Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
Standardization of Novel Siddha Formulation Vasantakusumakara Mathirai by HPTLC, Physiochemical, Phytochemical and Biochemical Analysis
Indhumathi M1*, Shanuvas A2 and Pitchiah Kumar M3
1SRF, AYUSH Project, Saveetha Medical College, Thandalam, Chennai, Tamilnadu, India
2Santhigiri Siddha Medical College, Trivandrum, Kerala, India
3State Licensing Authority [Indian medicine], Arumbakkam, Chennai, Tamilnadu, India
- *Corresponding Author:
- Indhumathi M
SRF, AYUSH Project, Saveetha Medical College
Thandalam, Chennai, Tamilnadu, India
Abstract
Siddha was an ancient system of medicine which belongs to south India especially Tamilnadu. Vasanthakusumakara mathirai (VKM) was a herbomineral formulation used for bronchial asthma, tuberculosis, common cold, fever etc. To establish a medicine for worldwide it need to be standardize. This drug was standardized by various parameters like physio chemical, phyto chemical, biochemical analysis and High Performance Thin Layer Chromatography (HPTLC). In physio chemical analysis, the pH, ash value, loss on drying disintegration time given that this drug has alkaline pH with high purity and prepared in the Good Laboratory Practise in hygienic manner. The phyto chemical analysis established the presence of chemical constituents which has been helpful for its pharmacological action. The presence of acid and basic radicals has been tested in biochemical analysis which has the potent radicals used to promote the action of this drug. The fingerprint of this drug was scanned by HPTLC at different wavelength. For resolve the contamination, microbial load has been done by agar plating method which shows that bacterial and fungal load are within the limit. This will promote the drug for further evaluation in future.
Keywords
Vasanthakusumakara mathirai, Siddha, Standardization, HPTLC.
Introduction
World Health Organization (WHO) has appreciated the importance of medicinal plants for public health care. The process of evaluating the quality and purity of herbo mineral drugs by means of various parameters like physical, chemical and biological observation is called standardization. Standardization of the this drug comes under the following categories
• Physico-chemical analysis
• Phyto chemical analysis
• Bio chemical analysis
• HPTLC
In physiochemical analysis, solubility, total ash, acid insoluble ash, water soluble ash, loss on drying, pH and disintegration time was determined. Phyto chemicals like alkaloids, glycosides, tannins, saponins, Diterpenes, triterpenes etc has been tested. Acid and basic radicals were tested in biochemical analysis.
Siddha is an Indian system of alternative medicine which has been developed by Siddhars. In Indian medicine commonly available many medicinal plants are used [1]. Vasanthakusumakara mathirai was a Siddha herbomineral formulation used for long time to cure Bronchial asthma, tuberculosis and also for common cold, fever etc [2]. This formulation contains so many raw drugs which have anti tuberculosis activity [3]. Before evaluating pharmacological activity standardization was important to give report of fingerprint about this drug.
Materials and Methods
Organoleptic evaluation
The Organoleptic characters of the sample were evaluated which include evaluation of the formulation by its colour, odour and size.
Colour examination
Ten tablets were taken into watch glasses and positioned against white back ground in white tube light. Its colour was observed by naked eye and wrote in results.
Odour examination
Ten numbers of tablets were smelled individually. The time interval among two smelling was kept two minutes to overturn the effect of previous smelling. Odour of VKM was noted in results table.
Size examination
The diameter of ten tablets was measured by Vernier calliper. The mean value of diameter was noted.
Physico-chemical investigation
Physico-chemical studies like total ash, water soluble ash, acid Insoluble ash, loss on drying at 105°C and pH were done at, Central Research Institute, Chennai.
Solubility test
A pinch of the sample (VKM) was taken in a dry test tube and shaken well with distilled water. A little amount of the sample (VKM) is shaken well with con Hcl and then Con H2SO4. Solubility was observed.
Determination of total ash
About 2 g of the ground drug (VKM) was accurately weighed in a silica dish and incinerated at a temperature not exceeding 450ºC until it was free from carbon, cooled and weighed. The percentage of ash with reference to the air-dried drug was calculated.
