Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 3
Study of the reactivity of 4-hydroxycoumarin towards 3-oxopyrazolidinium ylures and arylidenemalononitriles
Yassine H1*, Fatima A1, Abderrahim E Haib4, Rahhal E Ajlaoui1,2, Said A1 and El M Rakib1,32De´partement de Chimie, Faculte´ des Sciences Applique´es Ait Melloul, Universite´ IBN ZOHR, N10. BP 6146 Cite´ Azrou, Ait Melloul, 86150 Agadir, Morocco
3Higher School of Technology, Sultan Moulay Slimane University, BP 336, Fkih Ben Salah, Morocco
4Laboratory of Chemistry of Natural Substances, Faculty of Sciences Semlalia, Cadi Ayyad University, B.P. 2390, Marrakech,, Morocco
Yassine H, Laboratory of Molecular Chemistry, Materials and Catalysis, Faculty of Sciences and Technics, Sultan Moulay Slimane University, Be´ni-Mellal, BP 523, Morocco, Email: yassine.hakmaoui@gmail.com
Received: 02-Mar-2022, Manuscript No. dpc-22-55961; Accepted Date: Mar 04, 2022 ; Editor assigned: 04-Mar-2022, Pre QC No. dpc-22-55961; Reviewed: 21-Mar-2022, QC No. dpc-22-55961; Revised: 26-Mar-2022, Manuscript No. dpc-22-55961; Published: 04-Apr-2022, DOI: 10.4172/0975-413X.14.3.34-40
Abstract
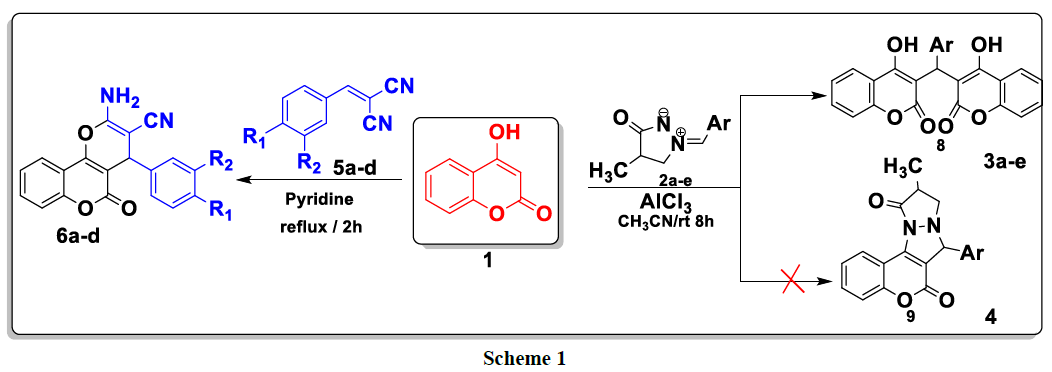
In the context of finding new heterocyclic systems pharmacological / biological activities, we were interested in this work in the study of the reactivity of hydroxycoumarin with respect to heterocyclic dipoles of the type: arylmethylidene-3-oxopyrazolidin-1-ium-2-ides 2a–e in acetonitrile in the presence of AlCl3 as catalyst and with arylidenemalononitriles 5a–d in refluxing pyridine. The biscoumarin 3a–e and pyranocomarin 6a–d were obtained with good to excellent yields (74–95%) and characterized by elemental analysis (Scheme 1).
Keywords
Biscoumarin, 4-hydroxycoumarin, Arylidenemalononitriles, pyranocomarin Cyclization, condensation, 4-methyl-3-oxo-1,2- pyrazolidiniums
Introduction
4-Hydroxycoumarin and its derivatives constitute a class of natural [1-6] products representing an interesting substrate in the pharmaceutical drug [7-13]; it has a wide range of biological applications because of their multitude of activities such as anticoagulant [14], Antioxidant [15, 16], anticorrosive [17], anti-inflammatory activities [18, 19], including antimicrobial [20-23], antiviral [24, 25], and anticancer [26-28]. In this regard, coumarin derivatives are important in organic synthesis studies [29-32], on our part due to the development of new therapeutic molecules, we have incorporated 4-hydroxycoumarin respectively with arylidénemalononitriles and 3-oxopyrazolidiniums ylures. This latter is stable as intermediates for the synthesis of various nitrogenous heterocycles, they react as 1,3-dipoles with certain dipolarophils, mainly with alkenes and alkynes and ketones [33]. Among the cyclic dipoles incorporating an N-N bond in a ring the most studied are the N-alkylidene-3- oxopyrazolidinium imides, which are stable and easily accessible. They have been used as 1,3-dipoles in different types of cycloadditions, not only [3 + 2] but also [3 + 3], [4 + 3] and [3 + 2 + 3], giving important products or intermediates for the preparation of many types of pharmaceuticals, agrochemicals and other biologically active compounds [34, 35]. Arylidénemalononitriles and these derivatives exhibit very significant pharmacological activity [36-39].
