Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Synthesis and Antimicrobial Screening of Novel Tetrazolo Triazolo Substituted Mercapto Quinoxalines
Srinivas B, Prasanna B and Ravinder M*Ravinder M, Department of Chemistry, Chaitanya Postgraduates College (Autonomous), Hanamkonda, Warangal, Telangana, India,
Abstract
A novel and convenient synthesis of substituted 6-(substituted thio) tetrazolo[1,5-a] triazolo[3,4-c] quinoxalines has been carried out. The newly synthesized compounds have been characterized by IR, 1HNMR, and mass spectral data followed by elemental analysis. All the newly synthesized heterocycles have been screened for their in vitro anti-microbial activity against Staphylococcus aureus and Bacillus subtilis as Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa as Gram-negative bacteria. They were also evaluated for their in vitro anti-fungal potential against Candida albicans. Few of them have exhibited the promising activity.
Keywords
Substituted tetrazolo[1,5-a]triazolo[3,4-c]quinoxaline-6-thiol, Aliphatic/aromatic alkyl halides, K2CO3, Dimethylformamide, Anti-microbial activity
Introduction
Quinoxaline derivatives are important class of nitrogen containing heterocyclic in medicinal chemistry. The compound containing quinoxaline nucleus exhibit a broad spectrum of biological activity such as anti-viral [1], anti-inflammetary, anti-protazoal [2], anti-helminthic [3], anti-cancer [4], anti-malarial [5] in addition to anti-depressant activites [6]. Some of the compounds Ferrocenic pyrrolo [1,2-a] quinoxalines exhibit in vitro antimalarial activity [7]. Few of the quinoxalines containing compounds shows cytotoxic activity in vitro, and substituted 2-furano-4(3H)-quinazolines and quinoxalines were evaluated as antitumor agents [8,9]. The compounds 1,2,4-triazolo[1,5-a]quinoxaline derivatives reported as adenosine receptor antagonist and some of quinoxaline derivatives were identified as Potent and Selective ADP-ribose-polymerase-1 and 2 inhibitor. Tetrazoles are in increasingly popular skeleton [10] with wide ranging applications. They have found use in pharmaceuticals as lipophilic spacers and carboxylic acid surrogates, which improves oral absorption [11]. Substituted-1,2,3,4-Tetrazoles were reported to possess anti neoeceptiveactivity [12-16], anti-bacterial [17], anti-fungal [18-20], anti-viral [21], anti-inflammatory [22,23] and anti-ulcer [24,25] activities.
Materials and Methods
All reagents and solvents were purchased from commercial suppliers and were dried and purified when necessary by standard techniques. All melting points were determined in open glass capillaries on a Gallenkamp melting point apparatus and are uncorrected. Mass spectra were obtained on an Agilent 1100 HPLC-MS instrument. 1H NMR spectra were run in DMSO-d6, with TMS as the internal standard, on a Bruker ARX-300 instrument operating at 400 MHz. IR spectra (KBr disks) were recorded on a Bruker IFS 55 instrument. Elemental analysis was performed with a Carlo-Erba 1106 Elemental analysis instrument.
