Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
Sulfonated Cellulose, Competent Catalyst for Synthesizing Polyhydroquinolinesin Presence of Dicationic Ionic Liquid (C4[mim]22[Br-])
Giribala Madhvrao Bondle1* and Sandeep TukaramAtkore22Department of Bio-chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004 Maharashtra, India
Giribala Madhvrao Bondle, Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004 Maharashtra, India, Tel: 9890766118, Email: gmbondle@gmail.com
Abstract
The four-component Hantzsch condensation reaction of dimedone, ethyl acetoacetate, ammonium acetate and various aromatic aldehyde in presence of sulfonated cellulose as a green and highly efficient catalyst in dicationic ionic liquid (C4[mim]2[Br-])to affords polyhydroquinoline derivatives in good to excellent yields. This reaction has been carried out in presence of (0.16 g) of sulfonated cellulose and 2-3 ml of dicationic ionic liquid at room temperature. The described novel synthesis method proposes several advantages of short reaction times, high yields, mild condition, high melting point, simplicity and easy workup compared to the traditional method of synthesis.
Keywords
Hantzsch reaction, Dimedone, Sulfonated cellulose, Dicationic ionic liquid
Introduction
Synthesis of heterocycles is of immense importance in design and discovery of new compounds for pharmaceutical applications. 1,4-Dihydropyridine (DHP), an important core unit of polyhydroquinolines which are used for the preparations of the drugs to treat cardiovascular disease, including hypertension [1]. In addition to this 1,4-DHP’s exhibit several other medicinal applications like neuroprotectant [2], platet anti-aggregator activity [3], as cerebral anti ischemic agents in the treatment of Alzheimer’s disease [4] and as a chemosensitizer in tumor therapy [5]. Also acts as calcium antagonists [6].
In 1882, Arthur Hantzsch reported the first synthesis of symmetrically substituted 1,4-dihydropyridines by the one-pot, four component condensations of two molecules of Ethylacetoacetate, aromatic aldehyde and ammonia [7]. The standard Hantzsch procedure does not need the intervention of any additive or reagent and the reaction was originally conducted either in acetic acid or at reflux in alcohol for rather long periods, resulting in low or modest yields of condensation products. Replacement of ammonia by ammonium acetate allowed the efficient synthesis of Hantzsch compounds in an aqueous medium as well as under solvent free conditions [8,9]. Realizing the importance of polyhydroquinoline derivatives in the synthesis of various drug sources.
Many reported characteristic method such as conventional heating [10,11], L-proline [12], various catalyst such as ammonium nitrate (CAN) [13], silica perchloric acid (HClO4-SiO2) [14], trimethylsilyl chloride [15], nickel nanoparticlel [16], FeF3 [17], K7[PW11CoO40] [18], p-TSA [19], solar heat [20], hafnium (IV) [21], SBA-Pr-SO3H [22], Baker’s yeast [23], iron (III) trifluoroacetate [24].
Ionic liquids are salts consisting of ions, which exist in the liquid state at ambient temperatures. They show reasonably high ionic conductivities. Ionic liquids have found great interest only recently. Ionic liquids are usually characterized by a wide electrochemical window of stability, a reasonable ionic conductivity (similar to most non-aqueous electrolytes). ILs typically consists of organic nitrogen-containing heterocyclic cations and inorganic anions [25]. Nevertheless, in the last few years, they become more attractive in other fields such as catalysis [26], formations of metal nanostructures [27], analytical chemistry [28] including sensors [29] and for electrochemical biosensors [30]. Due to their high polarities, the ionic liquids are expected to be very suitable solvents for the reactions between organo-soluble and water soluble reagents. The utility of the ionic liquids as solvents for various organic reactions [31,32] and polymerization reactions [33-35] has been studied. Generally, ILs are defined as those fused salts with a melting point less than 100ºC, with salts with higher melting points referred to as molten salts.
However, some of the reported methods for the synthesis of polyhydroquinoline derivatives have one or more disadvantages such as moisture sensitive, or highly toxic in the environment and unpleasant experimental procedure and reagents which are expensive. A mild and efficient catalyst for the synthesis of polyhydroquinoline is very desirable. Performing organic reactions in ionic liquid media has attracted much attention because of its wonderful properties. It would be significantly safe, cheap, non-toxic and environmentally friendly compared to organic solvent.