Result and Discussions
The treatment of methyl methacrylate with an excess of hydrazine hydrate under ethanol reflux allows to obtained the corresponding pyrazolidinone with a better yield, the condensation of this latter with aromatic aldehydes in ethanol at room temperature in the presence of an acid leads to 4- methyl-3-oxo-1,2-pyrazolidiniums ylures 2a – e with good yields. The compounds are prepared according to the procedure described in the literature [40]. We have succeeded in preparing differently substituted dipoles with good yields (Table 1).
| Entry | Ar | Time (h) | mp (°c) | Yield of 2a-e (%) | ||
|---|---|---|---|---|---|---|
| 2a | C6H5 | 12 | 123-125 | 80 |
|
|
| 2b | 4-ClC6H5 | 16 | 185-187 | 86 | 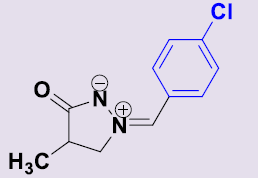 |
|
| 2c | 4-(CH3)2NC6H4 | 12 | 178-180 | 82 | 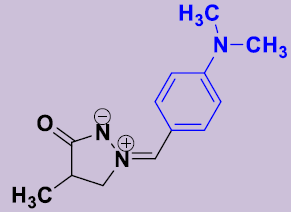 |
|
| 2d | 3-BrC6H4 | 24 | 162-164 | 78 | 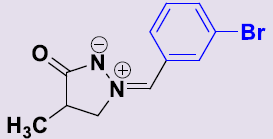 |
|
| 2e | 2-Furyl | 20 | 170-172 | 86 | 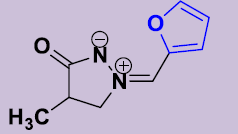 |
|
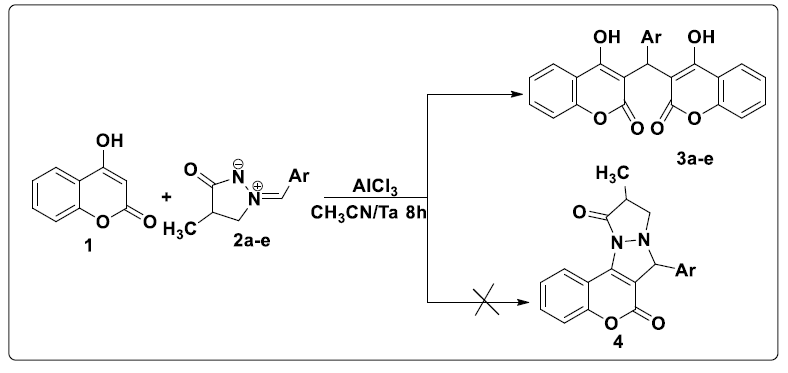
Contrary to was observed in the literature, the reaction of 4-hydroxycoumarin 1 with various ylures of 4-methyl-3-oxo-1,2-pyrazolidiniums 2a-e in acetonitrile and in the presence of AlCl3 as a catalyst at room temperature led to the formation of biscoumarins 3a-e with good yields (80-96%, (scheme 2). No cycloadduct 4 resulting from the addition of the dipole to the C = C double bond was detected under the above conditions.
In the 1H NMR spectrum of compounds 3a-e, more the signals of the aromatic protons, we note a signal in the form of a singlet between 6.20 and 6.30 ppm attributable to the proton of the methylene group CH. The chemical shift of carbon CH appears at around 36 ppm in the 13C NMR spectrum of compounds 3a-e (Table 2).