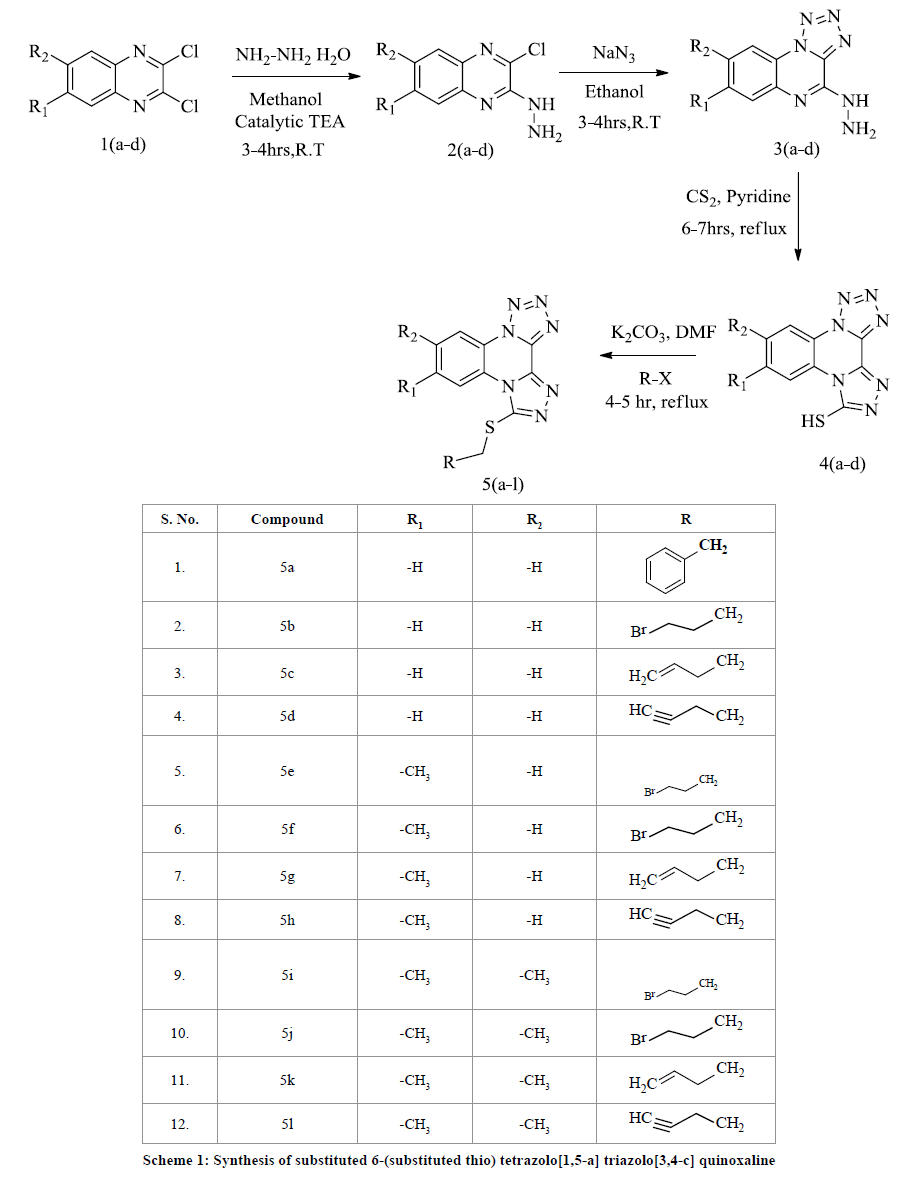
General procedure for the synthesis of substituted tetrazolo[1,5-a][1,2,4]triazolo[3,4-c] quinoxaline-6-thiol
To a stirred solution of substituted 4-hydrazinyltetrazolo [1,5-a] quinoxaline 3(a-b) (0.025 mol), Carbon disulphide (0.025 mol) in pyridine (10 mL) were added, than refluxed for 6-7 hr. The progress of the reaction was monitored by TLC. After completion of the reaction, the solid which separated was filtered and then recrystallized from ethanol to afford the pure product 4(a-d).
General procedure for the synthesis of substituted 6-(substitutedthio) tetrazolo[1,5-a] triazolo [3,4-c]quinoxaline A mixture of substituted tetrazolo[1,5-a][1,2,4]triazolo[3,4-c] quinoxaline-6-thiol 4(a-d) (0.05 mol) and anhydrous K2CO3 (0.3 mol), substituted aryl/alkyl halides (0.025 mol) in dimethylformamide (10 mL) were heated to 80-90oC for 4-5 h. The reaction was monitored by TLC, the solid was separated filtered, washed with water and recrystallized from ethanol to furnish the desired compounds 5(a-d).
