Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 1
Requalification of Friability Testing Apparatus
Chethan TP*, Kavya, Abhishek Singh, Rishu Yadav, Laxmi Yadav, Samruddhi Phansekar, Shailendra Gupta and Shubh am YadavChethan TP, Department of Pharmaceutical Quality Assurance, Priyadarshini College of Pharmacy, Koratagere, India, Email: chethu.sscp@gmail.com
Received: 28-Dec-2023, Manuscript No. DPC-23-123880; Editor assigned: 02-Jan-2024, Pre QC No. DPC-23-123880 (PQ); Reviewed: 16-Jan-2024, QC No. DPC-23-123880; Revised: 18-Jan-2024, Manuscript No. DPC-23-123880 (R); Published: 08-Feb-2024, DOI: 10.4172/0975-413X.16.1.183-191
Abstract
Re-qualification as a part of validation is the task performed to identify or check that utilities, equipment and ancillary systems can operate within limits for their intended use. Equipment qualification is a key element in the pharmaceutical quality system. In recent times regulatory agencies are more focusing on qualification of equipment. Re-qualification of the equipment starts from design of the equipment based on the user requirement specification and functional requirement specification. The review article provides information on design qualification which is done to identify whether the proposed design of facilities, system and equipment is suitable for intended purpose, installation qualification which is done to check whether the equipment is built and installed in compliance with design specification, operational qualification in which the process parameters shall be challenged to assure that product meets all requirements and finally performance qualification to demonstrate that the process will produce acceptable product consistently under normal operating conditions.
Keywords
Re-qualification; Operational qualification; Design qualification; Installation qualification; Performance qualification
Introduction
In the pharmaceutical industry, the concept of qualification plays a pivotal role in ensuring the safety, efficacy and reliability of pharmaceutical products. Qualification encompasses a systematic and rigorous approach to evaluating and verifying the various components, processes and personnel involved in the production and distribution of medications. It serves as a fundamental mechanism for meeting regulatory requirements, adhering to industry best practices and maintaining the highest standards of quality assurance [1-4].
Pharmaceutical qualification is a crucial process in the pharmaceutical industry that ensures equipment, systems and processes meet predefined standards and regulatory requirements. It encompasses various stages, including Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ). The goal is to guarantee the quality, safety and efficacy of pharmaceutical products by verifying that everything involved in their production and testing functions correctly and consistently. This rigorous qualification process helps maintain compliance with regulatory agencies like the FDA and ensures the reliability of pharmaceutical manufacturing. It is the action of providing and documenting that equipment or ancillary systems are properly installed, work correctly and leads to the expected results.
• Qualification is a part of validation, but the individual qualification steps alone do not constitute process validation.
• Qualification of analytical instrumentation is essential for accurate and precise measurement of analytical data.
Materials and Methods
What is qualification?
Qualifications are the criteria which an applicant must meet to be eligible for an opportunity award. Qualifications are used to narrow the field of all applicants to only the most qualified applicants.
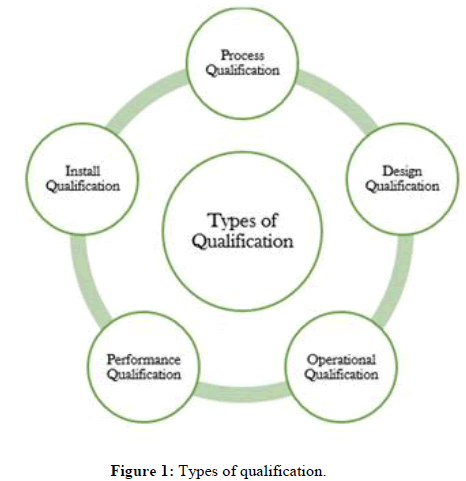
Types of qualification
Qualifications are created from the imported data on the import file, student answered questions on the general or conditional applications and/or apply to opportunity questions (Figure 1).
Process qualification: Process qualification is the qualification of manufacturing and production processes to confirm they can operate at a certain standard during sustained commercial manufacturing. Data covering critical process parameters must be recorded and analyzed to ensure critical quality attributes can be guaranteed throughout production. This may include testing equipment at maximum operating capacity to show quantity demands can be met. Once all processes have been qualified the manufacturer should have a complete understanding of the process design and have a framework in place to routinely monitor operations. Only after process qualification has been completed can the manufacturing process begin production for commercial use. Equally important as qualifying processes and equipment is qualifying software and personnel. A well-trained staff and accurate, thorough records helps ensure ongoing protection from process faults and quick recovery from otherwise costly process malfunctions. In many countries qualification measures are also required, especially in the pharmaceutical manufacturing field [5-10].