| Entry | Ar | Mp (°C) | Yield (%) | |
|---|---|---|---|---|
| 3a | C6H5 | 225-227 | 80 | 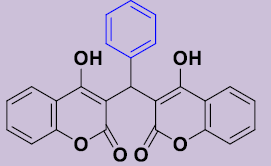 |
| 3b | 4-ClC6H5 | 250-252 | 86 | 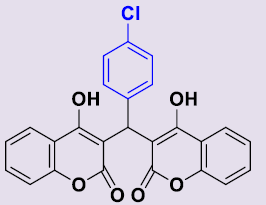 |
| 3c | 4-(CH3)2NC6H4 | 270-272 | 82 | 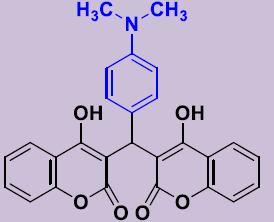 |
| 3d | 3-BrC6H4 | 258-260 | 90 | 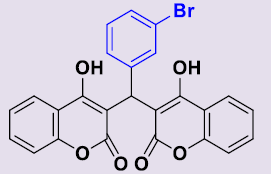 |
| 3e | 2-furyl | 245-248 | 96 | 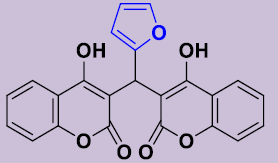 |
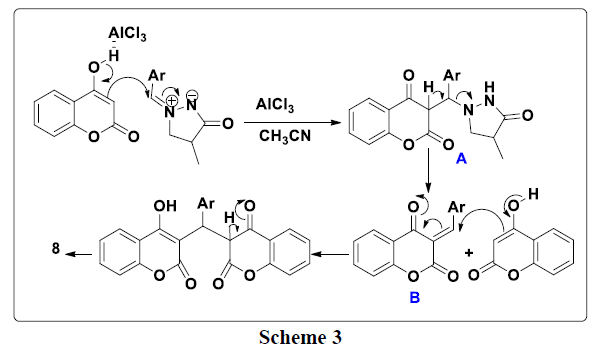
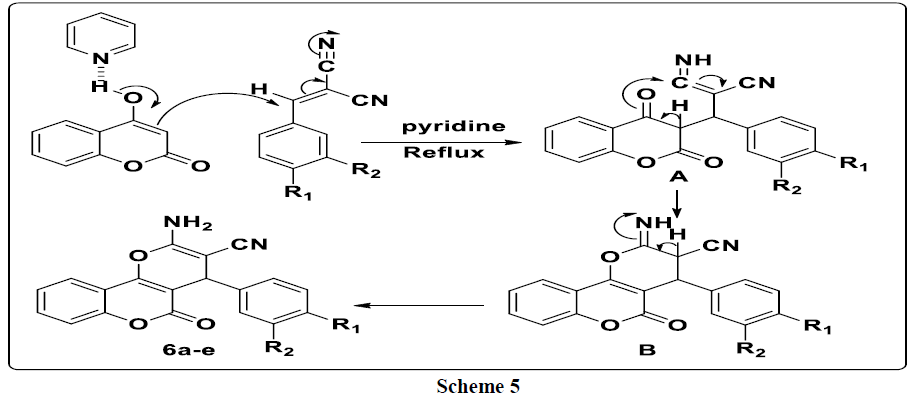
A plausible mechanism to explain the formation of the 3a-e compounds is provided in (Scheme 3). First, 4-hydroxycoumarin reacted with 4-methyl- 3-oxo-1, 2-pyrazolidinium 2a-e to give intermediate A. This the latter rearranges via the elimination of pyrazolidinone thus leading to the formation of intermediate B which reacts with a second molecule of 4-hydroxycoumarin to give bis-coumarin 3a-e.
Reactivity of arylidenemalonitriles towards 4-hydroxycoumarin
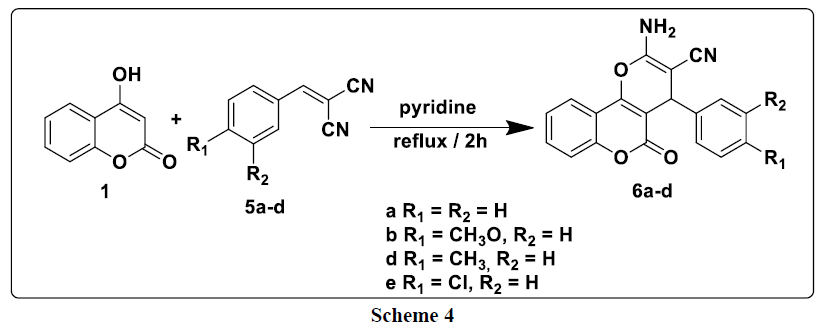
The first step of this study consists to reacted differently substituted aldehydes with malononitrile in ethanol reflux in the presence of phosphorus pentoxide as a catalyst; it leads to the formation of the corresponding arylidenemalononitriles 4a-d. This latter are then treated, in the second step, with 4-hydroxycoumarin 1 at reflux in pyridine to produce new products of the type: 2-amino-4- (aryl) -4H-benzo[h]chromene-3- carbonitriles 6a-d (Scheme 4).