6-(benzylthio)tetrazolo[1,5-a]triazolo[3,4-c]quinoxaline
Yield: 65%; m.p:242-244ºC; IR (KBr, cm-1): 1562 (C=N), 1589 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 4.89 (s, 2H, -CH2), 7.51-7.66 (m, 5H, Ar-H), 7.82-7.95 (m, 4H, Ar-H); MS (m/z): 334 (M+H); Anal.Calcd for C16H11N7S: C, 52.64.; H,3.33.; N, 29.41. Found: C, 52.62.; H, 3.30.; N, 29.40 5a.
6-((2-bromoethyl)thio)tetrazolo[1,5-a]triazolo[3,4-c]quinoxaline(5b)
Yield: 72%; m.p:224-226ºC; IR (KBr, cm-1): 1523 (C=N), 1552 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 3.71-3.75 (t, 2H, -CH2), 3.86-3.90 (t, 2H, -CH2), 7.53-7.61 (m, 4H, Ar-H); MS (m/z): 349 (M+H); Anal.Calcd for C11H8N7SBr: C, 37.73.; H,2.30.; N, 28.00. Found: C, 37.71.; H, 2.30.; N, 27.85.
6-(allylthio)tetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5c)
Yield: 64%; m.p:215-216ºC; IR (KBr, cm-1): 1542 (C=N), 1565 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 3.52 (d, 2H, -CH2), 5.05 (m, 1H, =CH), 5.20(d, 1H, =CH), 5.10(d, 1H, =CH), 7.42-7.51 (m, 4H, Ar-H); MS (m/z): 284 (M+H); Anal.Calcd for C12H9N7S: C, 50.87.; H, 3.20.; N, 39.61. Found: C, 50.83.; H, 3.18.; N, 39.61.
6-(prop-2-yn-1-ylthio)tetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5d)
Yield: 68%; m.p:243-245ºC; IR (KBr, cm-1): 1547 (C=N), 1574 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.53 (s, 1H, ≡ CH), 3.62 (s, 2H, -CH2), 7.80-7.82 (m, 4H, Ar-H); MS (m/z): 282 (M+H); Anal.Calcd for C12H7N7S: C, 51.24.; H, 2.51.; N, 34.86. Found: C, 51.23.; H, 2.48.; N, 34.83.
6-(benzylthio)-9-methyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5e)
Yield: 54%; m.p:273-275ºC; IR (KBr, cm-1): 1525 (C=N), 1554 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.36 (s, 3H, -CH3), 4.36 (s, 2H, -CH2), 7.52 (d, 1H, Ar-H),7.48 (d, 1H, Ar-H), 7.89 (d, 1H, Ar-H),7.45-7.55(m, 5H, Ar-H); MS (m/z): 334 (M+H); Anal. Calcd for C16H12N7S: C, 57.40.; H, 3.62.; N, 29.32. Found: C, 57.38.; H, 3.60.; N, 29.30.
6-((2-bromoethyl)thio)-9-methyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5f)
Yield: 63%; m.p:253-255ºC; IR (KBr, cm-1): 1555 (C=N), 1574 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.29 (s, 3H, -CH3), 3.45 (s, 2H, -CH2), 3.69 (s, 2H, -CH2), 7.48 (d, 1H, Ar-H),7.52 (d, 1H, Ar-H), 7.77 (d, 1H, Ar-H); MS (m/z): 363 (M+H); Anal.Calcd for C12H10N7SBr: C, 39.57.; H, 2.77.; N, 26.92. Found: C, 39.38.; H, 2.73.; N, 26.90.
6-(allylthio)-9-methyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5g)
Yield: 56%; m.p:274-276ºC; IR (KBr, cm-1): 1541 (C=N), 1554 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.32 (s, 3H, -CH3), 3.68 (s, 2H, -CH2), 5.60 (m, 1H, =CH), 5.20 (d, 1H, =CH), 5.10 (d, 1H, =CH), 7.49 (d, 1H, Ar-H),7.62 (d, 1H, Ar-H), 7.85 (d, 1H, Ar-H); MS (m/z): 298 (M+H); Anal.Calcd for C13H11N7S: C, 52.51.; H, 3.73.; N, 32.97. Found: C, 52.49.; H, 3.73.; N, 32.94.
