Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 6
Synthesis and Anti-inflammatory activity of novel 2-chloro-4-(aryl amino)-6, 7-dimethoxy quinazoline derivatives
Poonam Rani*, Manoj Kumar, Sonali and Bhupender NehraPoonam Rani, Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Haryana, India, Email: poonampharmagju@gmail.com
Received: 28-Oct-2023, Manuscript No. DPC-23-118630; Editor assigned: 31-Oct-2023, Pre QC No. DPC-23-118630 (PQ); Reviewed: 14-Nov-2023, QC No. DPC-23-118630; Revised: 17-Nov-2023, Manuscript No. DPC-23-118630 (R); Published: 15-Dec-2023, DOI: 10.4172/0975-413X.15.6.115-118
Abstract
To explore the anti-inflammatory potential of novel quinazoline consisted heterocyclic derivatives, a new series of 2-chloro-4-(aryl amino)-6, 7- dimethoxy quinazoline derivatives (1-4) were design and synthesized using multistep synthetic methodology. Structural elucidation of all synthesized molecules was performed by using FT-IR and 1H NMR spectral reports. All molecules were accessed for their anti-inflammatory potential against while taking diclofenac sodium as reference anti-inflammatory agent. Many compounds exhibited good anti-inflammatory activity in which compound 4 possessed the highest activity amongst the synthesized series with IC50 value of 1.772 μg/ml.
Keywords
Quinazoline; Cyclooxygenase-1; Cyclooxygenase-2; NSAIDs; Anti-inflammatory
Introduction
Heterocyclic compounds play a crucial role in both pharmaceutical and organic chemistry, and their synthesis has received a lot of attention [1]. They are essential for the identification of novel drug candidates because they can increase the chemical space already occupied by drugs and aid in the creation of new drug molecules [2]. The polarity, H-bonding, solubility, and lipophilicity of pharmaceuticals can be enhanced by the presence of heterocyclic moiety, which optimizes the ADME and Toxicity features of compounds [3]. The therapeutic effects of Non-Steroidal Anti- Inflammatory Medicines (NSAIDs) are produced by inhibiting Cyclooxygenase (COX) enzymes that produces Prostaglandins (PGs). COX enzyme inhibitors experience some side effects, such as stomach and renal toxicity to a greater or lesser extent [4]. There are two COX enzymes (COX 1 and COX-2). The constitutive COX-1 enzyme produces PGs that shield the kidney and stomach from the various types of harms [5]. Inflammatory stimuli like cytokines activate COX-1 enzyme, which then creates PGs that create inflammation, pain and swelling [6]. COX-1 is constitutively expressed in all tissues; it is constantly present and active. As far as is known, COX-2 only exhibits this behaviour in the kidney, brain, and ovaries. As COX-2 enzymes are expressed more and more in the afflicted tissues during inflammatory processes, the amount of pain-mediating prostaglandins produced also get increases [7].
The 4-oxoquinazoline family of anti-inflammatory drugs has been reported to have a wide variety of activities. It has been discovered that its derivatives exhibit a wide range of pharmacological actions, including COX-II inhibitors [8], analgesics [9], anti-viral [10], anti-bacterial [11], antifungal [12], anti-malarial [13] and anti-cancer [14] so that quinazolinone nucleus has been gaining popularity. In the present study, we synthesized new derivatives of quinazoline and evaluated their anti-inflammatory activity.
Materials and Methods
Chemistry
All the chemicals of analytical grade were used for the synthesis which were purchased from SRL (India) Hi-media (India), used without further purification. The synthesized derivatives were checked by using TLC, by using silica gel G and suitable solvent system to monitor the progress of all reaction’s procedures. The decibel melting point apparatus and an open capillary tube were used to measure the melting points of the intermediates and final compounds, which were then expressed in degree Celsius. Perkin Elmer IR spectrophotometer was used for IR spectra of the compound by using KBr pellets technique. For the 1HNMR, a proton nuclear magnetic resonance spectrum of the synthesized derivatives, Bruker Advance II 400 NMR spectrometer was used. Chemical shift were described in δ values downfield from TMS, while DMSO was used as solvent. NMR signal were expressed by the letter s, d, t, q and m, which respectively stand for singlet, doublet quartet and multiplet. Values for coupling constant (J) are given in hertz.
