Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 4
Regioselective synthesis of 1-alkyl-4-nitro-1H-imidazole using 2-methyl-5-nitroimidazole and 4 nitroimidazole as starting reagents
Yassine Hakmaoui1*, Fatima Asserne1, Abderrahim El Haib4, Rahhal El Ajlaoui1,2, Said Abouricha1 and El Mostapha Rakib1,32Departement de Chimie, Faculte des Sciences Appliquees Ait Melloul, Universite IBN ZOHR, N10. BP 6146 Cite Azrou, Ait Melloul, 86150 Agadir, Morocco
3Higher School of Technology, Sultan Moulay Slimane University, BP 336, Fkih Ben Salah, Morocco
4Laboratory of Chemistry of Natural Substances, Faculty of Sciences Semlalia, Cadi Ayyad University, B.P. 2390, Marrakech, Morocco
Yassine Hakmaoui, Laboratory of Molecular Chemistry, Materials and Catalysis, Faculty of Sciences and Technics, Sultan Moulay Slimane University, Be ni-Mellal, BP 523, Morocco, Email: yassine.hakmaoui@gmail.com
Received: 03-Apr-2022, Manuscript No. dpc-22-57024; Editor assigned: 05-Apr-2022, Pre QC No. dpc-22-57024 (PQ); Reviewed: 21-Apr-2022, QC No. dpc-22-57024; Revised: 27-Apr-2022, Manuscript No. dpc-22-57024 (R); Published: 04-May-2022, DOI: 10.4172/0975-413X.14.4.12-16
Abstract
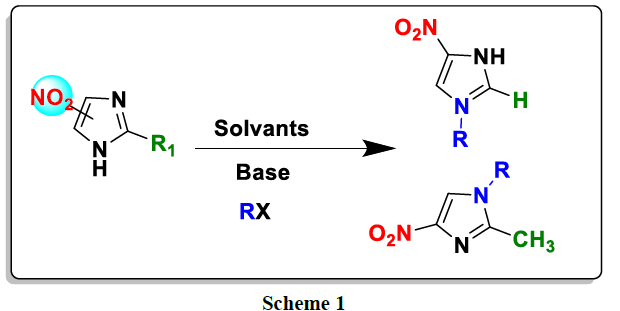
In this work, we were able to achieve a total regioselectivity for the alkylation in the N1 position of 2-methyl-5-nitro-1H-imidazole and in the N1 position of 4-nitro-1H-imidazole; it was carried out in different operating condition (base, solvent, temperature…) with different alkylating agents. The structures of the products obtained, they are established by 1H and 13C NMR spectroscopy. In parallel, a computational study it was carried out in accordance with the experimental results obtained (scheme 1).
Keywords
N-alkylation; regioselective; Nitroimidazole
Introduction
In recent years our laboratory has developed a research axis devoted mainly to the synthesis and biological evaluation of original heterocyclic compounds with potential therapeutic aim containing nitrogenous heterocyclics, we have studied the synthesis and reactivity of 4 and 5- nitoimidazole due to its wide range of applications such as antibacterial [1], antiviral [2], anti-inflammatory [3], anthelmintic [4], analgesic [5], antituberculosis [6], anti-cancer activities [7]. It known in the form of several valuable drugs for the treatment of several protozoan diseases as well as infections due to anaerobic bacteria [8], and important intermediates for organic synthesis. Some recent examples of the imidazole derivative are granisetron used in CRAC channel dysfunction [9, 10] drug for the treatment of human sleeping sickness [11] it is also used in the study to map hypoxia after stroke [12], is an important biological messenger involved in the regulation of many biological processes with physiological and pathological effects. Its use is not limited to the biological field alone, but also extends to the electronic field in new classes of materials [13, 14]. In the present work we have synthesized the novel 2-alkyl-4-nitroimidazole from 4 and 5 nitroimidazole by different alkylation conditions. These reactions gave several compounds depends on a number of factors, the most important factor is the nature of the starting molecule used, the reaction conditions used. We note that these reactions were regiospecific and the structures of the products were analyzed by NMR spectroscopy.