Multi-Component Reactions (MCRs) have emerged and powerful tool in modern synthetic organic chemistry allowingthe facile reaction of several new bonds in a one-pot reaction [36]. We would like to report a highly efficient and green four-component Hantzsch of sulfonated cellulose as a catalyst and dicationic ionic liquid as a solvent medium using various aromatic aldehyde, dimedone, ethyl acetoacetate and ammonium acetate to produce the polyhydroquinoline derivatives in good to excellent yields.
Materials and Methods
General experimental procedure for the synthesis of polyhydroquinoline derivatives
A mixture of aldehyde 1 (1 mmol), dimedone 2 (1 mmol), β-ketoester3 (1 mmol) and ammonium acetate (2 mmol), sulfonated cellulose (0.16 g) and dicationic ionic liquid (2-3 mL) was thoroughly mixed in a mortar and transferred to 50 ml round bottom flask and stirred for an appropriate time on magnetic stirrer till completion of reaction (TLC). The reaction mixture was diluted with water and extracted with ethyl acetate. The catalyst was filtered and washed with ethyl acetate. From the combine, filtrate solvent was removed under vacuum and the residue was recrystallized from ethanol to give the pure product. Also, the ionic liquid which is get dissolved in water is recovered by evaporating the water and can be reused again. The catalyst sulfonated cellulose [37] and dicationic ionic liquid [38] was prepared by using the known procedure given in the literature.
Spectral data of some representative compounds
Ethyl-1,4,7,8-tetrahydro-2,7,7-4-(phenyl)-5-(6H)-oxoquinoline-3-carboxylate (4a) Solid; IR (KBr) cm-1: 3292, 3210, 3074, 1699, 1602, 1067, 690; 1HNMR (CDCl3, 400 MHz): δ=0.90 (s, 3H,), 1.01 (s, 3H,), 1.20 (t, J=7.00 Hz, 3H, CH3-CH2O), 2.03-206 (m, 4H), 2.38 (s, 3H), 4.01 (q, J=6.8 Hz, 2H), 4.96 (s, 1H, Ar-CH), 6.39 (s, 5H, NH), 7.02-7.31 (m, 5H, Ar-H); m/z=340 [M+].
Ethyl-1,4,7,8-tetrahydro-2,7,7-4-(4-chlorophenyl)-5-(6H)-oxoquinolin-3-carboxylate(4b) Solid; IR (KBr) cm-1: 3290, 3205, 3074, 1705, 1608, 1073, 836; 1HNMR (CDCl3, 400 MHz): δ=0.91 (s, 3H), 1.03 (s, 3H), 1.24 (t, J=6.8 Hz, 3H, CH3-CH2O), 2.08-2.28 (m,4H), 2.35 (s, 3H, =C-CH3), 4.08 (q, J=6.7 Hz, 2H, OCH2CH3), 4.97 (s, 1H, Ar-CH), 6.40 (s, 1H, NH), 7.01 (d, J=8.2Hz, H, 2 x Ar- H), 7.31 (d, J=8.2Hz, 2H, 2 x Ar-H); m/z=374, 376[M+].
Ethyl-1,4,7,8-tetrahydro-2,7,7-4-(4-methylphenyl)-5-(6H)-oxoquinolin-3-carboxylate(4c) Solid; IR (KBr) cm-1: 3280, 3195, 3074, 1699, 1602, 1213, 1061; 1HNMR (CDCl3, 400 MHz): δ 0.90 (s, 3H), 1.05 (s, 3H), 1.21 (t, J=7.00 Hz, 3H, CH3-CH2O), 2.10(s, 3H, =C-CH3), 2.20-2.40 (m, 4H, 2 x CH 2 ), 2.38 (s, 3H, Ar-CH 3 ), 4.04 (q, 2H, J=7.00 Hz, OCH2CH3), 4.97 (s, 1H, Ar-CH), 6.40 (s, 1H, NH), 6.98 (d, J=8.2 Hz, 2H, Ar-H), 7.01 (d, J=8.2 Hz, 2H, ArH); m/z=354 [M+].