In the 1H NMR spectrum of compounds 6a-d, we note in particular the presence of a signal at around 4.38 ppm attributable to the proton of the CH group and a signal at 7.38 ppm due to the protons of the NH2 group (Table 3).
| Entry | R | Yields (%) | Mp (°C) |
|---|---|---|---|
| 6a | R1 = R2 = H | 95 | 258-260 |
| 6b | R1 = CH3O, R2 = H | 90 | f248-250 |
| 6d | R1 = CH3, R2 = H | 98 | 261-263 |
| 6e | R1 = Cl, R2 = H | 95 | 256-258 |
For explain the formation of compounds 6a-e, we have proposed the following mechanism: 4-hydroxycoumarin reacts with arylidenemalononitrile to give intermediate A. This latter rearranges via intramolecular cyclization leading to the formation of intermediate B, which rearranges to give the expected compounds 6a-e (Scheme 5).
Conclusions
Our study invented a new practical access to the synthesis of new comarine derivative, by the aldol condensation of 4-hydroxycoumarin with a dipole of the type: 4-methyl-3-oxo-1,2-pyrazolidiniums ylures in the acetonitrile at room temperature and in the presence of AlCl3 as catalyst. We optioned a coumarin dimer bearing an aryl substituent in the central part with an excellent yield. On the other hand, in the presence of arylidènemalononitriles with reflux of pyridine allowing access to various pyranocoumarins, we have developed a rapid and efficient study of the synthesis to access new heterocyclic systems with good yields, which can present promising biological properties.
Experimental Section
General procedure for synthesis of compound 2a-e
20 mmol of 4-methyl pyrazolidin-3-one and 20 mmol of the aromatic aldehyde dissolved in 15 ml of ethanol and then we added a few drops of TFA. The mixture stirred at room temperature for various times. After evaporating off the solvent, the crude product obtained recrystallized in ethanol, then washed with ethyl acetate, and filtered under vacuum.
General procedure for synthesis of compound 3a-e
In a 250 ml round-bottomed flask, the dipole (10 mmol), 4-hydroxycoumarin (10 mmol) and AlCl3 (2 mmol) are suspended with mechanical stirring in 25 ml of absolute acetonitrile at room temperature. After the completion of the reaction monitored by TLC, the solvent is removed under reduced pressure, the crude mixture is separated by chromography on silica gel (hexane-acetate = 4: 6).
General procedure for synthesis of compound 6a-d
4-Hydroxycoumarin (0.01 mol) and substituted arylidenemalononitriles (0.01 mol) added to 15 ml of the pyridine. The reaction mixture brought to reflux for 8 h. The crude product collected by filtration and recrystallized from ethanol, the appropriate product optioned in good yield.
(Z)-Benzylidène-4-méthyl-3-oxopyrazolidin-2-ium-1-ide 2a. White solid; mp 123–125 °C; 1H NMR (DMSO-d6, 300 MHz) : δ 1.15 (d, 3H, J = 7.2 Hz), 2.68–2.76 (m, 1H), 4.18 (dd, 1H, J = 7.6 Hz, 13.0 Hz), 4.73 (dd, 1H, J = 8.9 Hz, 13.0 Hz), 7.48–7.51 (m, 3H), 7.63 (s, 1H), 8.26–8.30 (m, 2H). 13C NMR (DMSO-d6, 75 MHz): δ 15.9 (CH3), 35.3 (CH), 64.6 (CH2N), 129.1 (2CH), 130.3 (C), 131.4 (2CH), 131.5 (CH), 132.5 (CH), 187.5 (CO).
(Z)-(4-Diméthylaminobenzylidene)-4-méthyl-5-oxopyrazolidin-2-ium-1-ide 2b. White solid; mp 178-180 °C; 1H NMR (DMSO-d6, 300 MHz): δ 1.13 (d, 3H, J = 7.2 Hz), 2.60–2.68 (m, 1H), 3.00 (s, 6H, N(CH3)2), 3.98-4.06 (m, 1H), 4.54-4.62 (m, 1H), 6.76 (d, 2H, J = 9.3 Hz), 7.41 (s, 1H), 8.10 (d, 2H, J = 9.3 Hz). 13C NMR (DMSO-d6, 75 MHz): δ 16.0 (CH3), 35.7 (CH), 40.0 (2CH3), 63.2 (CH2N), 111.6 (2CH), 117.5 (C), 133.5 (2CH), 133.9 (=CH), 152.2 (C), 186.1 (CO).