Determination of water soluble ash
Total ash was heated up to 600ºC with 25 ml of distilled water for 10 min and the residue was ignited in the furnace to get a constant weight. And the weight was calculated.
Determination of acid insoluble ash
The ash obtained was boiled for 5 min with 25 ml of dilute hydrochloric acid and insoluble matter was collected in an ash-less filter paper, washed with hot water and put up in flames to constant weight.
The percentage of acid-insoluble ash with reference to the air dried drug was note
Determination of moisture content (loss on drying)
This procedure was done to determine the amount of volatile matter in the drug. A sample of 10 g of the drug (VKM) was placed in a tarred evaporating dish after accurately weighting without preliminary drying.
The dish was dried at a temperature of 105ºC for about 5 h and again weighed. The drying and weighing procedure was repeated again and again until the difference between two successive weights was not more than 0.25%. And the weight was calculated.
pH value
Potentiometrically pH value was determined by a glass electrode and a pH meter. The pH of the VKM was written in results column.
Tablet disintegration test
Each VKM was placed in each of the six tubes of the basket present in the disintegration apparatus. The apparatus was operated by using water as the immersion fluid maintained at 35-39°C. At the end of the 30 min, the basket is lifted from the fluid and the state of the tablet is observed. The disintegration time of VKM was recorded [4].
Phyto chemical evaluation
Phytochemical are chemical compounds that are naturally present in plants. Phytochemical screening of the plant gives a vast idea about the chemical constituents present in the drug.
Preparation of the extract
5 g of VKM was taken in a 250 ml clean beaker and 50 ml of distilled water was added, boiled well and allowed to cool and filtered in a 100 ml volumetric flask and made up to 100 ml with distilled water. The VKM was subjected to the following phytochemical screening [5].
Alkaloids, carbohydrates, glycosides, saponins, tannins, phenolic compounds, phytosterol, diterpenes, triterpenes, flavonoid and quinones.
Biochemical analysis
The bio-chemical analysis was done to identify the acid and basic radicals present in the VKM.
Test for basic radicals
Test for potassium: To a pinch of the VKM 2 ml of sodium nitrate and 2 ml of cobalt nitrate solution in 30% glacial acetic acid was added and observed for the presence of yellow precipitate.
Test for calcium: To 2 ml of VKM extract, 2 ml of 4% ammonium oxide solution was added and observed for the formation of white precipitate.
Test for magnesium: To 2 ml of VKM extract, drops of sodium hydroxide solution was added and watched for the appearance of white precipitate.
Test for Ammonium: To 2 ml of VKM extract few ml of Nessler's reagent and excess of sodium hydroxide solution are added for the appearance of brown colour.
Test for sodium: hydrochloric acid was added with a pinch of the vkm, made as paste and introduced into the blue flame of bunsen burner and observed for the appearance of intense yellow colour.
Test for iron (ferrous): The VKM extract was treated with Conc. HNO3 and ammonium thiocyanate and waited for the appearance of blood red colour.
Test for zinc: To 2 ml of the VKM extract drops of sodium hydroxide solution was added and observed for white precipitate formation.
Test for aluminium: To the 2 ml of the VKM extract sodium hydroxide was added in drops and changes are noted.
Test for lead: To 2 ml of VKM extract 2 ml of potassium iodide solution was added and noted for yellow coloured precipitate.
Test for copper: A pinch of VKM was made into a paste with con. Hcl in a watch glass and introduced into the non-luminous part of the flame and noted for blue colour appearance. To 2 ml of VKM extract excess of ammonia solution was added and observed for the appearance of blue coloured precipitate.
Test for mercury: To 2 ml of the VKM extract sodium hydroxide solution was added and noted for yellow precipitate formation.
Test for arsenic: To 2 ml of the VKM extract 2 ml of sodium hydroxide solution was added and brown or red precipitate formation was noted.
Test for acid radicals
Test for sulphate: To 2 ml of the VKM extract 5% of barium chloride solution was added and observed for the appearance of white precipitate.
Test for chloride: The VKM extract was treated with silver nitrate solution and observed for the appearance of white precipitate.
Test for phosphate: The VKM extract was treated with ammonium molybdate and conc. HNO3 and observed for the appearance of yellow precipitate.
Test for carbonate: The VKM extract was treated with conc. Hcl and observed froth appearance of effervescence.
Test for fluoride and oxalate: To 2 ml of VKM extract 2 ml of dil. acetic acid and 2 ml calcium chloride solution was added and heated and watched for cloudy appearance.
Test for nitrate: To 1 g of the VKM, copper turnings was added and again conc. H2SO4 was added, heated and the test tube was tilted vertically down and observed for any changes.
Microbial load
Agar plating technique [6]
Principle
This method is based on the principle that when material containing bacteria are cultured, every viable bacterium develops into a visible colony on a nutrient agar medium. The number of colonies therefore is the same as the number of organisms contained in the VKM.
Dilution
A small measured volume of VKM is mixed with a large volume of sterile water called the Diluent or dilution blank. Dilution is usually made in multiples of ten. A single dilution is calculated as follows:
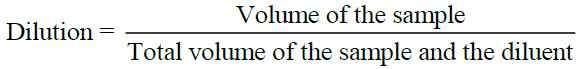
Procedure
1. Label the dilution blanks as 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, and 10-7.
2. Prepare the initial dilution by adding 1 ml of the VKM extract into a 9 ml dilution blank labeled 10-1 thus diluting the sample 10 times.
3. Mix the contents by rolling the tube back and forth between hands to obtain uniform distribution of organisms.
4. From the first dilution transfer 1 ml of the suspension while in motion, to the dilution blank 10-2 with a sterile and fresh 1 ml pipette diluting the original specimen to 100 times.
5. Repeat this procedure till the sample has been diluted 10,000,000 times using every time a fresh sterile pipette.
6 From the appropriate dilutions transfer 1 ml of suspension while in motion, with the respective pipettes, to sterile Petri dishes. Three Petri dishes are to use for each dilution.
7. Add approximately 15 ml of the nutrient medium, melted and cooled to 45°C, to each Petri dish containing the diluted VKM extract. Mix the contents of each dish by rotating gently to distribute the cells throughout the medium.
8. Allow the plates to solidify and incubate these plates in an inverted position for 24-48 hours at 37°C.
Observation
Observe all the plates for the appearance of bacterial colonies. Count the number of colonies in the plates. Calculate the number of bacteria per ml of the original suspension as follows:
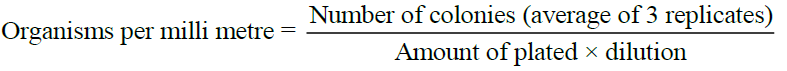
TLC/ HPTLC finger print studies
HPTLC finger printing was carried out as per the reference [7].
Preparation of spray reagent-vanillin-sulphuric acid reagent
Vanillin (1 g) was dissolved in ice cold ethanol (95 ml). Add to 5 ml of cooled concentrated sulphuric acid. Ice was added and stirred well. The solution was stored in refrigerator.
Chromatographic conditions
Instrument : CAMAG (Switzerland).
Sample Applicator : Camag Linomat - IV applicator with N2 gas flow.
Photo documentation System : Digi store-2 documentation system with Win Cat & video scan software.
Scanner : Camag HPTLC scanner - 3 (030618), Win Cats - IV.
Development Chamber : Camag HPTLC 10 × 10, 10 × 20 twin trough linear Development chamber.
Quantity applied : 5, 10 μl for extracts and 5 μl for standards
Stationary phase : Aluminium plate pre-coated with silica gel 60 (E. Merck)
Plate thickness : 0.2 mm.
Mobile Phase : For Chloroform extract-Toluene: Ethyl acetate (9:1) and ethanol extract - Toluene: Ethyl acetate (1:1).
Scanning wavelength : 575 nm
Laboratory condition : 26°C ± 5°C and 53 % relative humidity
The plate was developed up to a height of 8 cm, air dried, spots were observed under the UV light at 254 and 366 nm. Finally the plates were derivatized using vanillin-sulphuric acid reagent heated at 105° till colour spots appeared.
Results
Results of organoleptic characters (Table 1)
| S. No | Parameters | Results |
|---|---|---|
| 1 | Colour | Brown |
| 2 | Odour | Characteristic odour |
| 3 | State of matter | Solid |
| 4 | Consistency | Hard |
| 5 | Shape | Spherical |
Table 1: Organoleptic characters
Results of Physico-chemical analysis (Table 2)
| S .No. | Parameters | Results |
|---|---|---|
| 1 | pH | 8.63 |
| 2 | Total ash | 17.46% |
| 3 | Acid insoluble ash | 0.81% |
| 4 | Water soluble ash | 13.30% |
| 5 | Disintegration time | 22 min |
| 6 | Loss on Drying at 105°C | 11.04% |
Table 2: Physico-chemical analysis
pH value
The pH of VKM is 8.63. It is alkaline in nature. Our body functions are best when the pH is alkaline. Alkaline pH keeps our body tissue supple and reduces inflammation.
The advantages of alkaline pH [8] are as follows:
• Decrease aging process
• Alkaline medium enhance the mineral storage in order to buffer
• Increase utilization of oxygen level in body, which reduces fatigue, carry out cell repair, enhances ability of mitochondria function
• Also controls infection, inflammation of organs reduces microorganism toxicity (Decrease proliferation of microorganism).
Total ash
Total ash value will determine the amount of total inorganic content (ammonium, potassium, calcium, chloride, iron etc.) present in the drug after ignition is measured through it. The total ash value of VKM is 17.455%, which determines the presence of inorganic content.
Acid insoluble ash
The acid insoluble ash value of the drug denotes the amount of siliceous matter (dust, sand etc,) present in that drug. The quality of the drug is better if the acid insoluble ash value is low. Here, acid insoluble ash value of VKM is 0.805%. Hence, it represents the superior quality of the VKM.
Water soluble ash
Water soluble ash is a part of total ash value, which denotes the diffusion capacity of the drug. Here, the water soluble ash value of VKM is 13.295%, which represents easy facilitation of diffusion and osmosis mechanism.
Disintegration time
According to Ayush guidelines, the disintegration time does not exist more than 45 min. The disintegration time of VKM is 22 min. This implies a reasonable disintegration time, thereby a better absorbability and solubility is achieved.
Loss on Drying
It indicates the amount of volatile substance and moisture present in the drug. This also indicates the stability and shelf life of the drug. The loss on drying percentage of VKM is 11.04. Being a tablet, without incineration process, the volatile substances are slightly high in VKM.
Traditional test for pill
Character Inference
Non sticky on rolling +
No cracks over the surface after drying +
Shall be rolled uniformly over the plane surface +
Result of phytochemical analysis (Table 3)
| S. No. | Phytochemical | Test | Result |
|---|---|---|---|
| 1 | Alkaloids | Mayer’s test | + |
| 2 | Glycosides | Modified Borntrager’s test | + |
| 3 | Tannins | Gelatine test | + |
| 4 | Phenols | Alcoholic Ferric chloride test | + |
| 5 | Phytosterol | Ferric chloride acetic acid test | + |
| 6 | Triterpenes | Salkowiski’s test | + |
| 7 | Proteins and amino acids | Xanthoproteic test | + |
Table 3: phytochemical screening tests
From the primary phytochemical screening tests of VKM shows Alkaloids, Glycosides, Tannins, Phytosterol, Phenol, Triterpenes and Proteins.
Alkaloids [9]
Alkaloids have a wide range of pharmacological activity like Antibacterial. These alkaloids will enhance the action of VKM. Alkaloids possess antispasmodic, analgesic, bactericidal effects.
Glycosides
Many plants store chemicals in the form of inactive glycosides, such plant glycosides are used as medications. Inhibitory effect against Mycobacterium tuberculosis showed the conjugates of glycosides. The antitubercular activity is higher than drug Pyrizamide but lower than the drug Isoniazid [10].
Tannins
Tannins exhibits anti Mycobacterial activity by inhibiting the total growth and also of patient’s strain, which was fully sensitive to the standard anti tuberculosis drug [11]. Herbal preparation containing tannins are used for bronchitis and many others. Tannins are considered antioxidants, prevents the onset of degenerative diseases [12].
Phenols
Various bioactivities of phenolic compounds are responsible for their chemo preventive properties (anti-oxidant, antiinflammatory effects etc,) [13]. It will reduce the inflammation in tuberculosis. Phenols could be a source of natural anti- tuberculosis agents.
Triterpenes
Suppress the inflammatory process. Antioxidant and Antimicrobial activity. Antibacterial agent, these will destroy the bacterial growth, and proliferation of bacteria (M. tuberculosis) [14]. The substance inhibited 99% the growth of M. tuberculosis H37Rv, that oleanolic acid has a synergistic effect when combined with Isoniazid, Rifampicin or Ethambutol [15].
Proteins
Body uses protein to build and repair tissues, which are degenerated by the tubercular bacilli [16]. Substituted aminopyrimidine protein kinase B (PknB) inhibitors show activity against M. tuberculosis [17]. A synergistic effect of all these flavanoids, alkaloids, glycosides, tannins, phenols, saponins, increases the potency of the drug against Diabetes mellitus.
Result of bio chemical analysis
From the acid and basic radicals studies (Table 4) of VKM presents the following chemicals: Potassium, Ammonium and phosphate.
| Parameter | Result |
|---|---|
| Test for Potassium | + |
| Test For Ammonium | + |
| Test for Phosphate | + |
Table 4: Results of acid and basic radical studies
Potassium
Clavulanate potassium demonstrates synergic anti-bacterial activity [18].
Ammonium [19]
Ammonium is essential in the body as a building block for making proteins and other complex molecules. It is essential for much biological process and serves as a precursor for amino acid and nucleotide synthesis.
Phosphate [20]
Phosphate is an essential mineral primarily used for growth and repair of body cells and tissues. It is also required for a variety of biochemical processes including energy production and pH regulation. It is a component of DNA and RNA molecules. Hemoglobin, the important oxygen carrying protein in the bloodstream, also depends upon phosphorus contained in its structure for proper function. In TB patient, it will produce energy to the patient, regulate the pH and repairs the host cell.
Result of microbial load (Figures 1-4)
Bacterial load
Fungal load
These herbo-mineral drug are prepare from plant material they are prone to contamination. The contamination of herbal drugs by micro-organism not only cause bio deterioration but also reduces the efficacy of drugs.
The toxin produces by microbes makes herbal drugs unfit for human consumption because the contaminated drug may develop unwanted disease instead of disease being cured [21].
Here, the contamination of Mathirai has been examined by bacterial and fungal load.
o Total bacterial load in 10-4 dilution is 7 and in 10-6 dilution is 5.
o Total fungal load in 10-2 dilution is 4 and in 10-3 dilution is 1.
Here, the contamination of VKM is within the WHO norms. Hence, the drug is collected, prepared, stored and packed and decontaminated prior to formulation.
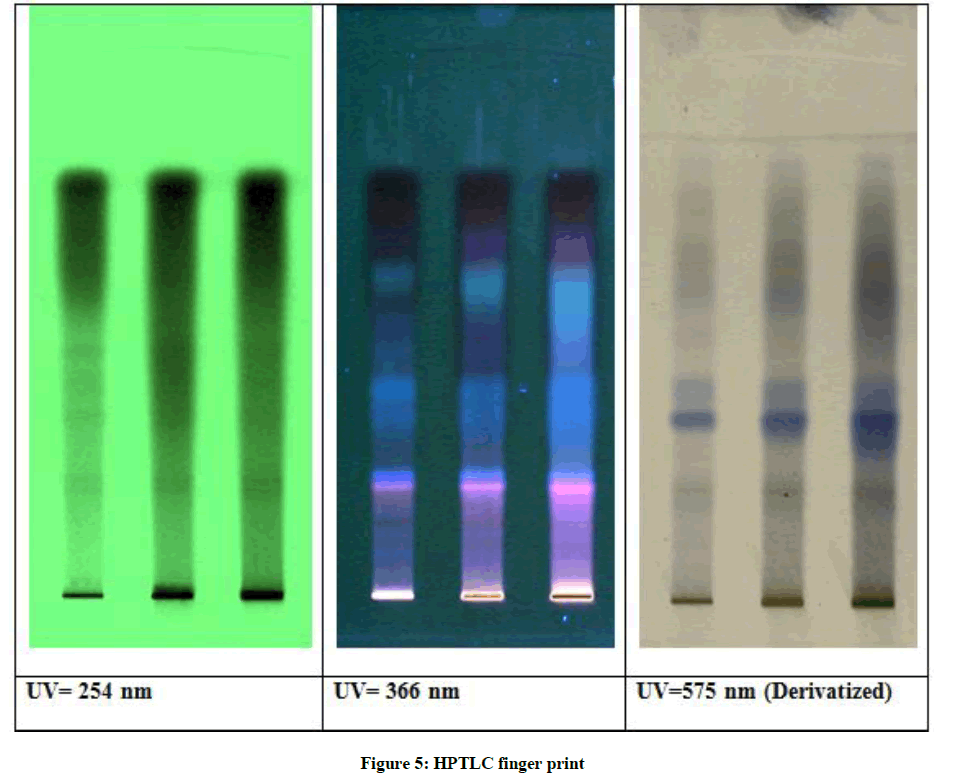
Result of HPTLC fingerprint study (Table 5 and Figure 5)
| Colour | Rf value(s) | Colour | Rf value(s) | Colour | Rf value(s) |
|---|---|---|---|---|---|
| Green | 0.21 | Magenta | 0.21 | Light Blue | 0.21 |
| Green | 0.22 | Blue | 0.22 | Brown | 0.22 |
| Green | 0.34 | Light Blue | 0.34 | Blue | 0.34 |
| Green | 0.4 | Light Blue | 0.4 | Light Blue | 0.42 |
| Green | 0.46 | Greenish Blue | 0.6 | Violet | 0.52 |
| Green | 0.78 | Light Blue | 0.6 |
Table 5: TLC photo documentation of chloroform extract of VKM
Stationary Phase - Silica Gel 60 F254
Mobile Phase - Toluene: Ethyl Acetate: Formic Acid (5: 2: 0.1 v/v/v)
Discussion
A qualitative fingerprinting of VKM has been performed by HPTLC method, which provides qualitative insights into the bioactive Constituents present in the drug. HPTLC shows separation of components present in the Chloroform extract of VKM. The method may be applied to identify the VKM from other manufacturing process.
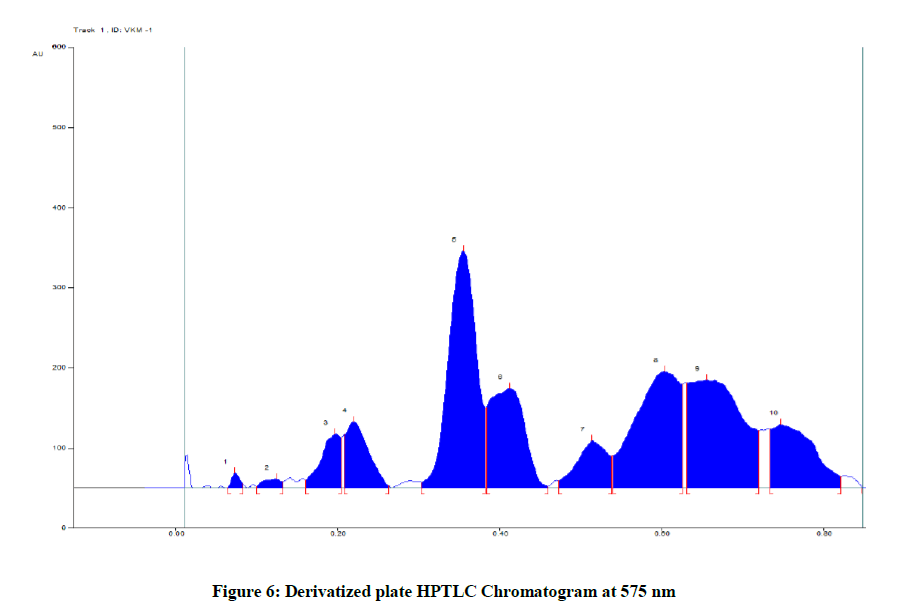
The present study revealed that VKM showed best results in Toluene: Ethyl Acetate: Formic acid: 5: 2: 1 solvent system. After scanning and visualizing the plates in absorbance mode at 254 nm, 366 nm and 575 nm (Table 6 and Figure 6) and visible light range, best results were shown at 575 nm.
| peak | Start Position | Start Height | Max Position | Max Height | Max % | End Position | End Height | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.06 Rf | 0.3 AU | 0.07 Rf | 18.5 AU | 1.81% | 0.08 Rf | 5.4 AU | 171.4 | 0.43% |
| 2 | 0.10 Rf | 1.9 AU | 0.12 Rf | 11.2 AU | 1.1% | 0.13 Rf | 8 AU | 233.7 | 0.59% |
| 3 | 0.16 Rf | 10 AU | 0.2 Rf | 67.9 AU | 6.65% | 0.21 Rf | 63.5 AU | 1515.2 | 3.84% |
| 4 | 0.21 Rf | 66.8 AU | 0.22 Rf | 83.2 AU | 8.15% | 0.26 Rf | 2.9 AU | 2132 | 5.4% |
| 5 | 0.30 Rf | 7.1 AU | 0.36 Rf | 296.5 AU | 29.05% | 0.38 Rf | 99.8 AU | 9309.5 | 23.59% |
| 6 | 0.38 Rf | 100.7 AU | 0.41 Rf | 124.3 AU | 12.18% | 0.46 Rf | 1.8 AU | 4864.1 | 12.33% |
| 7 | 0.47 Rf | 8.9 AU | 0.52 Rf | 59.2 AU | 5.8% | 0.54 Rf | 40.1 AU | 2010.2 | 5.09% |
| 8 | 0.54 Rf | 40.5 AU | 0.61 Rf | 145.4 AU | 14.24% | 0.63 Rf | 29.6 AU | 7288.9 | 18.47% |
| 9 | 0.63 Rf | 131.3 AU | 0.66 Rf | 134.8 AU | 13.21% | 0.72 Rf | 72.4 AU | 8092.8 | 20.51% |
| 10 | 0.74 Rf | 73.8 AU | 0.75 Rf | 79.7 AU | 7.81% | 0.82 Rf | 14.3 AU | 3840.5 | 9.73% |
Table 6: Peak Table at 575 nm (Track 1, ID: VKM-1)
TLC plate showed different colour phyto constituents of chloroform extract of VKM. The bands revealed presence of six greenish, five light blue, two blue, one greenish blue, onemagenta, one brown and one violet band showing the presence of steroids, terpenoids, alkaloids, flavanoids, tannins, lignans and saponins.
The results from HPTLC finger print scanned at wavelength 575 nm for chloroform extract of VKM. There are ten polyvalent phyto constituents and corresponding ascending order of Rf values start from 0.06 to 0.74 in which highest concentrations of the phyto constituents was found to be 29.05% and 14.24 % with its corresponding Rf value were found to be 0.30 and 0.54 respectively.
Conclusion
The medicine VKM has been subjected to various standardization methods to establish that the work of Siddhars to be true. In physio chemical analysis, drug has alkaline pH which will supple our body tissue, total ash value 17.455% shows the presence of inorganic contents, acid insoluble ash 0.805% represent superior quality of this drug, water soluble ash value 13.295% determines diffusion capacity of this drug, disintegration time was 22 min shows the better absorbability and solubility. In phyto chemical analysis, presence of alkaloids, glycosides, tannin, phenols and triterpenes enhance the pharmacological activity. The result of HPTLC shows a finger print at wavelength 575 nm, Rf value starts from 0.06 to 0.74, 0.30 and 0.54. The result gives a way for further research in future.
Acknowledgement
I express my special thanks to Mrs. R. Shakila M.Sc, Research officer, Dept of Chemistry, Central Research Institution of Siddha, Chennai for his valuable precious help to conduct Physico chemical, Phytochemical and chemical analysis of the drug and help towards the successful completion of the entire Study.
References
- M. Indhumathi, A. Shanuvas, Am. J. Pharm. Health. Res., 2018, 6(03).
- K.N.K. Mudhaliyar, K.S. Uthamarayan, S.V. Thiratu, Indian Medicine and Homeopathy, First (Edn.), 1933, 40.
- A. Shanuvas, M. Indhumathi, Eur. J. Pharmaceut. Med. Res., 2018, 5(3), 217-224.
- D.R. Lohar, Protocol for testing: Ayurvedic, Siddha and Unani Medicines, Pharmacopoeial Laboratory for Indian Medicine, Ghaziabad.
- P. Tiwari, B. Kumar, M. Kaur, G. Kaur, H. Kaur, Int. Pharmaceut. Sci., 2011, 1, 98-106.
- J.N. Eloff, D.T. Ntloedide, R.V. Brummelen, Afr. J. Tradit. Complement. Altern. Med., 2011, 8(5), 1-12.
- K.R. Aneja, Practical text book of microbiology, CBS publishers and Distributors, 2005, Pp: 467.
- S. Collins, Alkaline Ph, reviewed by M.D. singer, March 11, 2016, available at drsheiland.com/what-is-alkaline-and-why-is-it-important.
- P. Kittakoop, C. Mahidol, S. Ruchirawat, Curr. Top. Med. Chem., 2014, 14(2), 239-252.
- V.E. Kataev, U. Strobykina II, O.V. Andreeva, B.F. Garifullin, R.R. Sharipova, V.F. Mironov, R.V. Chestnova, Bioorg Khim., 2011, 37(4), 542-51.
- K. Asres, F. Bucar, S. Edelsbrunner, T. Kartnig, G. Höger, W. Thiel, Phytother. Res., 2001, 15(4), 323-326.
- B. Uttara, A.V. Singh, P. Zamboni, R.T Mahajan, Curr. Neuro. Pharmacol., 2009, 7(1), o65-74.
- N. Kickler, G. Gambarota, R. Mekle, R. Gruetter, R. Mulkern, J. Magn. Reson., 2010, 2013(1), 108-112.
- M.M. Caan, Clin. Microbial. Res., 1999, 12(4), 564-582.
- D. Martins, L. Lucas Carrion, Biomed Research International., 2013, 605831.
- www.m.webmd.com/men/features/benefits-protein
- T.M. Chapmen, N. Bouloc, R.S. Buxton, J. Chugh, K.E.A. Lougheed, S.A. Osborne, B. Saxty, S.J. Smerdon, D.L. Taylor, D. Whalley, Bioorg. Med. Chem. Lett., 2012, 22(9), 3349-3353.
- C. Pointek, M. Paczkowska, J. Cielecka-Piontek, D. Szymanowska-Powałowska, M. Paczkowska, P. Łysakowski, P. Zalewski, P. Garbacki, J. Antibiot., 2015, 68(1), 35-39.
- K.L. Manchester, Sites of Hormonal Regulation of Protein Metabolism, (In Edi.) H.N. Munro, J.B. Allison, Mammalian protein metabolism 4, New York, Academic Press, 1964, 229.
- K.O Soetan, C.O Olaiya, O.E. Oyewole, Afr. J. Food Sci., 2010, 4(5), 200-222.
- K. Nandna, K.S. Rajeev, S. Bhaduria, Int. J. Pharmaceut. Quality Assurance., 2011, 3(4), 15-17.