Hanan A Soliman, Makarem M Said et al., Russ J Gen Chem. 2020, 90(3): p. 460-469.
- Yousif MNM, Soliman HA, Said MM et al., Russ J Gen Chem. 2020, 90(4): p. 767.
- Ahmed A Fayed, Saleh A Bahashwan, Mahmoud NM Yousif et al., Russ J Gen Chem. 2019, 89(9): p. 1887-1895.
- Mahmoud NM Yousif , Ibrahim F Nassar, Nabil M Yousif et al., Russ J Gen Chem. 2019, 89(8): p. 1673–1682.
- Ahmed A Fayed, Mahmoud NM Yousif, Taha T Abdelgawad et al., Chem Heterocycl Compd, 2019, 55(8): p. 773–778.
- Mohamed TM Nemr , Mahmoud NM Yousif and Jan Barciszewski. Archiv Der Pharmazie, 2019, 352(80): p. 1-7.
- Mahmoud NM Yousif, Ahmed A Fayed and Nabil M Yousif. Egypt J Chem. 2019, 62(8): p. 1759-1766.
- Fayed AA, Bahashwan SA, Yousif MNM et al., Russ J Gen Chem. 2019, 89(6): p. 1209-1217.
- Mahmoud NM Yousif, Hoda AR Hussein, Nabil M Yousif et al., J Appl Pharm Sci. 2019, 9(1): p. 6-14.
- Mahmoud NM Yousif, Ahmed A Fayed and Nabil M Yousif. Der Pharma Chemica. 2018, 10(8): p. 105-109.
- Mahmoud NM Yousif, Wael A El-Sayed, Hebat-Allah S Abbas et al., J Appl Pharm Sci. 2017, 7(11): p. 21-32.
- Hanan A Soliman, Mahmoud NM Yousif, Makarem M Said et al., Der Pharma Chemica. 2014, 6(3): p. 394-410.
- Abdel-Rahman BA El-Gazzar, Mervat M El-Enany and Mahmoud N Mahmoud. Bioorg Med Chem. 2008, 16: p. 3261-3273.
- Konakanchi R, Kankala S and Kotha LR. Synthetic Communications. 2018, 48(14): p. 1777-1785.
- Kankala S, Pagadala R, Maddila S et al., RSC Advances. 2015, 5: p. 105446-105452.
- Mahmoud NM Yousif, Abdel-Rahman BA El-Gazzar, Ahmed A Fayed et al., J Appl Pharm Sci. 2020, 10(12): p. 35-43.