(Z)-(3-Bromobenzylidene)-4-méthyl-3-oxopyrazolidin-2-ium-1-ide 2c. White solid; mp 162-164°C; 1H NMR (DMSO-d6, 300 MHz): δ 1.16 (d, 3H, J = 7.2 Hz), 2.70–2.77 (m, 1H), 4.18-4.25 (m, 1H), 4.71-4.78 (m, 1H), 7.47 (t, 1H, J = 8.1 Hz), 7.63 (s, 1H), 7.69 (dd, 1H, J = 8.1 Hz, 0.9 Hz), 8.09 (d, 1H, J = 8.1 Hz), 8.68 (t, 1H, J = 1.5 Hz); 13C NMR (DMSO-d6, 75 MHz): δ 15.8 (CH3), 35.1 (CH), 64.8 (CH2N), 122.3 (C), 130.3 (CH), 130.5 (CH), 131.2 (CH), 132.4 (C), 132.9 (CH), 133.9 (CH), 187.7 (CO).
(Z)-(4-Chlorobenzylidene)-4-méthyl-3-oxopyrazolidin-2-ium-1-ide 2d. White solid; mp 185-187 °C; 1H NMR (DMSO-d6, 300 MHz):δ 1.15 (d, 3H, J = 7.2 Hz), 2.68–2.77 (m, 1H), 4.16-4.23 (m, 1H), 4.69-4.77 (m, 1H), 7.58 (d, 2H, J = 8.7 Hz), 7.64 (s, 1H), 8.29 (d, 2H, J = 8.7 Hz); 13C NMR (DMSO-d6, 75 MHz): δ 15.8 (CH3), 35.2 (CH), 64.6 (CH2N), 129.2 (C), 129.3 (2CH), 131.1 (=CH), 132.9 (2CH), 135.9 (C), 187.5 (CO).
(Z)-4-Méthyl-2-(furylidene)-3-oxopyrazolidin-2-ium-1-ide 2e. White solid; mp 170-172 °C; 1H NMR (DMSO-d6, 300 MHz): δ 1.14 (d, 3H, J = 7.2 Hz), 2.67–2.80 (m, 1H), 4.06-4.13 (m, 1H), 4.62-4.70 (m, 1H), 6.77 (dd, 1H, J = 4.0 Hz, 3.4 Hz), 7.58 (d, 1H, J = 4.1 Hz), 7.75 (s, 1H), 7.95 (d, 1H, J = 3.0 Hz). 13C NMR (DMSO-d6, 75 MHz): δ 15.8 (CH3), 36.0 (CH), 63.0 (CH2N), 113.8 (CH), 119.5 (CH), 121.4 (CH), 146.6 (C), 147.1 (CH), 187.1 (CO).
2-(Benzylidene)malononitrile 5a. White solid; mp 88-90°C; 1H NMR (DMSO-d6): δ 7.57–7.68 (m, 3H, H-Ar), 7.91–7.95 (m, 2H, H-Ar), 8.52 (s, 1H, H-vinyl). 13C NMR (DMSO-d6): δ 82.1 (C), 113.7 (CN), 114.6 (CN), 129.9 (2CH), 131.0 (2CH), 131.8 (C), 134.8 (CH), 162.0 (CHvinyl).
2-(4-Methoxybenzylidene)malononitrile 5b. yellow solid; mp 110–112°C; 1H NMR (DMSO-d6): δ 3.86 (s, 3H, CH3O), 7.14 (d, 2H, J = 9.0 Hz), 7.93 (d, 2H, J = 9.0 Hz), 8.34 (s, 1H, H-vinyl).13C NMR (DMSO-d6): δ 56.4 (CH3O), 77.3 (C), 114.3 (CN), 115.2 (CN), 115.6 (2CH), 124.6 (C), 133.8 (2CH), 160.9 (CHvinyl), 164.8(=CO).
2-(4-Methylbenzylidene)malononitrile 5c. White solid; mp 130–132°C; 1H NMR (DMSO-d6): δ 2.39 (s, 3H, CH3), 7.41 (d, 2H, J = 8.1 Hz), 7.84 (d, 2H, J = 8.1 Hz), 8.45 (s, 1H, H-vinyl). 13C NMR (DMSO-d6): δ 21.9 (CH3), 80.4 (C), 113.9 (CN), 114.8 (CN), 129.2 (C), 130.6 (2CH), 131.1 (2CH), 146.1 (C), 161.8(CHvinyl).