9-methyl-6-(prop-2-yn-1-ylthio)tetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5h)
Yield: 61%; m.p:254-256ºC; IR (KBr, cm-1): 1552 (C=N), 1570 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.42 (s, 3H, -CH3), 2.89 (s, 1H, ≡ CH), 3.68 (s, 2H, -CH2), 7.58 (d, 1H, Ar-H),7.71 (d, 1H, Ar-H), 7.86 (d, 1H, Ar-H); MS (m/z): 296 (M+H); Anal. Calcd for C13H9N7S: C, 52.87.; H, 3.03.; N, 33.26. Found: C, 52.85.; H, 3.00.; N, 33.26.
6-(benzylthio)-9,10-dimethyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5i)
Yield: 59%; m.p:322-324ºC; IR (KBr, cm-1): 1529 (C=N), 1566 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.49 (s, 3H, -CH3), 2.56 (s, 3H, -CH3), 4.68 (s, 2H, -CH2), 7.72 (1, 1H, Ar-H),7.75 (s, 1H, Ar-H), 7.52-7.60 (m, 5H, Ar-H); MS (m/z): 362 (M+H); Anal.Calcd for C18H15N7S: C, 59.82.; H, 4.18.; N, 27.13. Found: C, 59.80.; H, 4.16.; N, 27.13.
6-((2-bromoethyl)thio)-9,10-dimethyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5j):
Yield: 69%; m.p:310-312ºC; IR (KBr, cm-1): 1536 (C=N), 1576 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.52 (s, 3H, -CH3), 2.63 (s, 3H, -CH3), 4.10 (s, 2H, -CH2), 4.35 (s, 2H, -CH2),7.45 (1, 1H, Ar-H),7.55 (s, 1H, Ar-H); MS (m/z): 378 (M+H); Anal.Calcd for C13H12N7SBr: C, 41.28.; H, 3.20.; N, 21.12. Found: C, 41.28.; H, 3.18.; N, 21.10.
6-(allylthio)-9,10-dimethyltetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5k)
Yield: 51%; m.p:274-276ºC; IR (KBr, cm-1): 1555 (C=N), 1578 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.54 (s, 3H, -CH3), 2.49 (s, 3H, -CH3), 3.87 (s, 2H, -CH2), 5.01 (m, 1H, =CH), 5.10 (d, 1H, =CH), 6.00 (d, 1H, =CH), 7.51 (d, 1H, Ar-H),7.57 (d, 1H, Ar-H); MS (m/z): 312 (M+H); Anal.Calcd for C14H13N7S: C, 54.00.; H, 4.21.; N, 31.49. Found: C, 54.00.; H, 4.20.; N, 31.45.
9,10-dimethyl-6-(prop-2-yn-1-ylthio)tetrazolo[1,5-a][1,2,4]triazolo[3,4-c]quinoxaline(5l):
Yield: 53%; m.p: 312-314ºC; IR (KBr, cm-1): 1548 (C=N), 1574 (C-N); 1HNMR(400MHz, DMSO-d6, δ ppm): δ 2.42 (s, 3H, -CH3), 2.49 (s, 3H, -CH3), 2.83 (s, 1H, ≡ CH), 3.58 (s, 2H, -CH2), 7.58 (d, 1H, Ar-H),7.68 (d, 1H, Ar-H); MS (m/z): 310 (M+H); Anal.Calcd for C14H11N7S: C, 54.36.; H, 3.58.; N, 31.69. Found: C, 54.36.; H, 3.58.; N, 31.67.