Anti-inflammatory activity
Protein denaturation method: In this method, egg albumin solution was used to evaluate the in vitro anti-inflammatory activity. A phosphate buffer of 7.4 pH was used to maintain the pH of the solution. Either distilled water or DMSO could be used as a control [15,16].
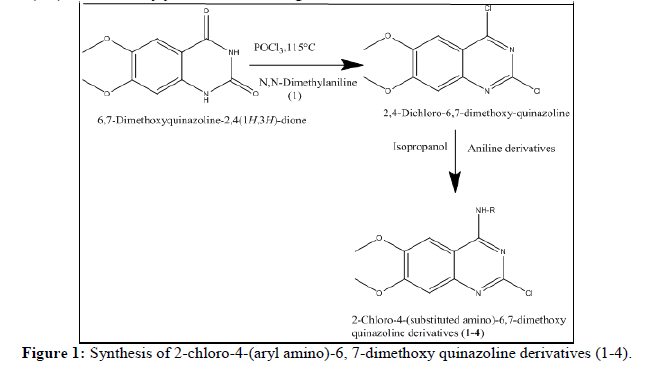
In the present work, we synthesized a series of quinazoline amine derivatives (1-4) from 6, 7-dimethoxy quinazolin-2, 4-diones via a two-step reaction.
Procedure for synthesis of 2,4-dichloro-6,7-dimethoxy quinazoline: A mixture of 6,7-dimethoxy quinazolin-2,4-diones (1, 2.0 g) and phosphorous oxychloride (POCl3, 6 mL) was refluxed in the presence of N,N-dimethylaniline (0.6 mL) for 5 h. The reaction mixture was allowed to cool to room temperature and poured into ice-cold water with stirring. The precipitates obtained were filtered and washed with distilled water.
General procedure for synthesis of 2-chloro-4-(aryl amino)-6, 7-dimethoxy quinazoline derivatives (1-4)
2, 4-dichloro-6, 7-dimethoxy quinazoline (2) (4 mmol) was refluxed with aniline derivatives (4 mmol) in isopropanol (5 ml) for 6 h to obtain the final quinazoline derivatives (1-4) in satisfactory yield as shown in Figure 1.
2-chloro-N-(3-chloro-2-methylphenyl)-6, 7-dimethoxyquinazolin-4-amine (1)
% Yield 83; mp 284°C; IR (KBr, cm-1): 3193 (N-H str. of secondary amine), 2921 (aliphatic C-H stretch), 1628 (C=N of quinazoline ring), 1539 (aromatic C=C stretch), 766 (C-Cl strong), 768 (out of plane).
1H NMR (400 MHz, DMSO, δ ppm)): δ 10.15 (s, 1H, NH), 8.27-7.21(m, aromatic H), 4.29-3.79 (M, H of OCH3), 2.35 (s, 3H, CH3).
2-Chloro-N-(2, 4-dimethylphenyl)-6, 7-dimethoxyquinazolin-4-amine (2)
% Yield 60; mp 254°C; IR (KBr, cm-1): 3239 (N-H str. of secondary amine), 2938 (aliphatic C-H stretch), 1626 (C=N of quinazoline ring), 1403 (aromatic C=C stretch), 768 (C-Cl strong), 840 (disubstituted CH3), 613 (out of plane).
1H NMR (400 MHz, DMSO, δ ppm): 9.98 (s, 1H, NH), 7.96-7.11 (m, aromatic H), 4.19- 3.93 (m, H of OCH3), 2.37-2.31 (m, H, CH3).