Ethyl-1,4,7,8-tetrahydro-2,7,7-4-(3-nitrophenyl)-5-(6H)-oxoquinolin-3-carboxylate(4e) Solid; IR (KBr) cm-1: 3299, 2958, 1687, 1610, 1164, 757; 1HNMR (CDCl3, 400 MHz): δ=0.92 (s, 3H, C-CH3), 1.05 (s, 3H, C-CH3), 1.21 (t, J=7.2 Hz, 3H, CH3-CH2O), 2.10- 2.31 (m, 4H, 2x CH2), 2.36 (s, 3H, =C-CH3), 4.06 (q, J=6.8 Hz, 2H, OCH2), 4.95 (s, 1H, Ar-CH), 6.36 (s, 1H, NH), 6.70 (d, J=8.4Hz, 1H, Ar-H), 7.02-7.15 (m, 2H, 2 x Ar-H), 7.38 (d, J=8.4Hz, 1H, Ar-H); m/z=385[M +].
Ethyl-1,4,7,8-tetrahydro-2,7,7-4-(4-hydroxyphenyl)-5-(6H)-oxoquinolin-3-carboxylate(4j) IR (KBr) cm-1: 3365, 2955, 1700, 1645, 1590, 1480, 1385, 1220, 782; 1HNMR (CDCl3, 400 MHz) d: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J =7.2 Hz, 3H), 2.09–2.22 (m, 3H), 2.20–2.34 (m, 4H), 4.06 (q, J= 7.8 Hz, 2H), 4.98 (s, 1H), 5.61 (s, 1H), 6.10 (s, 1H), 6.65 (d, J= 8.0 Hz, 2H), 7.17 (d,J =7.8 Hz, 2H) m/z= 355 [M+].
Results and Discussions
Here in, we described the synthesis of poyhydroquinoline derivatives via four-component Hantzsch condensation reaction in presence of dicationic ionic liquid (C4[mim]22[Br-]) as solvent media and sulfonated cellulose as a catalyst by grinding method (Scheme 1).
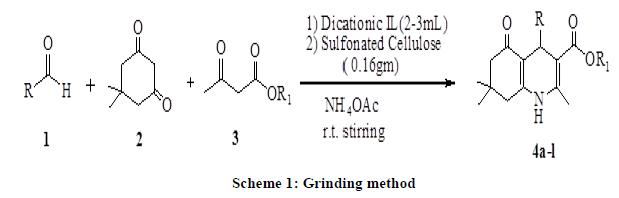
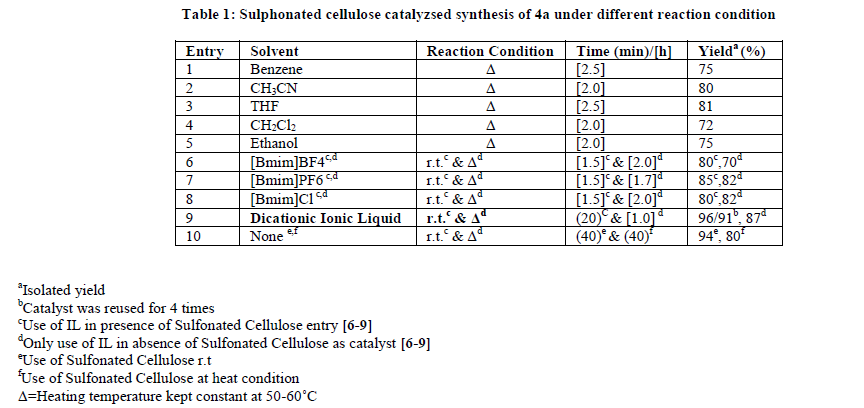
In recent years, the use of heterogeneous catalysts has received considerable interest in various disciplines including organic synthesis. They are advantageous over their homogenous counterparts that in most of the cases the catalyst can be recovered easily and reused [39]. Similarly, the avoidance of the solvent helps in making the process environmentally benign and economically viable. Chlorosulphonic acid supported on natural polymer cellulose [40] has been proved to be the novel heterogeneous catalyst of choice, due to its ease of preparation using readily available reagents as well as its high catalytic efficiency. In continuation with our previous work on solid-supported reagents [40-47], we wish to report here sulfonated cellulose as a novel catalyst for the synthesis of polyhydroquinoline derivatives (Scheme 1). We started our study on one pot Hantzsch condensation for the model reaction involving benzaldehyde, dimedone and ethylacetoacetate to afford corresponding polyhydroquinoline (4a) by using sulfonated cellulose as a catalyst and their comparative effects on the synthesis of 4a in presence of different solvent conditions under room temperature stirring as well as heating conditions. The results are presented in Table 1 indicate optimization experiments. Entries 1-10 show the effect of various solvents on the reaction. Solvent-free condition (entry 10), whereas by using dicationic ionic liquid (entry 9) is the best-suited condition in term of yields and time, indicates the scope and generality of the present protocol.
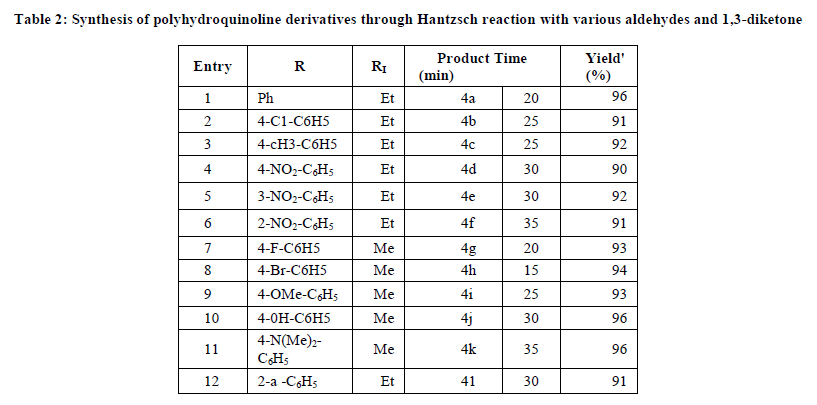
This procedure offers easy access to substituted polyhydroquinoline derivatives with a variety of substitution patterns. Aromatic aldehydes carrying either electron donating or withdrawing substituent’s afforded the corresponding polyhydro quinolines in good to excellent yields. The results of synthesis of various derivatives under room temperature condition using sulfonated cellulose and dicationic IL are summarized in Table 2.
Conclusion
In conclusion, we have described herein sulfonated cellulose and dicationic ionic liquid as an extremely efficient catalyst for the preparation of tetrahydroquinolines. The important features of the present method arethe use of an inexpensive catalyst, room temperature conditions, short reaction times, high yields, re-use of catalyst.
Acknowledgments
Authors are thankful to Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad for providing the facility and for the timely help.
References
[1] H. Nakayama, Y. Kasoaka, Heterocycles., 1996, 42, 901.
[2] V. Klusa, Drugs Future., 1995, 20, 135.
[3] R.G. Bretzel, C.C. Bollen, E. Maeser, K.F. Federlin, Am. J. Kidney. Dis., 1993, 21, 53.
[4] R.G. Bretzel, C.C. Bollen, E. Maeser, K.F. Federlin, Drugs Future., 1992, 17, 465.
[5] R. Boer, V. Gekeler, Drugs Future., 1995, 20, 499.
[6] M.F. Gordeev, D.V. Patel, E.M. Gordon, J. Org. Chem., 1996, 61, 924.
[7] R.A. Janis, P.J. Silver, D.J. Triggle, Adv. Drug. Res., 1987, 16, 309.
[8] P.P. Mager, R.A. Coburn, A.J. Solo, D.J. Triggle, H. Rothe, Drug. Design. Dis., 1992, 8, 273.
[9] A. Sausins, G. Duburs, Heterocycles., 1988, 27, 269.
[10] R. Manmhold, B. Jablonka, W. Voigdt, K. Schoenafinger, E. Schraven,. J. Med. Chem., 1992, 27, 229.
[11] A.C. Gaudio, A. Korokovas, Y. Takahata, J. Pharmaceut. Sci., 1994, 83, 1110.
[12] Hantzsch, A., Der Chemie., 1882, 215, 1.
[13] G.W. Wang, J.J. Xia, C.B. Miao, X.L. Wu, Bull. Chem. Soc. Jpn., 2006, 3, 454.
[14] M.A. Zolfigol, M. Safaiee, Synletter., 2004, 827.
[15] J.B. Sainani, A.C. Shah, Indian. J. Chem., 1994, 33B, 526.
[16] M. Sufirez, E. Ochoa, Y. Verdecia, B. Verdecia, L. Moran, N. Martin, M. Quinteiro, C. Seoane, J.L. Soto, H. Novoa, N. Blaton, O.M. Peters, Tetrahedron., 1999, 55, 875.
[17] N.N. Karade, V.H. Budhewar, S.V. Shinde, W.N. Jadhav, Lett. Org. Chem., 2007, 16.
[18] C.S. Reddy, M. Raghu, Chinese. Chem. Lett., 2008, 19, 775.
[19] M. Maheswara, V. Siddaiah, G.L. Damu, C. Venkata Rao, Arkivoc., 2006, 2, 201.
[20] G. Sabitha, G.S.K. Reddy, C.S. Reddy, J.S. Yadav, Tetrahedron Lett., 2003, 44, 4129.
[21] S.B. Sapkal, K.F. Shelke, B.B. Shingate, M.S. Shingare, Tetrahedron Lett., 2009, 50, 1754.
[22] R. Surasani, D. Kalita, A.V. Dhanunjaya-Rao, K. Yarbagi, K.B. Chandrasekhar, J. Fluor. Chem., 2012, 135, 91.
[23] M.M. Heravi, K. Bakhtiari, N.M. Javadi, F.F. Bamoharram, M. Saeedi, H.A. Oskooie, J. Mol. Catal. A: Chem., 2007, 264, 50.
[24] S.R. Cherkupally, R. Mekalan, Chem. Pharmaceut. Bulletin., 2008, 56, 1002.
[25] R.A. Mekheimer, A.A. Hameed, K.U. Sadek, Green Chem., 2008, 10, 592.
[26] M. Hong, C.H. Cai, W.B. Yi, J. Fluor. Chem., 2010, 131, 111.
[27] G.H. Mohammadi-Ziarani, A.R. Badiei, Y. Khaniania, M. Haddadpour, Iranian J. Chem. Chem. Eng., 2010, 29, 1.
[28] A. Kumar, R.A. Maurya,. Tetrahedron. lett., 2007, 48, 3887.
[29] H. Adibi, H.A. Samimi, M. Beygzadeh, Catal. Commun., 2007, 8, 2119.
[30] D. Zhao, M. Wu, Y. Kou, E. Min, Catal. Today., 2002, 74, 157.
[31] T. Welton, Coord. Chem. Rev., 2004, 248, 2459.
[32] A.I. Bhatt, A. Mechler, L.L. Martin, A.M. Bond, J. Mat. Chem., 2007, 17, 2241.
[33] J.F. Liu, J.A. Jonsson, J.B. Jiang, Trends Anal. Chem., 2005, 24, 20.
[34] R. Wang, T. Okajima, F. Kitamura, T. Ohsaka, Electroanalysis., 2004, 16, 66.
[35] Y. Liu, I. Shi, M. Wang, Z. Li, H. Lui, J. Li, Green. Chem., 2005, 7, 655.
[36] J.D. Holbrey, K.R. Seddon, Cleaner Production Processes., 1999, 1, 223.
[37] A.J. Carmichael, D.M. Haddleton, S.A.F. Bon, K.R. Seddon, Chem. Commun., 2000, 1237.
[38] F. Matloubi-Moghaddam, H. Saeidian, Z. Mirjafary, A. Sadeghi, J. Iranian Chem. Soc., 2009, 6, 317.
[39] P. Wassercheid, W. keim, Angewandte Chem. Int. Edn. Engl., 2002, 39, 3772.
[40] T. Biedron, P. Kubisa, Macromol. Rapid. Commun., 2001, 22, 1237.
[41] J.H. Clark, Acc. Chem. Res., 2002, 35, 791.
[42] V.T. Kamble, V.S. Jamode, N.S. Joshi, Tetrahderon. Lett., 2006, 47, 5573.
[43] V.T. Kamble, B.P. Bandgar, N.S. Joshi, Synletter., 2006, 2719.
[44] V.T. Kamble, K.R. Kadam, N.S. Joshi, Catal. Commun., 2007, 8, 498.
[45] V.T. Kamble, B.P. Bandgar, D.B. Muley, J. Mol. Catal. A: Chem., 2007, 268, 70.
[46] A. Shaabani, A. Rahmati, Z. Badri, Catal. Commun., 2008, 9, 13.
[47] J.L. Aderson, R. Ding, A. Ellern, D.W. Armstrong, J. Am. Chem. Soc., 2005, 127.