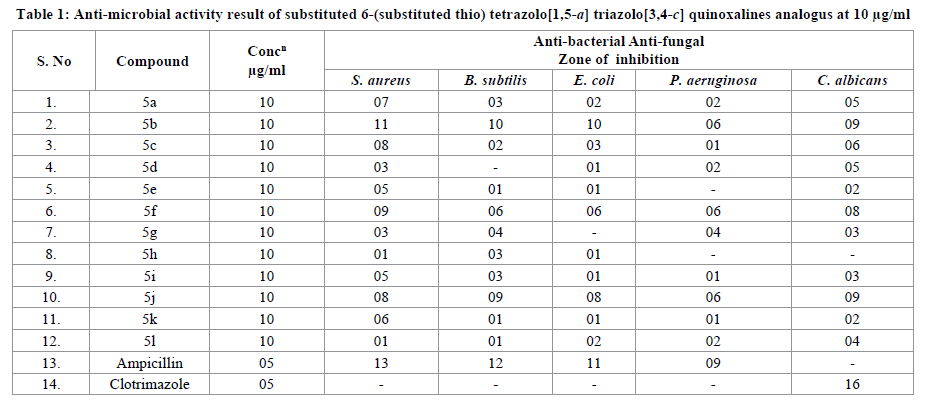
Anti-microbial activity
All newly synthesized compounds were evaluated for their in vitro anti-bacterial activity against Staphylococcus aureus and Bacillus subtilis as Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa as Gram-negative bacteria. They were also evaluated for their in vitro antifungal potential against Candida albicans. The agar Disc-diffusion method was used to evaluate anti-microbial activity. The compounds were dissolved in DMSO to 10 μg/mL concentration solutions used. The compounds were placed aseptically on Muller-Hinton Agar for the both Gram positive and Gram negative bacteria and Saboround dextrose agar for fungi and incubated for 24 h at 37C. At the end of the incubation period, the diameter of the growth of inhibition zones was measured. Ampicillin was used as standard antibacterial while clotrimazole was used as the antifungal reference.
Result and Discussion
Chemistry
The synthesis of substituted 6-(substituted thio) tetrazolo[1,5-a] triazolo[3,4-c] quinoxaline illustrated in the Scheme 1. The starting compound 4-hydrazinyl tetrazolo quinoxalines 3(a-d) prepared according to previous literature methods. The hydrazine compound was treated with carbon disulphide in pyridine at refluxing temperature. The intermediate compounds 4(a-d) were again treated with substituted alkyl/aryl halides in a mixture of anhydrous K2CO3, dimethyl formamide were refluxed for 4-5 h. The synthesized compounds were characterized by IR, 1HNMR and Mass spectroscopy. The IR spectrum of compounds 5(a-l) showed a characteristic absorption bands within the 1562 cm-1 and 1589 cm-1 shows a characteristic absorption band due to C=N, C-N group. The 1HNMR spectrum of compound (5b) shows a triplet at 3.71-3.75 δ ppm another triplet at 3.86-3.90 δ ppm due to two ethylene protons, a multiplate between 7.53-7.61 δ ppm due to aromatic protons.
Biological activity
Anti-microbial activity
Anti-bacterial activity results (Table 1) revealed that, among the synthesized compounds 5b, 5f, and 5j showed potent activity against gram-positive bacterial strains S. aureus, B. subtilis and gram-negative bacterial strains E. coli and P. aeruginosa on comparing with the standard drugs Ampicillin. Compound 5c and 5k showed moderate activity against grampositive bacterial strains S. aureus, B. subtilis and gram-negative bacterial strains E. coli and P. aeruginosa. The anti-fungal screening data revealed that the 5b, 5f and 5j compounds showed good activity against the tested fungal strains C. albicans on comparing with the standard drugs clotrimazole. The antifungal screening results revealed that 5c moderate activity against C. albicans.
Conclusion
We have synthesized a new series of substituted 6-(substituted thio) tetrazolo[1,5-a] triazolo[3,4-c] quinoxalines analogus. All the synthesized compounds 5(a-l) were screened for anti-microbial activity. Among all the synthesized compounds 5b, 5f and 5jshowed good activity and 5c and 5k showed moderate activity against, B. subtilis and gram-negative bacterial strains E.coli and P.aeruginosa. 5b, 5f and 5j showed appreciable activity and 5c shows moderate activity against tested fungal strains C. albicans organism.
Acknowledgements
The authors are thankful to the chairman Dr. Ch.V.Purushottam Reddy and Dr.K.Veera venkataiah, Prinicipal of chaitanya postgraduate college (Autonomous), Hanamkonda, Warangal, for providing research facilities and University Grant Commission for providing financial assistance (Minor research Project-No-MRP-6085/15(SERO/UGC).