2-(4-Chlorobenzylidene)malononitrile 5d. White solid; mp 148–150°C; 1H NMR (DMSO-d6): δ 7.67 (d, 2H, J = 8.4 Hz), 7.92 (d, 2H, J = 8.4 Hz), 8.51 (s, 1H, H-vinyl). 13C NMR (DMSO-d6): δ 82.7 (C), 113.5 (CN), 114.5 (CN), 130.2 (2CH), 130.6 (C), 132.6 (2CH), 139.5 (C), 160.6 (CHvinyl).
3, 3'-[(Phényl) méthylene]bis(4-hydroxy-2H-chromen-2-one) 3a. White solid; mp 225-227°C; 1H NMR (DMSO-d6): 6.28 (s, 1H; CH), 7.07-7.28 (m, 9H, H-Ar), 7.48-7.51 (m, 2H, H-Ar), 7.80-7.83 (m, 2H, H-Ar), 11.58 (s, 2H, OH). 13C NMR (DMSO-d6): 36.6 (CH), 103.9 (2C), 115.9 (2CH), 120.4 (2C), 123.3 (2CH), 124.6 (2CH), 125.3 (CH), 127.1 (2CH), 128.2 (2CH), 131.4 (2CH), 142.8 (C), 153.0 (2C), 165.1 (2C), 168.2 (2CO).
3, 3'-[(4-Chlorophényl)méthylene]bis(4-hydroxy-2H-chromen-2-one) 3b. White solid; mp 190-192°C; 1H NMR (DMSO-d6): 6.24 (s, 1H; CH), 7.09-7.28 (m, 8H, H-Ar), 7.48-7.54 (m, 2H, H-Ar), 7.80-7.83 (m, 2H, H-Ar), 11.64 (s, 2H, OH). 13C NMR (DMSO-d6): 36.2 (CH), 103.6 (2C), 115.9 (2CH), 120.3 (2C), 123.4 (2CH), 124.6 (2CH), 128.1 (2CH), 129.0 (2CH), 129.8 (C), 131.5 (2CH), 141.9 (C), 153.0 (2C), 164.9 (2C), 168.2 (2CO).
3, 3'-[(4-(Diméthylaminophenyl) méthylene]bis(4-hydroxy-2H-chromen-2-one) 3c. White solid; mp 270-272°C; 1H NMR (DMSO-d6): 2.89 (s, 6H, N(CH3)2), 6.20 (s, 1H; CH), 6.77-6.81 (m, 2H, H-Ar), 6.99 (d, 2H, H-Ar), 7.20-7.30 (m, 4H, H-Ar), 7.47-7.53 (m, 2H, H-Ar), 7.81 (dd, 2H, H-Ar), 11.45 (s, 2H, OH). 13C NMR (DMSO-d6): 35.8 (CH), 42.3 (NCH3), 104.1 (2C), 115.9 (2CH), 120.4 (2C), 123.3 (2CH), 124.5 (2CH), 127.9 (2CH), 131.3 (2CH), 141.9 (C), 152.9 (2C), 165.0 (2C), 168.1 (2CO).
3, 3'-[(3-Bromophényl)méthylene]bis(4-hydroxy-2H-chromen-2-one) 3d. White solid; mp 258-260°C; 1H NMR (DMSO-d6): 6.27 (s, 1H; CH), 7.13-7.30 (m, 8H, H-Ar), 7.49-7.55 (m, 2H, H-Ar), 7.83 (dd, 2H, H-Ar), 11.58 (s, 2H, OH). 13C NMR (DMSO-d6): 36.5 (CH), 103.3 (2C), 116.0 (C), 120.3 (2C), 121.8 (2CH), 123.5 (2CH), 126.4 (CH), 129.6 (CH), 130.5 (CH), 131.6 (2CH), 146.9 (C), 152.9 (2C), 164.9 (2C), 168.3 (2CO).
3, 3'-[(Furyl)méthylene]bis(4-hydroxy-2H-chromen-2-one) 3e. White solid; mp 245-248°C; 1H NMR (DMSO-d6): 6.24 (s, 1H; CH), 7.14-7.34 (m, 7H, H-Ar), 7.32-7.43 (m, 2H, H-Ar), 7.68-7.82 (m, 2H, H-Ar), 11.76 (s, 2H, OH). 13C NMR (DMSO-d6): 32.1 (CH), 102.0 (2C), 102.3 (CH), 105.6 (CH), 115.9 (2CH), 116.2 (2C), 123.2 (2CH), 124.6 (2CH), 130.0 (2CH), 140.1 (CH), 152.9 (2C), 164.4 (2C), 168.3 (2CO).