Result and Discussions
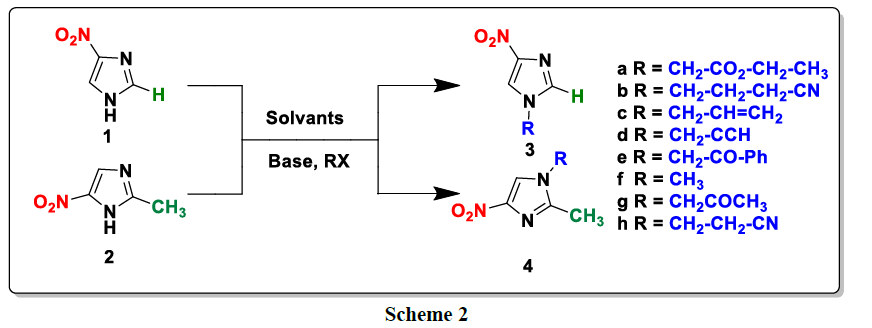
For the alkylation of 4 and 5-nitroimidazole, we used several alkylating agents under different operating conditions (base, solvent and temperature). In all cases, we obtain a single product alkylimidazole 3a-h and 4a-h (Scheme 2). We report in (Tables 1) (Tables 2), the operating conditions used for each alkylating reagent as well as the yields of the N-alkylated derivatives of 4 5-nitroimidazole.
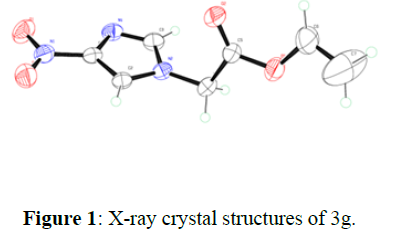
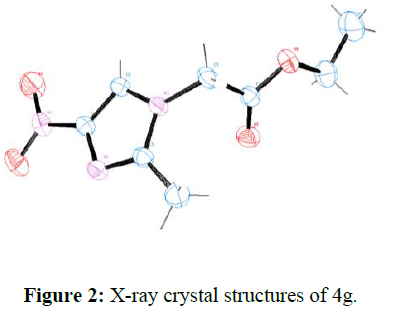
All structures were determined by examination of their NMR data. Furthermore, the structures of products 4g and 3g is unambiguously confirmed through single crystal X-ray crystallographic analysis (Figure. 1).
The yields of the alkylated products are determined after purification by chromatography. The results of (Table 1) show that the alkylation of 4-nitroimidazole in the presence of K2CO3 as base in acetonitrile gives better yields compared to the other conditions: K2CO3 / DMSO, K2CO3 / DMF, KOH / DMSO, KOH / DMF and KOH / acetonitrile. The yields are low in the presence of KOH as the base in DMF, DMSO and acetonitrile. On the other hand in the case of 5-nitroimidazole the effect of the base has a slight influence on the yield except in the case of propargyl where the yield does not exceed 28% with KOH on the other hand with K2CO3 it is 40% in acetonitrile as solvent . Yields of N-alkylated products are better in acetonitrile in the presence of K2CO3 compared to using other conditions.
| Entry | Alkylating agent | Base | Yield according to the solvent (%) | Tim (h) | T°C | ||
|---|---|---|---|---|---|---|---|
| DMSO | DMF | Acétonitrile | |||||
| 3a | Ethyl bromoethylacetate | K2CO3 | 40% | 35% | 50% | 8 | rt |
| KOH | 30% | 36% | 48% | ||||
| 3b | 4-bromobutanitrile | K2CO3 | 32% | 40% | 46% | 7 | rt |
| KOH | 20% | 30% | 34% | ||||
| 3c | Allyl bromide | K2CO3 | 12% | 20% | 40% | 9 | rt |
| KOH | 12% | 20% | 38% | ||||
| 3d | Propargyl bromide | K2CO3 | 20% | 25% | 40% | 8 | rt |
| KOH | 15% | 16% | 25% | ||||
| 3e | bromoacétophénone | K2CO3 | 40% | 48% | 60% | 6 | rt |
| KOH | 20% | 35% | 40% | ||||
| 4a | Ethyl bromoethylacetate | K2CO3 | 41% | 40% | 56% | 6 | rt |
| KOH | 41% | 45% | 50% | ||||
| 4b | 4-bromobutanitrile | K2CO3 | 41% | 40 | 56% | 8 | rt |
| KOH | 35% | 38% | 48% | ||||
| 4c | Allyl bromide | K2CO3 | 30% | 24% | 44% | 8 | rt |
| KOH | 36% | 40% | 40% | ||||
| 4d | Propargyl bromide | K2CO3 | 23% | 20% | 40% | 8 | rt |
| KOH | 12% | 12% | 28% | ||||
| 4e | bromoacétophénone | K2CO3 | 54% | 65% | 67% | 7.5 | rt |
| KOH | 26% | 38% | 41% | ||||
To study the influence of temperature on the alkylation reaction, we performed the alkylation reaction of 4 and 5-nitroimidazole with several alkylating agents under the above conditions (Table 2).