2-Chloro-6, 7-dimethoxy-N-(2-methyl-4-nitrophenyl) quinazolin-4-amine (3)
% Yield 80; mp 232°C; IR (KBr, cm-1): 3176 (N-H str. of secondary amine), 2942 (aliphatic C-H stretch), 1628 (C=N of quinazoline ring), 1529 (NO2), 1439 (aromatic C=C stretch), 736 (C-Cl strong), 853 (out of plane).
1H NMR (400 MHz, DMSO, δ ppm): 10.15 (s, 1H, NH), 8.27-7.21(m, aromatic H)4.29-3.79 (m, H of OCH3), 2.35 (s, 3H, CH3).
2-Chloro-N-(2, 3-dimethlyphenyl)-6, 7-dimethoxy quinazolin-4-amine (4)
% Yield 88; mp 262°C IR (KBr, cm-1): 3234 (N-H str. of secondary amine), 2937 (aliphatic C-H stretch), 1627 (C=N of quinazoline ring), 1438 (aromatic C=C stretch), 771 (C-Cl strong), 743 (out of plane).
1H NMR (400 MHz, DMSO, δ ppm): 9.98 (s, 1H, NH), 7.96-7.11 (m, aromatic H), 4.19- 3.93 (m, H of OCH3), 2.37-2.31 (m, H, CH3).
In vitro anti-inflammatory activity
For in vitro anti-inflammatory activity, Diclofenac sodium was used as a standard drug. Both synthesized compounds (1-4) and standard drug were used for the preparation of different concentrations (500, 250, 125, 62.25, 31.25μg/mL) in DMSO and 1ml of each resulting solutions were taken in different test tubes. Then 1.4 ml of freshly prepared phosphate buffer (pH 6.4) and 0.1ml egg albumin from fresh egg was transferred in each test tube containing different solutions for a BOD for 15 minutes at 37°C ± 20°C and then heated for 5 minutes at 70°C temperature. Then, the test tubes were cooled at room temperature and absorbance was determined by UV spectroscopy at 660 nm wavelength.
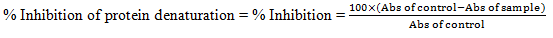
IC50 values were also determined; IC50 is the concentration that elicits the half maximal response.
Results and Discussion
Chemistry
A series of quinazoline derivatives (1-16) were synthesized from 6, 7-dimethoxy quinazolin-2, 4-diones in 2 steps, as shown in Figure 1. Intermediate 2, 4-dichloro-6, 7-dimethoxy quinazoline was synthesized from 6, 7-dimethoxy quinazolin-2,4-diones as per the procedure described in the literature. After that, the proposed derivatives (1-4) were synthesized by refluxing suitable aniline derivatives with intermediate 2, 4-dichloro- 6, 7-dimethoxy quinazoline in isopropanol for 6 hours.
Biological evaluation
All derivatives were evaluated for their anti-inflammatory activity by the protein denaturation method. Diclofenac sodium was used as the standard drug, and the percentage inhibition of protein denaturation was noted down, and from that, IC50 values were determined.
Based on anti-inflammatory activity, it was concluded that almost derivatives had good potency as compared to the standard as shown in Table 1. Among all synthesized derivatives, compound 4 was more potent. The potency of the compound 4 might be due to the presence of a hydrophobic group (Table 1).
