Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Study on Molecular Interaction of N, N Dimethylimidodicarbonimidic Diamide at 308 K of Various Concentrations by Ultrasonic Method
Balakrishnan J1*, Prakash N1, Raghu Y2 and Ekambaram S3
1Department of Chemistry, Saveetha School of Engineering, SIMATS University, Chennai-602105, Tamil Nadu, India
2Department of Physics, Saveetha School of Engineering, SIMATS University, Chennai-602105, Tamil Nadu, India
3Department of Chemistry, RKM Vivekananda College, Chennai-600004, Tamil Nadu India
- *Corresponding Author:
- Balakrishnan J
Department of Chemistry
Saveetha School of Engineering, SIMATS University
Chennai-602105, Tamil Nadu, India
Abstract
To attempted sound shown on sound bounds of N,N Dimethylimidodicarbonimidic diamide, water system, on different concentration’s measured, weight by volume percentage [0.2%, 0.4%, 0.6%, 0.8% and 1.0%] at temperature of 308K using ultrasonic interferometer. The parameters similar ultrasonic velocity, compactness, Adiabatic compressibility, Sound Impedance, permitted distance, relation time, adsorption factor wada constant are measured and it is supportive for parallel of molecular interface of binary blend N, N Dimethylimidodicarbonimidic diamide water system.
Keywords
N, N Dimethylimidodicarbonimidic diamide, Sound impedance, Adiabatic compressibility, Adsorption factor, Permitted distance, Binary blend.
Introduction
Ultrasonic velocity data have been found to be quite useful in evaluating a large number of thermodynamic and acoustical and acoustical parameters of liquids and their mixtures. The common velocity (v) of the ultrasonic wave in certain water sample increases as the wave length (λ) of the ultrasonic wave, which shows that there is a strong molecular interaction between water molecules and the velocity decreases in the wave length which shows that nearby is a weak molecular interaction among water molecules.
The adiabatic compressibility (βs), in the free distance (Lf), and the absorption factor (α/f2) increases with decrease in the velocity, which shows that it’s weak molecular interaction among water molecules [1]. Similarly, the above acoustical parameters are decreases with increase in the velocity which appearances that a strong molecular interaction between water molecules [1]. The adiabatic compressibility is the fractional decrease of volume per unit increase of pressure, when no heat flows in or out [2]. Adiabatic compressibility (β) exhibits an exact reverse trend as of ultrasonic velocity for the solutions studied at dissimilar concentrations [3]. The available solvent molecules for the next inward component get decreased and every solvent has a limit for the compression as regulating compressibility value [4]. Solvents examined were chosen to cover a wide range of adiabatic compressibility [5]. This favors increase in intermolecular distance into solute and solvent molecules making relatively wider gaps the molecules and becoming the main cause of impediment in the dissemination of ultrasonic waves [4]. The molecular interactions are between solute and solvent into intermolecular hydrogen bonding.
The intermolecular free length (Lf) also follows the same trend as that of adiabatic compressibility in solutions studied at different concentrations and free length is greater at low concentration which could be due to solvent self-association [6]. The decrease in the distance with increase in ultrasonic velocity beside concentration increases intermolecular force between solute and solvents [7]. Absorption coefficient is a reduction coefficient is a distinguishing parameter of the medium and it depends on outside condition like temperature, pressure and frequency of measurement [4].
The experimental application of using mixing solvent, solvent in trade and organic processes in case of solvent or solvent provides a wide range of mixture with desirable properties [3,4,6]. N,N Dimethylimidodicarbonimidic diamide is oral diabetic medicine which helps to control blood sugar level, kidney damage, nerve problems. Loss of limbs and sexual functional problems. Water solvent with the combination of N, N Dimethylimidodicarbonimidic diamide with water at various concentrations for the correlation of molecular interaction [2,5,8-16].
Materials and Methods
Solvent was purified by double distillation water and solution of N, N Dimethylimidodicarbonimidic diamide with different concentration by weight by volume percentage is prepared at room temperature (308K). All the solution allowed association at constant temperature in constant temperature. The density of solution was carried out using a pycnometer of bulb capacity of 10 ml. The accuracy of density was found to be +0.001 g/cc. The viscosities of binary mixture were determines using an Oswald Viscometer (sigma chemical instruments). The ultrasonic velocities of pure solvent, solvent-solute mixture using a single crystal path interferometer at 2 MHz (Mittal Enterprises, New Delhi). The ultra sound properties were studied.