On examining (Table 2), we noticed that heating the reaction to a temperature of 60ºC markedly improved the yields of the products obtained under all conditions. Alkylation in acetonitrile as a solvent and in the presence of K2CO3 leads to the N-alkylated derivatives of imidazole in good yields (66-85%). Temperature also has an influence on the kinetics of the alkylation reaction. Reactions last from one hour to three hours. Also the N-alkylated derivatives of 5-nitroimidazole are obtained with good yields, in particular in the case of the use of acetonitrile as solvent in the presence of K2CO3. The excellent yield is obtained in the case of the reaction of 5-nitroimidazole with ethyl bromoethylacetate, the N-alkyl derivative is isolated with a yield of 96%.
| Entry | Alkylating agent | Base | Yield according to the solvent (%) | Tim (h) | T°C | ||
|---|---|---|---|---|---|---|---|
| DMSO | DMF | Acétonitrile | |||||
| 3a | Ethyl bromoethylacetate | K2CO3 | 56% | 45% | 85% | 1 | 60 |
| KOH | 43% | 53% | 62% | ||||
| 3b | 4-bromobutanitrile | K2CO3 | 38% | 41% | 54% | 1,5 | 60 |
| KOH | 28% | 36% | 44% | ||||
| 3c | Allyl bromide | K2CO3 | 47% | 60% | 74% | 3 | 60 |
| KOH | 35% | 48% | 61% | ||||
| 3d | Propargyl bromide | K2CO3 | 43% | 59% | 66% | 3 | 60 |
| KOH | 38% | 42% | 50% | ||||
| 3e | bromoacétophénone | K2CO3 | 51% | 66% | 84% | 1 | 60 |
| KOH | 32% | 40% | 53% | ||||
| 4a | Ethyl bromoethylacetate | K2CO3 | 80% | 88% | 96% | 1 | 60 |
| KOH | 70% | 81% | 90% | ||||
| 4b | 4-bromobutanitrile | K2CO3 | 78% | 80% | 85% | 1 | 60 |
| KOH | 60% | 68% | 78% | ||||
| 4c | Allyl bromide | K2CO3 | 46% | 49% | 75% | 8 | 60 |
| KOH | 36% | 40% | 62% | ||||
| 4d | Propargyl bromide | K2CO3 | 40% | 44% | 75% | 8 | 60 |
| KOH | 20% | 18% | 62% | ||||
| 4e | bromoacétophénone | K2CO3 | 73% | 86% | 91% | 0.5 | 60 |
| KOH | 55% | 60% | 74% | ||||
To obtain information on the influence of an alkylating agent at the 3-position of 2-methyl-5-nitroimidazole, we have collected the 13C NMR chemical shifts of some compounds in the following (Table 3).
| Entry | Substitute | Chemical shift (δ) ppm | |||
|---|---|---|---|---|---|
| C2 | C4 | C5 | CH3 | ||
| 3 | H | 145,17 | 119,45 | 147,28 | 14,99 |
| 4a | CH2CO2C2H5 | 145,45 | 122,13 | 147 | 12,84 |
| 4c | CH2=CH-CH2 | 144,9 | 130,9 | 138,9 | 12,89 |
| 4d | HCCCH2 | 145,45 | 122,13 | 122,14 | 13,06 |
| 4e | Ph-CO-CH2 | 145,79 | 134,50 | 146,62 | 12,88 |
| 4f | CH3 | 145,8 | 123,49 | 145,9 | 12,87 |
It can be seen that the chemical shift of the C2 atom of the cycle is less influenced, on the other hand the chemical shift of the C4 carbon depends on the nature of the substituent, it varies between 119.45, for the non-alkylated product, and 134.5 in the case of the alkylating agent bromoacetophenone. Also, the chemical shift of C5 is sensitive to the alkylating agent; it varies between 122.14 and 147.28. Finally, the chemical shift of the methyl carbon atom is between 12.84 and 14.99 ppm.
Conclusions
We have shown that the alkylation reaction of 4-nitroimidazole and 5-nitroimidazole is sensitive to the position of the nitro group and to the operating conditions used. At room temperature, a low yield is observed. This yield is best when working in acetonitrile at 60ºC in the presence of K2CO3 as the base. We was observed Regioselectivity in favor of N-3 alkylation of 2-methyl-5-nitroimidazole due to the steric effect of the nitro group. On the other hand, in the case of 4 nitroimidazole the alkylation of N-1 is favored. The desired objective is the preparation of new substituted derivatives with potential pharmacological properties.