| Compound number | % Inhibition | |||||
|---|---|---|---|---|---|---|
| 500 µg/ml | 250 µg/ml | 125 µg/ml | 62.5 µg/ml | 31.25 µg/ml | IC50 µg/ml | |
| 1 | 60.7182 | 47.8963 | 45.0424 | 38.4579 | 24.9474 | 2.2027 |
| 2 | 27.9322 | 26.0402 | 26.364 | 22.3743 | 13.4197 | 2.9102 |
| 3 | 49.3319 | 45.3423 | 43.813 | 43.0664 | 40.2598 | 2.8393 |
| 4 | 55.2416 | 47.7213 | 37.4798 | 35.4457 | 19.3532 | 1.772 |
| Std. | 75.3169 | 62.9641 | 56.6333 | 48.8606 | 44.4325 | 2.2027 |
Table 1: Anti-inflammatory activity of 2-chloro-4-(aryl amino)-6, 7-dimethoxy quinazoline derivatives
Conclusion
In conclusion, we have synthesized four new quinazoline (1-4) and determined their physicochemical (melting point) and spectral characterization using FTIR and 1H-NMR spectroscopy. Outcomes of anti-inflammatory activity highlighted that almost tested derivatives presented good to potent anti-inflammatory activity. Whereas, compound 4 was found to be most active anti-inflammatory candidate with most promising results. Also, we conclude that the quinazoline derivatives had comparable anti-inflammatory activity to the standard drug. The bulky substituent on the phenyl ring made a significant contribution to the anti-inflammatory activity. Some more derivatives with bulky groups in the second position of the quinazoline ring might be developed for better anti-inflammatory activity.
Acknowledgement
The authors acknowledge the Vice Chancellor, Guru Jambheshwar University of Science & Technology, Hisar for his constant support and motivation.
Conflict of Interest
The authors declare no conflict of interest.
References
- Martins MA, Frizzo CP, Moreira DN, et al. Chem Rev. 2008; 108(6): p. 2015-2050.
[Crossref] [Google Scholar] [PubMed]
- Barker A, Kettle JG, Nowak T, et al. Drug Discov Today. 2013; 18(5-6): p. 298-304.
[Crossref] [Google Scholar] [PubMed]
- Meanwell NA. Chem Res Toxicol. 2016; 29(4): p. 564-616.
[Crossref] [Google Scholar] [PubMed]
- Buttgereit F, Burmester GR, Simon LS. American J Med. 2001; 110(3): p. 13-19.
[Crossref] [Google Scholar] [PubMed]
- Grosch S, Niederberger E, Geisslinger G. Expert Opin Investig Drugs. 2017; 26(1): p. 51-61.
[Crossref] [Google Scholar] [PubMed]
- Masferrer JL, Isakson PC, Seibert K. Gastroenterol Clins. 1996; 25(2): p. 363-372.
[Crossref] [Google Scholar] [PubMed]
- Tristan-Manzano M, Guirado A, Martínez-Esparza M, et al. Curr Med Chem. 2015; 22(26): p. 3075-3108.
[Crossref] [Google Scholar] [PubMed]
- Farag DB, Farag NA, Esmat A, et al. Med Chem Comm. 2015; 6(2): p. 283-299.
- Alafeefy AM, Kadi AA, Al-Deeb OA, et al. Eur J Med Chem. 2010; 45(11): p. 4947-4952.
[Crossref] [Google Scholar] [PubMed]
- Xie D, Shi J, Zhang A, Lei Z, et al. Bioorg Chem. 2018; 80(17): p. 433-443.
[Crossref] [Google Scholar] [PubMed]
- Zayed MF, Hassan MH. Saudi Pharm J. 2014; 22(2): p. 157-162.
[Crossref] [Google Scholar] [PubMed]
- Shalaby AA, El Khamry AM, Shiba SA, et al. J Pharm Med Chem. 2000; 333(11): 365-372.
[Crossref] [Google Scholar] [PubMed]
- Birhan YS, Bekhit AA, Hymete A. BMC Res Notes. 2019; 72(19): p. 99-102.
[Crossref] [Google Scholar] [PubMed]
- Abuelizz HA, Marzouk M, Ghabbour H, et al. Saudi Pharm J. 2017; 25(7): 1047-1154.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. Sci Rep. 2017; 7(1): p. 427-717.
[Crossref] [Google Scholar] [PubMed]
- Al-Wabli R, Fouad M, El-Haggar R. Antiallergy Agents Med Chem. 2018; 17(2): p. 115-124.
[Crossref] [Google Scholar] [PubMed]