References
[1] M. Loriga, S. Piras, P. Sanna, G. Paglietti, Farmaco, 1997, 52, 157.
[2] X. Hui, J. Desrivot, C. Bories, P.M. Loiseau, X. Frank, R. Hocquimiller, B. Figadere, Bioorg. Med. Chem. Lett., 2006, 16, 815.
[3] G. Sakata, K. Makino, Y. Karasawa, Heterocycl., 1988, 27, 2481.
[4] C.W. Lindsley, Z. Zhao, W.H. Leister, R.G. Robinson, S.F. Barnell, D. Defeozones, R.E. Jones, G.D. Hartman, J.R. Huff, H.E.
Huber, M.E. Duggan, Bioorg. Med. Chem. Lett., 2005, 15, 761.
[5] J. Guillon, G. Philippe, M. Labaied, P. Sonnet, J.M. Legar, P.D. Poultain, C. Serghraet, J.J. Christian, J. Med. Chem., 2004, 47, 1997.
[6] R. Sarges, H.R. Howerd, R.C. Browne, L.A. Label, J. Med.Chem., 1990, 33, 2240.
[7] J. Guillon, S. Moreau, E. Mouray, V. Sinou, I. Forfar, Bioorg. Med. Chem., 2008, 15, 16(20), 9133.
[8] M.N. Noolvi, H.M. Patel, V. Bhardwaj, A. Chauhan, Eur. J. Med. Chem., 2011, 46(6), 2327.
[9] D. Catarzi, V. Colotta, C. Montopoli, J. Med.Chem., 2005, 48 (25), 7932.
[10] R.N. Butler, A.R. katritzky, C.W. Reas, E.F.F. Serivan, Comprehensive Heterocyclic Chem., Pergamon, Oxford, UK, 1996.
[11] H. Singh, A.S. Chawala, V.K. Kaporr, D. Paul, R.K. Malhotra, Prog. Med. Chem., 1980, 17, 151.
[12] P. Kumar, E.E. Khans, Drug. Des. Discov., 1994, 11(1), 15.
[13] P. Vicini, L. Amoretti, E. Baroelli, M. Chiavarini, M. Impicicatore, Farmaco, 1986, 41, 111.
[14] K.D. Stewart, Bio. Org. Med. Chem. Lett., 1998, 3, 8(5), 529.
[15] A. Rajasekharan, P.P. Thampi, Eur. J. Med. Chem., 2004, 39, 273.
[16] S.C. Bachar, S.C. lahiri, Pharmazie., 2004, 59, 435.
[17] R.M. Demarinis, J.R.E. Hoover, G.L. Dunn, P. Actor, J.V. Uri J.A. Weisbach, J. Antibiotics., 1973, 28, 463.
[18] T. Iehikawa, M. Yamada, M. Yamaguchi, T. Kitazski, Y.Matsushitha, K. Higashikawa, K. Itoh, Chem. Pharm. Bull., 2001, 49, 1110.
[19] J. Matysiak, A. Niewiadomy, E. Krajeswaska Kulak, G. Macik-Niewiadonmy, Farmakco, 2003, 58, 455.
[20] R.S. Upadhyaya, S. Jain, N. Simha, N. Kishore, R. Chandra, S.K. Arora, Eur. J. Med. Chem., 2004, 39, 579.
[21] A. Dlugosz, Pharmazie., 1995, 50, 180.
[22] S.M. Ray, S.C. Lahiri, J. Indian. Chem. Soc., 1990, 67, 324.
[23] A. Rajesekaran, P.P. Thampi, Acta. Pharma. Ture., 2003, 45, 235.
[24] I. Ueda, K. Ishii, K. Sinazaki. M. Htanaka, Chem. Pharm. Bull., 1991, 39, 1430.
[25] K. Terashima, T. Tanimura, H. Shimamura, A. Kawase, K. Uenishi, Y. Tanaka, I. Kamasaki, Y. Ishizuka, M. Sato, Chem. Pharm. Bull., 1995, 43, 1042.