Process qualification should cover the following aspects of manufacturing:
• Facility
• Utilities
• Equipment
• Personnel
• End-to-end manufacturing
• Control protocols and monitoring software
Design qualification: Design Qualification (DQ) is a crucial phase in the design and development process of a product or system, especially in industries like pharmaceuticals, biotechnology and manufacturing. It ensures that the design meets the intended requirements and specifications before moving on to further stages of development. Here's a simplified outline of the steps involved in a typical design qualification process:
• Define user requirements: Begin by clearly defining the user or stakeholder requirements. What does the product need to achieve? What are the critical parameters and specifications it must meet?
• Develop design specifications: Based on user requirements, create detailed design specifications that outline how the product or system will fulfil those requirements. This includes technical specifications, materials, components and functionality.
• Design review: Conduct a comprehensive design review with a cross functional team, including engineers, designers and relevant stakeholders. This step ensures that the design aligns with the user requirements and that any potential issues or risks are identified.
• Risk assessment: Perform a risk assessment to identify and evaluate potential risks associated with the design. This could include safety concerns, compliance with regulations and any other relevant factors.
• Prototype and testing: Build prototypes or conduct simulations to verify that the design functions as intended. Testing should cover various scenarios and conditions to ensure reliability and performance.
• Documentation: Maintain thorough documentation throughout the DQ process. This includes records of design changes, test results, risk assessments and design reviews.
• Approval: Seek approval from relevant stakeholders and regulatory authorities, if applicable. This step confirms that the design is qualified to move forward.
• Validation: Once DQ is complete, the design can proceed to the validation phase, where it is tested under real world conditions to ensure it meets user requirements.
• Continuous monitoring: After implementation, the design continues to be monitored to ensure it performs as expected throughout its lifecycle.
• Change control: Implement a robust change control process to manage any modifications or updates to the design post-qualification.
Design qualification is a critical step to ensure that a product or system is reliable, safe and meets its intended purpose. It helps minimize risks, ensures compliance with regulations and provides a solid foundation for subsequent phases of development and production.
Operational qualification: Operational Qualification (OQ) is a critical phase in the validation process, particularly in regulated industries such as pharmaceuticals, biotechnology and manufacturing. OQ ensures that equipment, systems or processes operate effectively and consistently within their specified operational limits. Here's an overview of the operational qualification process:
• Establishing test criteria: Define the specific criteria and parameters that need to be tested during the OQ phase. These criteria are typically based on the equipment or system's design specifications, user requirements and regulatory guidelines.
• Test protocols: Develop detailed test protocols that outline the procedures for conducting OQ testing. These protocols should include step-by-step instructions, acceptance criteria and the equipment and personnel responsible for performing the tests.
• Performance testing: Execute a series of tests to evaluate the equipment or system's performance under various operating conditions. This may include testing different operational modes, running the equipment at maximum and minimum settings and assessing its response to normal and abnormal situations.
• Data collection: Collect comprehensive data during the testing process, including measurements, observations and any deviations or issues encountered. It's essential to maintain accurate and detailed records.
• Data analysis: Analyze the collected data to determine whether the equipment or system meets the predefined acceptance criteria. Any discrepancies or failures should be thoroughly investigated and documented.
• Documentation: Maintain comprehensive records of the OQ testing, including test protocols, test results, data analysis and any corrective actions taken to address identified issues.
• Review and approval: Review the OQ documentation and test results with relevant stakeholders, including quality assurance personnel and regulatory authorities if necessary. Seek approval for the successful completion of the OQ phase.
• Requalification: Periodic requalification may be required to ensure that the equipment or system continues to operate within specifications. This may be triggered by factors such as equipment maintenance, software updates or changes in operating conditions.
• Operational qualification is crucial to ensure that equipment and systems perform consistently and reliably within their specified operational parameters. It provides confidence that the equipment or process is capable of consistently producing the desired results and is a fundamental step in achieving regulatory compliance and product quality in regulated industries.
Performance qualification: Performance Qualification (PQ) is a critical phase in the validation process, particularly in regulated industries such as pharmaceuticals, biotechnology and manufacturing. PQ demonstrates that equipment, systems or processes consistently produce results that meet predetermined acceptance criteria and are suitable for their intended use. Here's an overview of the performance qualification process:
• Test criteria and acceptance criteria: Define the specific test criteria and acceptance criteria for the equipment, system or process. These criteria should be based on user requirements, design specifications and industry regulations.
• Test protocols: Develop detailed test protocols that outline the procedures for conducting PQ testing. These protocols should include step-by-step instructions, acceptance criteria and the equipment and personnel responsible for performing the tests.
• Performance testing: Execute a series of tests under actual or simulated operational conditions to verify that the equipment or system consistently meets the predetermined criteria. This involves running the equipment or process for an extended period to assess its performance over time.
• Data collection: Collect comprehensive data during the testing process, including measurements, observations and any deviations or issues encountered. It's essential to maintain accurate and detailed records.
• Data analysis: Analyze the collected data to determine whether the equipment, system or process consistently meets the predefined acceptance criteria. Any discrepancies or deviations from expected performance should be investigated and documented.
• Documentation: Maintain comprehensive records of the PQ testing, including test protocols, test results, data analysis and any corrective actions taken to address identified issues.
• Review and approval: Review the PQ documentation and test results with relevant stakeholders, including quality assurance personnel and regulatory authorities if necessary. Seek approval for the successful completion of the PQ phase.
• Continuous monitoring and maintenance: After successful PQ, the equipment, system or process should be subject to ongoing monitoring and maintenance to ensure that it continues to perform within specifications. Periodic requalification may also be necessary.
• Performance Qualification is a critical step in demonstrating that equipment, systems or processes are capable of consistently producing results that meet quality and regulatory requirements. It provides assurance that the equipment or process is suitable for its intended purpose and is a fundamental component of ensuring product quality and regulatory compliance in regulated industries.
Installation qualification: Installation Qualification (IQ) is one of the key steps in the validation process, particularly in regulated industries like pharmaceuticals, biotechnology and manufacturing. IQ ensures that equipment, systems or facilities have been installed correctly and in accordance with their design specifications. Here's an overview of the installation qualification process:
• Documentation review: Begin by reviewing documentation related to the equipment or system being installed. This includes design specifications, user requirements and any relevant Standard Operating Procedures (SOPs).
• Installation verification: Physically verify that the equipment or system has been installed correctly. This involves confirming that it is in the right location, connected properly and in compliance with design specifications.
• Calibration and configuration: Ensure that any instruments or sensors are calibrated and configured according to the manufacturer's recommendations and user requirements.
• Utility services: Verify that all necessary utility services (e.g., power, water, gas) are properly connected and functioning as required.
• Environmental conditions: Check and document environmental conditions such as temperature, humidity and cleanliness to ensure they meet the specified criteria for the equipment or system.
• Safety checks: Confirm that safety features and precautions are in place and operational. This includes emergency stop buttons, safety interlocks and alarms.
• Functional testing: Perform basic functional tests to ensure that the equipment or system operates as expected. This may include running diagnostic tests and verifying that all components are functioning correctly.
• Documentation and records: Maintain detailed records of the IQ process, including photographs, test results and any deviations or issues encountered.
• Review and approval: Once the installation has been successfully qualified, review the IQ documentation and seek approval from relevant stakeholders and regulatory authorities if required.
Installation qualification is a critical step to ensure that the equipment or system is set up correctly and is ready for further validation activities, such as Operational Qualification (OQ) and Performance Qualification (PQ). It provides assurance that the infrastructure is in place to support the intended processes and functions within regulated industries, where precision and compliance are paramount.
Requalification: In accordance with the WHO prequalification procedure, holders of prequalified Finished Pharmaceutical Products (FPPs) should submit a quality review five years after the date of prequalification or will be invited to do so earlier if WHO considers this to be necessary. This procedure forms part of the maintenance of the prequalified FPP and is called “requalification”. The procedure is applicable only to FPPs that have been requalified via the full assessment (multisource generic) route.
WHO assesses the data and information submitted in the quality review to verify that the prequalified product continues to meet current norms and standards. It also uses the data and information supplied to assess the consistency of product quality and manufacturing processes over the identified period. Requalification is a process in which a person or entity undergoes evaluation or testing to determine if they still meet certain qualifications or standards. It is often used in various contexts such as professional certifications, employment and regulatory compliance to ensure that individuals or organizations continue to meet the necessary criteria. Requalification may involve examinations, training or other assessments to maintain a specific status or certification.
Need of requalification: In the pharmaceutical industry, requalification is essential for several reasons:
• Regulatory compliance: Pharmaceutical companies are subject to strict regulatory requirements to ensure the safety and efficacy of their products. Requalification helps maintain compliance with these regulations, such as Good Manufacturing Practices (GMP), which require periodic validation and revalidation of equipment and processes.
• Product quality and safety: Ensuring that equipment, facilities and processes remain in a state of control is crucial for maintaining the quality and safety of pharmaceutical products. Requalification helps identify and rectify any deviations or issues that may affect product quality.
• Process changes: Pharmaceuticals often undergo process changes or upgrades to improve efficiency or comply with new regulations. Requalification is necessary to verify that these changes do not compromise product quality or safety.
• Equipment maintenance: Pharmaceutical equipment must be regularly maintained to ensure accuracy and reliability. Requalification helps confirm that equipment functions correctly and consistently.
• Risk management: Requalification serves as a risk management tool by identifying potential sources of contamination, errors or product deviations. Addressing these issues proactively helps minimize the risk of product recalls or adverse events.
• Product lifecycle: Over time, pharmaceutical products may remain on the market for many years. Requalification helps ensure that manufacturing processes and product quality remain consistent throughout the product's lifecycle.
• Continuous improvement: Requalification provides opportunities for continuous improvement by identifying areas where processes or equipment can be optimized for better performance, cost-efficiency and safety [11,12].
Overall, requalification in the pharmaceutical industry is critical for maintaining product quality, patient safety and regulatory compliance throughout the entire lifecycle of pharmaceutical products and processes.
The primary objective of the re-qualification process for the friability tester is:
• To ensure the continued accuracy, precision and reliability of the equipment in accordance with regulatory requirement and industrial standards.
• To assess and document the performance of the equipment, identifying any deviation from specifications and implementing necessary corrective actions to maintain the integrity of test results (Tables 1-6).
| Sr. No | Checks to be performed | Acceptance criteria | Observation | Checked by |
|---|---|---|---|---|
| 1. | Check for any scratches on the machine body and drums | It shall not have any scratches on machine body and drums | ||
| 2. | Check for electrical connection | It shall not observe a loose or damage connection | ||
| 3. | Check for levelling of the platform | Air bubble of level indicator should be at centre | ||
| 4. | Check drum properly locked with knob on the shaft | Drum should be properly locked with knob on the shaft |
Table 1: Installation verification check list.
| Sr. No. | Parameter | Acceptance criteria | Observation | Checked by |
|---|---|---|---|---|
| 1. | Room temperature | 15°C to 30°C | ||
| 2. | Room environmental checks | Away from direct sunlight | ||
| Free from vibration | ||||
| No corrosive gases | ||||
| 3. | Table space | Width: 15” Length: 18” Height: 20” | ||
| 4. | Point for electrical connection | Single phase of 230 V AC 50 Hz | ||
| 5. | Base/table level | Levelled sturdy, with no vibration | ||
| 6. | Earthing | Shall be provided |
Table 2: Site inspection checklist
| Sr. No | Parameter | Acceptance criteria | Observation | Checked by |
|---|---|---|---|---|
| 1 | Phase voltage | Single phase, 220/230 VAC, 50/60 Hz |
Table 3: Utility verification checklist
| Sr. No. | Test | Acceptance criteria | Observation | Checked by |
|---|---|---|---|---|
| 1. | Speed | 25 Revolutions Per Minute (RPM) | ||
| 2. | Count range | 01 to 99 revolutions. | ||
| 3. | Type of the drum | Electro lab AD drum and abrasion drum. | ||
| 4. | Power supply | Single phase, 220/230 V AC, 50/60 Hz | ||
| 5. | Dimension | 350 mm (L) × 310 mm × 430 mm (H) | ||
| 6. | Weight | 12 Kg. (approx.) | ||
| 7. | No. of drums | 01 Nos. | ||
| 8. | No. of discharge trays | 01 Nos. |
Table 4: Technical specification checklist.
| Sr. No. | Name | Acceptance criteria (As per USP general chapter-1216) | Observation | Checked by |
|---|---|---|---|---|
| 11 | Drums | One side removable | ||
| Material | Transparent synthetic polymer with polished internal surfaces |
Table 5: Drums specification check list.
| Sr. No. | Operation | Acceptance criteria | Observations | Checked by |
|---|---|---|---|---|
| 1 | Switch ‘ON’ the power switch. | The drum shall initialize itself to the loading position. The display shall now show ‘start” | ||
| 2 | Press ‘RESET’ key. | The instrument shall initialize and they will stop at the loading position. |
Table 6: Operational verification checklist.
Results and Discussion
Protocol for re-qualification
Performance of instrument
Performance of instrument is checked by taking different size or shape of tablets (different size or shape of tablets causes a regular and irregular tumbling). Initially weighed tablets are transferred gently into the drum from the side slit provided on the drums. The TIME mode is set for 4 minutes i.e., 100 rotations. Calculated the friability and result is recorded in following Tables 8-14.
% Friability=(Initial weight in gm-final weight in gm)/Initial weight in gm × 100
• For regular tumbling, set the instrument at normal position, as there is not any irregular tumbling of tablets.
• For irregular tumbling, set the instrument with 10° tilt with the bench top to prevent any irregular tumbling of tablets.
Acceptance criteria: Performance of instrument shall be found satisfactory.
| Product name: | ||||||
| No. | Time set in minute | Initial weight in gm | Final weight in gm | Friability | Acceptance criteria | Checked by |
|---|---|---|---|---|---|---|
| 1 | NMT 1% | |||||
| 2 | ||||||
| 3 | ||||||
Table 7: Data protocol for normal position.
Calculation for normal position:
% Friability=(Initial weight in gm-final weight in gm)/Initial weight in gm × 100
| Product name: | ||||||
| No. | Time set in minute | Initial weight in gm | Final weight in gm | Friability | Acceptance criteria | Checked by |
|---|---|---|---|---|---|---|
| 1. | NMT 1% | |||||
| 2. | ||||||
| 3. | ||||||
Table 8: Data protocol for 10° tilt position.
Calculation for 10° tilts position:
% Friability=(Initial weight in gm-final weight in gm)/Initial weight in gm × 100
Re-qualification report
| Sr. No | Checks to be performed | Acceptance criteria | Observation |
|---|---|---|---|
| 1. | Check for any scratches on the machine body and drums | It shall not have any scratches on machine body and drums | No scratches found |
| 2. | Check for electrical connection | It shall not observe a loose or damage connection | No loose or damage connection found |
| 3. | Check for levelling of the platform | Air bubble of level indicator should be at centre | In the level on the platform |
| 4. | Check drum properly locked with knob on the shaft | Drum should be properly locked with knob on the shaft | Properly locked with knob on the shaft |
Table 9: Installation verification check list.
| Sr. No | Parameter | Acceptance criteria | Observation |
|---|---|---|---|
| 1 | Phase voltage | Single phase, 220/230 VAC, 50/60 Hz. | Pass |
Table 10: Utility verification checklist.
| Sr. No. | Test | Acceptance criteria | Observation |
|---|---|---|---|
| 1. | Speed | 25 Revolutions Per Minute (RPM) | Pass |
| 2. | Count range | 01 to 99 revolutions. | Pass |
| 3. | Type of the drum | Electro lab AD drum and abrasion drum. | Pass |
| 4. | Power supply | Single Phase, 220/230 V AC, 50/60 Hz | Pass |
| 5. | Dimension | 350 mm (L) × 310mm × 430 mm (H) | Pass |
| 6. | Weight | 12 Kg. (approx.) | Pass |
| 7. | No. of drums | 01 Nos. | Pass |
| 8. | No. of discharge trays | 01 Nos. | Pass |
Table 11: Technical specification checklist.
| Sr. No. | Name | Acceptance criteria (As per USP general chapter-1216) | Observation |
|---|---|---|---|
| 1 | Drums | One side removable | Single side |
| Material | Transparent synthetic polymer with polished internal surfaces | Pass |
Table 12: Drums specification check list.
Report: Drum Specification check point was verified; observations were noted above.
| Sr. No. | Operation | Acceptance criteria | Observations |
|---|---|---|---|
| 1. | Switch ‘ON’ the power switch. | The drum shall initialize itself to the loading position. The display shall now show ‘Start” | Pass |
| 2. | Press ‘RESET’ key. | The instrument shall initialize and they will stop at the loading position. | Pass |
Table 13: Operational verification checklist.
Report: Operational verification check point was verified; observations were noted above.
Performance of instrument
Acceptance criteria: Performance of instrument shall be found satisfactory.
| Product Name | DOLO 650 | |||||
|---|---|---|---|---|---|---|
| Sr. No. | Time | Initial weight in gm | Final weight in gm | Friability | Acceptance criteria | Checked |
| Set in minute | by | |||||
| 1. | 04 Min | 6.845 | 6.842 | 0.04% | NMT 1% | |
| 2. | 04 Min | 6.831 | 6.831 | 0.02% | ||
| 3. | 04 Min | 6.83 | 6.81 | 0.20% | ||
Table 14: Data for normal position.
Calculation for normal position:
% Friability=(Initial weight in gm-final weight in gm)/Initial weight in gm × 100
• 1st trail=(6.845-6.842)/6.845 *100=0.04%
• 2nd trail=(6.831-6.829)/6.831*100=0.02%
• 3rd trail=(6.830-6.810)/6.830 *100=0.2%
Report: All 3 trails of tablets (DOLO 650) are within the acceptance criteria. Hence the tablets pass the friability test as per USP.
Requalification
The requalification has been carried for the friability tester by using two different shaped tablets of same dose. The performance of the equipment
tested. Results were found satisfactory and with the acceptance criteria [13-15]. In two variations performance qualification carried out i.e.,
• At normal position
• At 10° tilt position
Conclusion
The samples (marketed tablets) of different shapes taken for the performance qualification, the friability tester passed the all the parameters of Installation, operational and performance qualification. After reviewing the results of all parameters, the parameters of installation, operational and performance qualification, following points are concluded.
• The friability tester passed the all the parameters of Installation and operational qualification without any failure.
• Coming to performance qualification, at normal position the equipment taken for 3 trails, all the trails are within the limit and equipment
as per the USP.
• At 10° tilt position the equipment taken for 3 trails, all the trails are within the limit and equipment as per the USP.
• From the above results we can conclude that the equipment able to achieve continued accuracy, precision and reliability of the equipment
in accordance with regulatory requirement and industrial standards.
Acknowledgment
The authors are grateful to the Blatter herbarium, St. Xavier’s college, Mumbai for helping us with the authentication of the plant samples. We are also thankful to the entire staff at Anchrome enterprise Pvt. Ltd. Mulund (E), Mumbai for lending their knowledge and expertise in the HPTLC analysis.
Declaration of Interest Statement
Authors declare that there is no conflict of interest.
References
- Singh H, Rana AC, Saini S, et al. Int Res J Pharm. 2012; 4(2): p. 63-70.
- Singh CN, Rana AC, Seema S, et al. Int Res J Pharm. 2012; 17(5): p. 114-119.
- Pranitha K, Gupta NV, Anis S, et al. Int J Pharmtech Res. 2012; 4(1): p. 49-55.
- Deepika P, Amul M. Int J Pharm Erud. 2019; 17(6): p. 56-58.
- Zayed MA, A El-Shal M, Abdel Azime M. Egypt J Chem. 2017; 60(6): p. 1177-1188.
- Pandharmise P, Kulkarni A, Naiyar S, et al. Int J Pharm Tech Res. 2011; 3 (7): p. 1515-1520.
- Patell VB, Rathwal MR, Patel K. Int. J Pharm Sci Res Biomed Sci. 2011; 2(4): P.15-17.
- Devi NK, Reddy VV, Mrudula BS, et al. Res J Pharm Dos for Technol. 2010; 2(2): p. 189-192.
- Venkata Raveendranath T, Kumar KK, Madhuri L, et al. Int J Res Pharm Biomed Sci. 2010; 1(2): p. 109-123.
- Raghunandanan R. Pharm Times. 2009; 41(4): p. 15-18.
- Ku MS, Lu Q, Li W, Chen Y. Int J Pharm. 2011; 416(1): p. 16-24.
- Frick A, Moller H, Wirbitzki E. Eur J Pharm Biopharm. 1998; 46(3): p. 305-311.
[Crossref] [Google Scholar] [PubMed]
- Deng G, Ashley AJ, Brown WE, et al. Pharm Res. 2008; 25(5): p. 1100-1109.
[Crossref] [Google Scholar] [PubMed]
- Stotz I, Lamanna G, Hettrich H, et al. Rev Sci Instrum. 2008; 79(12). P.55-57.
[Crossref] [Google Scholar] [PubMed]
- Woertz K, Tissen C, Kleinebudde P, et al. Int J Pharm. 2011; 417(1-2): p.256-271.
[Crossref] [Google Scholar] [PubMed]