Results and Discussion
From the variation of densities, viscosities and ultrasonic parameters with concentration and temperature, a qualitative interpretation of the intermolecular interactions (Table 1) in the above binary mixtures can be proposed. An increase in the density of a solution with dilution is the expected trend [9]. As the concentration increases the densities of solution of N, N Dimethylimidodicarbonimidic diamide water system also increases.
| Concentration of Solution | Density of Solution | Viscosity× 10-3 | Ultrasonic velocity | Adiabatic Compressibility (β) × 10-7 | Acoustic Impedance | Free Length Lf × 10-10 | Relaxation time × 10-9 | Absorption Co-efficient × 10-11 | Wada constant |
|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.9796 | 8.7592 | 1635 | 3.8198 | 1601.84 | 6.5476 | 4.4587 | 5.3769 | 941.67 |
| 0.4 | 0.9812 | 8.8238 | 1768 | 3.2605 | 1734.76 | 6.0495 | 3.8359 | 4.2784 | 940.14 |
| 0.6 | 0.9814 | 8.8814 | 1788 | 3.1873 | 1754.74 | 5.9812 | 3.7743 | 4.1356 | 928.94 |
| 0.8 | 0.9931 | 9.0438 | 1883 | 2.8414 | 1869.55 | 5.5474 | 3.4263 | 3.5891 | 928.87 |
| 1 | 0.994 | 9.1362 | 1996 | 2.2518 | 1984.02 | 5.324 | 3.0763 | 3.0392 | 928.03 |
Table 1: Study Molecular interaction of N,N Dimethylimidodicarbonimidic diamide at 35ºC of various concentration by ultrasonic method
Adiabatic comperssibility (β)
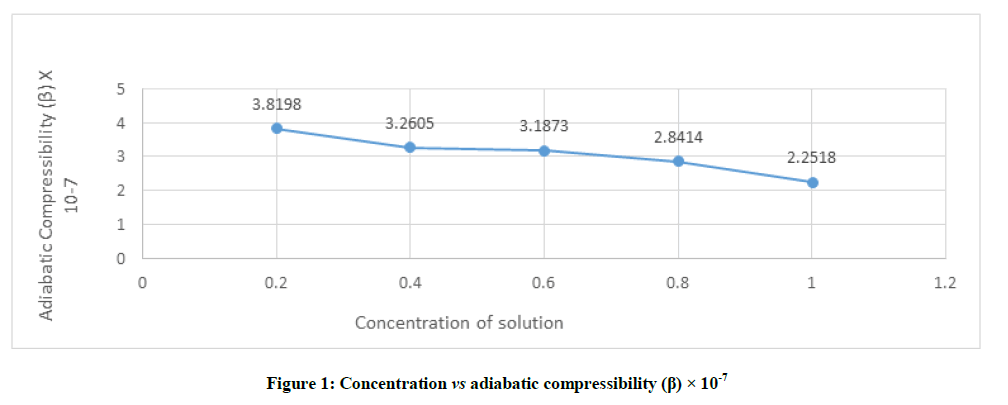
β=1/ U2ρ Kg-1ms2, where U is ultrasonic velocities and ρ is density of liquid mixtures. The adiabatic compressibility (β) exhibits an exact reverse trend as that of ultrasonic velocity for the solutions studied at different concentrations. As shown in Figure 1, the adiabatic compressibility (β) of the studied solution varies from 2.2518 to 3.8198 and the highest value is recorded for the lowest concentration (0.2) of studied sample. The compressibility is greater at low concentration and it decreases with increase in concentration of solution. It confirms the presence of molecular association among solute-solvent molecule through intermolecular H-boding.