2-Amino-4, 5-dihydro-5-oxo-4-phénylpyrano [3,2-c]chromene-3-carbonitrile 6a. White solid; mp 242-244°C; 1H NMR (DMSO-d6): 4.45 (s, 1H, CH), 7.27-7.35 (m, 5H, H-Ar), 7.40 (s, 2H, NH2), 7.45-7.52 (m, 2H, H-Ar), 7.7 (td, 1H, H-Ar), 7.91 (dd, 1H, H-Ar). 13C NMR (DMSO-d6): 37.4 (CH), 58.5 (=C), 104.5 (C), 113.4 (C), 117.0 (CH), 119.7 (CN), 122.9 (CH), 125.1 (CH), 127.6 (CH), 128.1 (2CH), 128.9 (2CH), 133.4 (CH), 143.7 (C), 152.6 (C), 153.9 (C), 158.5 (C), 160.0 (C=O).
2-Amino-4, 5-dihydro-4-(4-methoxyphenyl)-5-oxopyrano [3,2-c]chromene-3-carbonitrile 6b. White solid; mp 248-250°C; 1H NMR (DMSO-d6): 3.72 (s, 3H, OCH3), 4.40 (s, 1H, H-4), 6.87 (d, 2H, J = 8.1 Hz, H-Ar), 7.18 (d, 2H, J = 8.1 Hz, H-Ar), 7.37 (s, 2H, NH2), 7.45 (d, 1H, J = 8.3 Hz, H-Ar), 7.49 (t, 1H, J = 7.8 Hz, H-Ar), 7.70 (t, 1H, J = 7.7 Hz, H-Ar), 7.89 (d, 1H, J = 7.7 Hz, H-Ar). 13C NMR (DMSO-d6): 55.9, 59.1, 105.1, 113.8, 114.7, 117.4, 120.2, 123.3, 125.5, 129.6, 133.7, 136.3, 152.9, 153.9, 158.8, 159.2, 160.4.
2-Amino-4, 5-dihydro-5-oxo-4-p-tolylpyrano[3,2-c]chromene-3-carbonitrile 6c. White solid; mp 235-237°C; 1H NMR (DMSO-d6): 2.27 (s, 3H, CH3), 4.41 (s, 1H, H-4), 7.10-7.16 (m, 4H, H-Ar), 7.38 (s, 2H, NH2), 7.44-7.52 (m, 2H, H-Ar), 7.7 (td, 1H, H-Ar), 7.90 (dd, 1H, H-Ar). 13C NMR (DMSO-d6): 21.1 (CH3), 37.0 (CH), 58.6 (=C), 104.6 (C), 113,5 (C), 117.0 (CH), 119.7 (CN), 122.9 (CH), 125.1 (CH), 128.0 (2CH), 129.5 (2CH), 133.4 (CH), 136.7 (C), 140.8 (C), 152.6 (C), 153.7 (C), 158.4 (C), 160.0 (C=O).
2-Amino-4-(4-chlorophényl)-4,5-dihydro-5-oxopyrano[3,2-c]chromene-3-carbonitrile 6d. White solid; mp 256-258°C; 1H NMR (DMSO-d6): 4.50 (s,1H, H-4), 7.31 (d, 2H, J = 8.2 Hz, H-Ar), 7.36 (s, 2H, NH2), 7.38 (s, 2H, H-Ar), 7.44 (d, 1H, J = 8.2 Hz, H-Ar), 7.49 (t, 1H, J = 7.6 Hz, H-Ar), 7.71 (t, 1H, J = 7.8 Hz, H-Ar), 7.92 (d,1H, J = 7.8 Hz, H-Ar). 13C NMR (DMSO-d6) : 58.6, 104.4, 113.8, 117.3, 119.8, 123.4, 125.4, 129.3, 130.4, 132.6, 133.7, 143.1, 153.0, 154.4, 158.9, 160.3.
REFERENCES
- Rosselli S, Maggio AM, Faraone N et al., Nat Prod Commun. 2009, 4(12): p.1934578X0900401219.
- Shabbir M, Sultani SZ, Jabbar A et al., Phytochemistry. 1997, 44(4): p.683-685.
- Fuller RW, Bokesch HR, Gustafson KR et al., Med Chem Lett. 1994, 16: p.1961.