Experimental Section
General procedure for the synthesis of N-alkyls of 4-nitro and 5-nitroimidazole:
Procedure 1: solvent used DMSO or DMF. To a solution of 4 (5) nitroimidazole (7.87 mmol) in (DMSO or DMF), is added potassium hydroxide or K2CO3 (8.7 mmol). After stirring for 15 minutes, the alkylating agent (15.74 mmol) is added dropwise. After disappearance of the starting material indicated by TLC, the mixture was added to ice-water and extracted with ethyl acetate. The organic phase is washed with brine and dried over magnesium sulfate. The solvent is evaporated off in vacuo. The resulting residue is purified by column chromatography (EtOAc / hexane09 / 01).Procedure 2: using CH3CN as solvent.
Procedure 2: solvent used CH3CN. To a solution of 4 (5) nitroimidazole (7.87 mmol) in (CH3CN), is added potassium hydroxide or K2CO3 (8.7 mmol). After stirring for 15 minutes, the alkylating agent (15.74 mmol) is added dropwise. After disappearance of the starting material indicated by TLC, the resulting mixture is evaporated. The crude product was dissolved with EtOAc (50 mL); it washed with water and brine, and dried over magnesium sulfate. The solvent is evaporated off in vacuo. The resulting residue was purified by column chromatography (EtOAc / hexane 9/1).
Ethyl 2-(4-nitro-1H-imidazol-1-yl)acetate 3a: RMN1H (300 MHz, DMSO-d6): δ 8.37 (d, J = 1.4 Hz, 1H, H2), 7.84 (d, J = 1.4 Hz, 1H, H5), 5.09 (s, 2H, H1’), 4.19 (q, J = 7.1 Hz, 2H, H1’’’), 1.23 (t, J = 7.1 Hz, 3H, H1’’’’).
13C NMR (75 MHz, DMSO-d6): δ 167.60 (C1’’, C=O), 146.87 (C4-NO2), 138.35 (C5), 122.69 (C2), 61.61 (C1’’’, CH2), 48.34 (C1’, CH2), 14.01 (C1’’’’, CH3).
4-(4-Nitro-1H-imidazol-1-yl)butanenitrile 3b: RMN1H (300 MHz, DMSO-d6): δ 8.46 (d, J = 1.5 Hz, 1H, H2), 7.89 (d, J = 1.4 Hz, 1H, H5), 4.14 (t, J = 7.0 Hz, 2H, H1’), 2.55-2.50 (m, 2H, H1’’), 2.14-2.08 (m, 2H, H1’’’).
13C NMR (75 MHz, DMSO-d6): δ 147.15 (C4), 137.51 (C5), 121.67 (C2), 119.78 (C1’’’’), 46.31 (C1’), 25.62 (C1’’’), 13.63 (C1’’).
1-Allyl-4-nitro-1H-imidazole 3c: RMN1H (300 MHz, DMSO-d6): δ 8.37 (d, J = 1.5 Hz, 1H, H-2), 7.86 (d, J = 1.4 Hz, 1H, H-5), 6,12˗5.99 (m, 1H, H1’’), 5.30˗5.14 (m, 2H, H1’’’), 4.75˗4.72 (m, 2H, H1’).
13C NMR (75 MHz, DMSO-d6): δ 147.03 (C4), 137.38 (C5), 133.05 (C1’’), 121.60 (C2), 118.88 (C1’’’), 49.56 (C1’).
4-Nitro-1-(prop-2-ynyl)-1H-imidazole 3dRMN1H (300 MHz, DMSO-d6): δ 8.42 (d, J = 1.5 Hz, 1H, H-2), 7.94 (d, J = 1.5 Hz, 1H, H-5), 5.05 (d, J = 2.6 Hz, 2H, H1’), 3.68 (t, J = 2.6 Hz, 1H, H1’’’).
13C NMR (75 MHz, DMSO-d6): δ 147.1 (C4), 137.0 (C5), 121.2 (C2), 77.7 (C1’’’), 77.4 (C1’’), 36.9 (C1’).
2-(4-Nitro-1H-imidazol-1-yl)-1-phényléthanone 3e: 1H NMR (300 MHz, DMSO-d6): δ 8.33 (d, J = 1.4 Hz, 1H, H2), 8.07-8.03 (m, 2H, Ho-Ph), 7.81 (d, J = 1.4 Hz, 1H, H5), 7.78-7.72 (m, 1H, Hp-Ph), 7.65-7.60 (m, 2H, Hm-Ph), 5.88 (s, 2H, H1’).
13C NMR (75 MHz, DMSO-d6): δ 192.24 (C1’’, C=O), 146.79 (C4-NO2), 138.53 (C5), 134.35 (Cp-Ph), 133.98 (C1’’’), 129.09 (Cm-Ph), 128.07 (Co-Ph), 123.04 (C2), 54.01 (C1’).
1-Méthyl-4-nitro-1H-imidazole 3f : 1H NMR (300MHz, DMSO-d6): δ 8.35 (d, J = 1.3 Hz, 1H, H-2), 7.80 (d, J = 1.2 Hz, 1H, H-5), 3.75 (s, 3H, N1-CH3).
13C NMR (75 MHz, DMSO-6): δ 146.9 (C4-NO2), 138.1 (C5), 122.6 (C2), 34.2 (N1-CH3).
1-(4-Nitro-1H-imidazol-1-yl)propan-2-one 3g: 1H NMR (300 MHz, DMSO-d6): δ 8.22 (d, J = 1.4 Hz, 1H, H2), 7.70 (d, J = 1.4 Hz, 1H, H5), 5.16 (s, 2H, H1’, CH2), 2.20 (s, 3H, H1’’’, CH3).
13C NMR (75 MHz, DMSO-d6): δ 200.93 (C1’’, C=O), 146.68 (C4), 138.17 (C5), 122.65 (C2), 56.30 (C1’, CH2), 26.91 (C1’’’, CH3).
3-(4-Nitro-1H-imidazol-1-yl)propanenitrile 3h: 1H NMR (300 MHz, DMSO-d6): δ 8.48 (d, J = 1.5 Hz, 1H, H2), 7.94 (d, J = 1.4 Hz, 1H, H5), 4.39 (t, J = 6.6 Hz, 2H, H1’), 3.17 (t, J = 6.6 Hz, 2H, H1’’).
13C NMR (75 MHz, DMSO-d6): δ 147.15 (C4), 137.60 (C5), 121.49 (C2), 118.12 (C1’’’), 43.05 (C1’), 18.97 (C1’’).
2-(4-Nitro-1H-imidazol-1-yl)-4-methylbenzyl 3i: 1H-NMR (300 MHz, DMSO-d6): δ 8.46 (d, J = 1.5 Hz, 1H, H2), 7.98 (d, J = 1.5 Hz, 1H, H5), 7.28 (d, J = 8.0 Hz, 2H, Ho-Ph), 7.19 (d, J = 8.0 Hz, 2H, Hm-Ph), 5.24 (s, 2H, H1’), 2.28 (s, 3H, CH3).
13C-NMR (75 MHz, DMSO-d6): δ 147.15 (C4), 137.69 (C-CH3), 137.28 (C5), 133.26 (C1’’), 129.45 (Cm-Ph), 127.99 (Co-Ph), 121.44 (C2), 50.48 (C1’), 20.70 (CH3).
Ethyl 2-(2-méthyl-4-nitro-1H-imidazol-1-yl)acetate 4a : 1H NMR (300 MHz, CDCl3): 1.30 (t, 3H, CH3, J = 7.2 Hz), 2.40 (s, 3H; CH3), 4.27 (q, 2H, CH2, J = 7.2 Hz), 4.69 (s, 2H, NCH2), 7.74 (s, 1H, H-5).
13C NMR (75 MHz, CDCl3): 12.8 (CH3), 14.1 (CH3), 48.1 (NCH2), 62.8 (CH2O), 120.7 (CH), 145.5 (C), 165.9 (CO).
1-Allyl-2-methyl-4-nitro-1H-imidazole 4c: 1H NMR (300 MHz, CDCl3): 2.5 (s, 3H; CH3), 5.1 (d, 2H, NCH2), 5.4 (m, 2H, =CH2), 5.87 (m, 1H, HC=), 7.93 (s, 1H, H-5).
13C NMR (75 MHz, CDCl3): 12.9 (CH3), 49.5 (NCH2), 119.8 (=CH2), 130.4 (CH), 130.9 (CH), 138.9 (C), 154.9 (C).
2-Méthyl-4-nitro-1-(prop-2-yn-1-yl)-1H-imidazole 4d: 1H NMR (300 MHz, DMSO-d6): 2.38 (s, 3H, CH3), 3.62 (t, 1H, J = 2.4 Hz, ≡CH), 4.97 (d, 2H, J = 2.4 Hz, NCH2), 8.29 (s, 1H, H-5).
13C NMR (75 MHz, DMSO-d6): 13.0 (CH3), 36.6 (NCH2), 77.4 (≡CH), 77.9 (≡C-), 122.1 (CH), 145.4 (C).
2-(2-Méthyl-4-nitro-1H-imidazol-1-yl)-1-phényléthan-1-one 4e: 1H NMR (DMSO-d6): 2.36 (s, 3H; CH3), 5.81 (s, 2H, NCH2), 7.58-7.63 (m, 2H, H-Ar), 7.70-7.74 (m, 1H, H-Ar), 8.02-8.08 (m, 2H, H-Ar), 8.20 (s, 1H, H-5).
13C NMR (75 MHz, DMSO-d6): 12.9 (CH3), 53.7 (NCH2), 123.7 (CH), 128.6 (2CH), 129.4 (2CH), 134.5 (CH), 134.8 (C), 145.79 (C), 146.6 (C), 192.9 (CO).
1,2-Diméthyl-4-nitro-1H-imidazole 4f : 1H NMR (300 MHz, CDCl3): 2.3 (s, 3H; CH3), 3.63 (s, 3H, N-CH3), 8.24 (s, 1H, C4-H).
13C NMR (75 MHz, CDCl3): 12.9 (CH3), 33.9 (N-CH3), 123.5(CH), 140.1 (C), 145.9 (C).
1-(2-Méthyl-4-nitro-1H-imidazol-1-yl)propan-2-one 4g: 1H NMR (300 MHz, DMSO-d6): δ 8.11 (s, 1H, H-5), 5.13 (s, 2H, H1’, CH2), 2.22 (s, 3H, H1’’’-CH3), 2.20 (s, 3H, H2-CH3).
13C NMR (75 MHz, DMSO-d6): δ 201.06 (C1’’, C=O), 145.81 (C4), 145.19 (C2), 123.01 (C5), 55.61 (C1’, CH2), 27.06 (s, C1’’’, CH3), 12.37 (s, C2-CH3).
1-Ethyl-2-méthyl-5-nitro-1H-imidazole 4i : 1H NMR (300 MHz, CDCl3): 1.50 (t, 3H; J = 7.2 Hz, CH3), 2.5 (s, 3H, CH3), 4.0 (q, 2H, J = 7.2 Hz, NCH2), 7.95 (s, 1H, H-5).
13C NMR (75 MHz, CDCl3): 12.9 (CH3), 15.1 (CH3), 33.7 (NCH2), 133.2 (CH), 138.4 (C), 153.1 (C).
REFERENCES
- Matysiak J, Niewiadomy A, Krajewska-Kułak E et al ., Il Farmaco. 2003, 58(6) : p. 455-461.
- Cheng J, Xie J and Luo X. Bioorg Med Chem Lett. 2005, 15(2): p.267-269.
- Singh P, Kumar R and Tewari AK. Chem Eur J. 2020, 11(1): p.50-59.
- Kapoor VK, Dubey S and Mahindroo N. 2000.
- Khabnadideh S, Rezaei Z, Khalafi-Nezhad A et al., Bioorg Med Chem Lett. 2003, 13(17): p.2863-2865.
- Mallia MB, Subramanian S, Mathur A et al., Bioorg Med Chem Lett. 2008, 18(19): p.5233-5237.
- Boyanova L, Kolarov R and Mitov I. Expert Rev Anti-Infect Ther. 2007, 5(4): p.685-701.
- Tian C, Du L, Zhou Y et al., Future Med Chem. 2016, 8(7): p.817-832.
- Jairaman A and Prakriya M. Channels. 2013, 7(5): p.402-414.
- Kaiser M, Bray MA, Cal M et al., Antimicrob Agents Chemother. 2011, 55(12): p.5602-5608.
- Takasawa M, Moustafa RR and Baron JC. Stroke. 2008, 39(5): p.1629-1637.
- Wang TT, Zeng GC, Zeng HP et al., Tetrahedron. 2009, 65(32): p.6325-6329.
- Serpell CJ and Beer PD. Cryst Growth Des. 2013, 13(7): p.2866-2871.
- Hakmaoui Y, Rakib EM, Mojahidi S et al., IUCrData. 2016, 1(4): p.x160588.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Crossref