Acoustic impedance (Z)
The specific acoustic impedance is related to density and ultrasonic velocity by the relation
Z = Uρ Kgm-2S-1
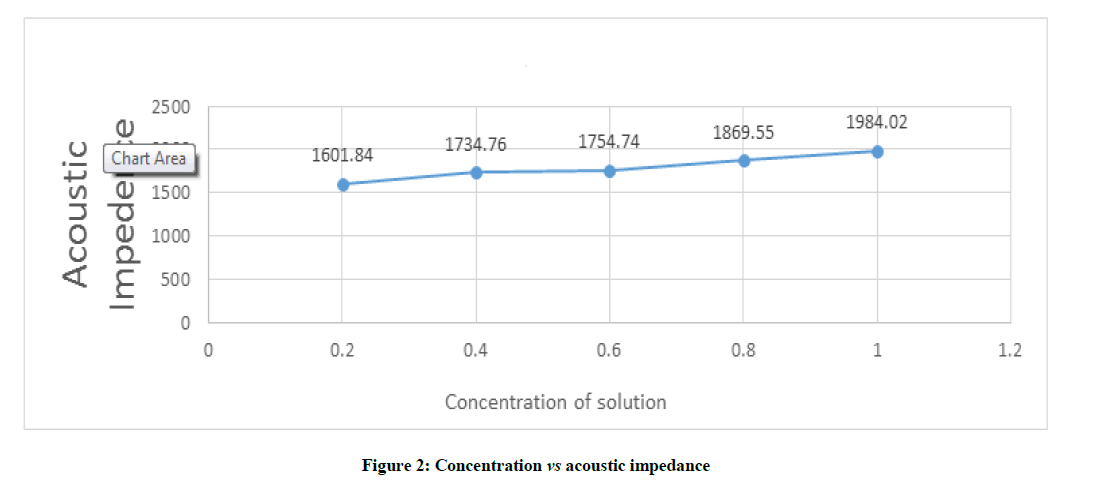
Figure 2 depicts that the acoustic impedance increases as concentration increases and vice versa. The value of Acoustic Impedance varies from 1601.84 to 1984.02 with corresponding variation in concentration of studied sample from 0.2 to 1.0.
Intermolecular free length (Lf)
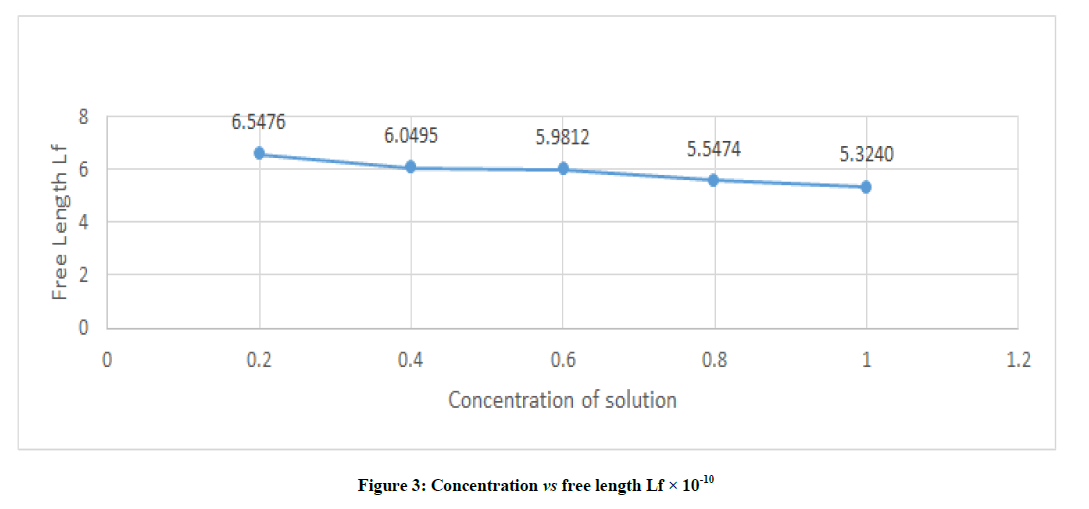
The free length is the distance covered by sound wave between the surface of neighboring molecules and is related to ultrasonic velocity and Lf=K / (ρ U)1/2 m, K=(93.785+0.345 T ) X 10-8. From Figure 3, it was found that the intermolecular free length decreases with increases in concentration and this behavior is very gradual. The range if intermolecular free length of studied sample shows variations in concentration from 0.2 to 1.0 from 6.5476 to 5.3240. This shows the specific inter-actins existing in the solution.
Relaxation time (τ)
The relaxation time and adsorption coefficient are directly interrelated. The adsorption of sound wave is the result of time lag between the passing’s of ultra-sonication and return of molecule to their equilibrium position. It is computed using the relation
τ = 4η / 3 ρU2 sec
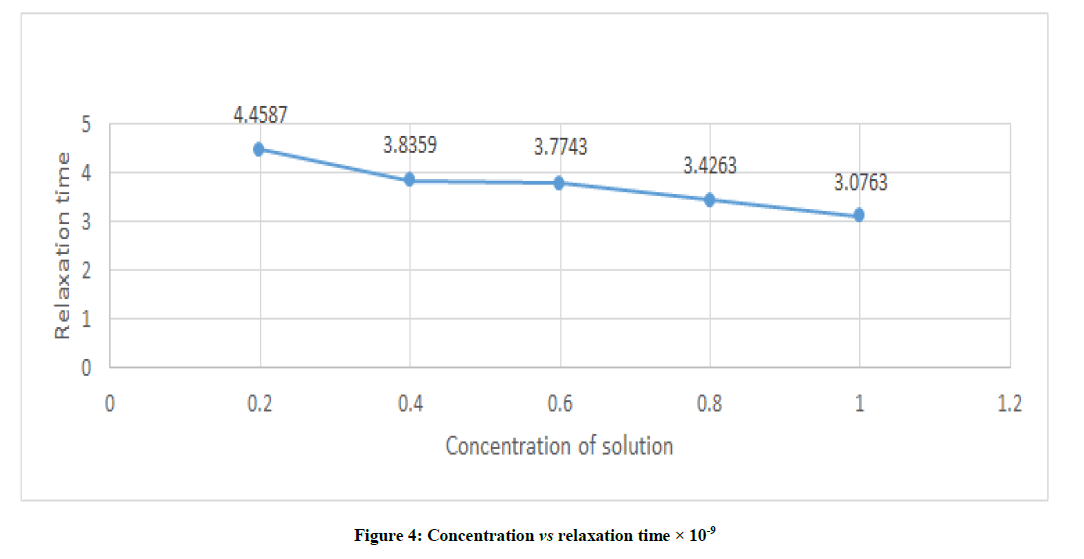
From Figure 4, it is clear that the relaxation time decreases from 4.4587 to 3.0763 with increase in concentration of studied sample from 0.2 to 1.0.
Absorption coefficient (α/f2)
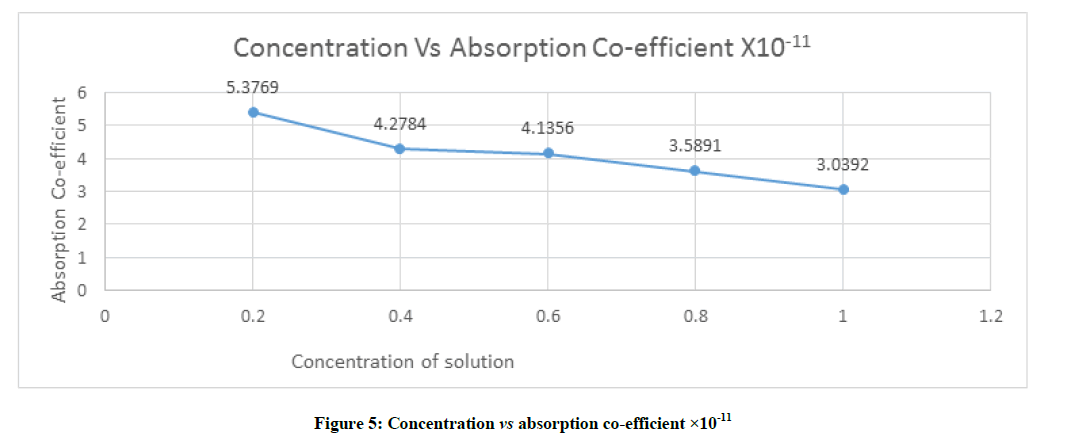
Absorption coefficient is also called attenuation coefficient and it is characteristic parameter of medium and depends on the external condition like temperature pressure and frequency of measurement which is shown in Figure 5 and given by the formula
(α/f2) = 8Π2η/[3ρU3] Npm-1s2)
From the Figure 5 the cohesive energy of different concentration solvent–solute mixture decreases from 5.3769 to 3.0392 with concentration of range 0.2 to 1.0.
Molar compressibility or Wada’s constant (B)
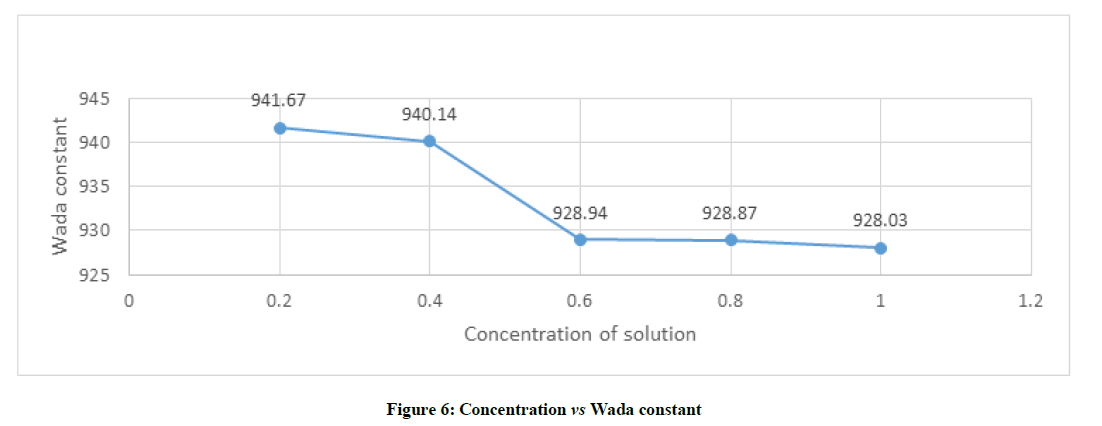
Molar compressibility is dependent on adiabatic compressibility and density which is given by formula B=(M/ρ) K-1/7 from the Figure 6 depicts that wada constant decreases with increases in concentration in general. But is found that when the concentration of solution varies from 0.4 to 0.6, the amount of decrease of wada constant is more. The concentration increases from 0.6 to 0.8 or from 0.8 to 1.0, wada constant decreases very least amount or it can be said that almost constant. This kind of behavioural change wada constant which shows there is specific molecular interaction s at a particular concentration which are responsible for increase in absorption and transmission property of the sample. It can also be concluded that molecular interactions in studied sample at particular concentration re due to complex formation on the basis of hydrogen bonding.
Conclusion
From the data obtained based on the molar concentration of solution N,N Dimethylimidodicarbonimidic diamide–water system, the concentration at 0.4% and 0.6% of shows variation among the other molar concentration, which indicate effective molecular interaction taking place in the concentration between 0.4 to 0.6%.
Acknowledgement
I thank department of Chemistry, Physics, Management of Saveetha Institute of Medical and Technical Sciences for given me the opportunity, constant encouragement, support to do the work and make this article to be published.
References
- C.C. Kanagam, J. Balakrishnan, R.T. Selvan, Indian J. Phys. Chem., 2011, 6(1).
- S. Thirumaran, J.E. Jayakumar, Indian J. Pure Applied Phys., 2009, 47, 265.
- J. Balakrishnan, S. Ekambaram S. Rajesh, V. Balasubramanian, J. Elixir Appl. Chem., 2013, 65, 20063- 20067.
- P.S. Nikam, M.C. Jadhav, M.J. Hasan, J. Molec. Liq., 1998, 76, 1.
- V. Kannappan, R. Jayasanthi, E.J.P. Malar, J. Phys. Chem. Liq., 2000, 40 133.
- P.S. Nikam, T.R. Mahale, M. Hasan, J. Acoustica Acta., 1998, 84, 579.
- S. Thirumaran, K. Ramya, Asian J. Chem., 2009, 21(8), 6359.
- P.S. Nikam, T.R. Mahale, M. Hasan, Indian J. Pure Appl. Phy., 1999, 37, 92.
- O.P. Chimankar, R. Shiriwas, V.A. Tabhane, J. Chemical Pharmaceut. Res., 2011, 3(3), 587-596.
- S. Thirumaran, P. Anbuselvi, Asian J. Chem., 2009, 21(9), 7261.
- S. Thirumaran, D. George, J. Eng. Applied Sci., 2008, 27(2), 281.
- S.R. Aswale, S.S. Aswale, A.B. Dhote, J. Chemical Pharmaceut. Res., 2011, 3(6), 233-237.
- K. Sreekanth, D. Sravanakumar, M. Kondaiah, J. Chemical Pharmaceut. Res., 2011, 3(4), 229-241.
- J. Balakrishnan, V. Balasubramanian, J. Chemical Pharmaceut. Res., 2012, 4(8).
- J. Balakrishnan, V. Balasubramanian, J. Chemical Pharmaceut. Res., 2012, 4(9).
- J. Balakrishnan, G. Ramachandran, R. Rajeswari, V. Balasubramanian, J. Elixir Appl. Chem., 2013, 65, 19719-19723.