- Spino C, Dodier M and Sotheeswaran S. Bioorg Med Chem Lett. 1998, 8(24): p.3475-3478.
- Mead JAR, Smith JN and Williams RT. Biochem J.1958, 68(1): p.67.
- Venugopala KN, Rashmi V and Odhav B. BioMed Res Int. 2013.
- Lacy A and O'Kennedy R. Curr Pharm Des. 2004, 10(30): p.3797-3811.
- Musa MA, Badisa VL, Latinwo LM et al., Anticancer Res. 2010, 30(11): p.4613-4617.
- Musa MA, Joseph MY, Latinwo LM et al., Anticancer Res. 2015, 35(2): p.653-659.
- lina AA, wageeh AY, farkaad AK et al., Plos one. May 14, (2019)
- Musa MA, Badisa VL and Latinwo LM. Anticancer Res. 2014, 34(4): p.1601-1607.
- Desai NC, Karkar TJ, Vekariya RH et al., Indian J Chem Sect B. 2020, 59(2): p.231-237.
- Abdelhafez OM, Amin KM, Batran RZ et al., Bioorg Med Chem. 2010, 18(10): p.3371-3378.
- Araújo SG, Pinto MEA, Silva NL et al., Biochemistry and Biotechnology Reports. 2013, 2(1): p.35-43.
- Al-Amiery A, Shaker L and Gaaz T. Biointerface Res Appl Chem. 2021, 11: p.10393-10401.
- Tasic ŽZ, Petrovic Mihajlovic MB, Radovanovic MB et al., Chemical Papers. 2019, 73(9): p.2103-2132.
- Fouda AS, Rashwan SM, Kamel MM et al., JMES. 2016, (8): 2658-2678.
- M Hosni, Hanaa and Abdulla Mohamed M. Acta Pharm Sin B. 2008, 58(2): p.175-186.
- Khan IA, Kulkarni MV, Gopal M et al., Bioorg Med Chem Lett. 2005, 15(15): p.3584-3587.
- Sahoo CR, Sahoo J, Mahapatra M et al., Arab J Chem. 2021, 14(2): p.102922.
- Mulwad V, Shirodkar JJ. Chem. 2002, 41B: 1263–1267.
- Abunada NM, Hassaneen HM, Samaha AS et al., J Braz Chem Soc. 2009, 20(5): p.975-987.
- El-Saghier AM, Naili MB, Rammash BK et al., Arkivoc. 2007, 16: p.83-91.
- Reddy MVR, Rao MR, Rhodes D et al., J Med Chem. 1999, 42(11): p.1901-1907.
- Mishra S, Pandey A and Manvati S. Heliyon. 2020, 6(1): p.e03217.
- Boger DL, Soenen DR, Boyce CW et al., J Org Chem. 2000, 65(8): pp.2479-2483.
- Neagoie C, Vedrenne E, Buron F et al., Eur J Med Chem. 2012, 49: 379–396.
- Rajabi M, Hossaini Z, Khalilzadeh MA et al., J Photochem Photobiol B: Biol. 2015, 148: p.66-72.
- Chandel M, Roy SM, Sharma D et al., J Lumin. 2014, 154: p.515-519.
- Gerasov AO, Shandura MP and Kovtun YP. Dyes and Pigments. 2008, 79(3): pp.252-258.
- Karci F and Ertan N. Dyes Pigm. 2005, 64(3): p.243-249.
- Shintani R and Fu GC. J Am Chem Soc. 2003, 125(36): p.10778-10779.
- Saberi MR, Vinh TK, Yee SW et al., J Med Chem. 2006, 49(3): p.1016-1022.
- Hayashi S, Hirao A, Imai A et al., J Med Chem. 2009, 52(3): p.610-625.
- Patel D, Jain M, Shah SR et al., ChemMedChem. 2011, 6(6): p.1011-1016.
- Sagara Y, Ishige K, Tsai C et al., J Biol Chem. 2002, 277(39): p.36204-36215.
- Duan J, Cui J, Yang Z et al., J Neuroinflammation. 2019, 16(1): p.1-16.
- Turpaev K, Ermolenko M, Cresteil T et al., Biochem Pharmacol. 2011, 82(5): p.535-547.
- Xin Y, Zhao J, Gu J et al., J Fluor Chem. 2011, 132(6): p.402-408.
- Turk C. Ann Arbor, MI: Michigan Publishing, University of Michigan Library. 2002.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google scholar, Crossref
Indexed at, Google Scholar Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref