Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Recent Advances in the Construction of Indole Scaffolds
Fateh V Singh* and Saeesh R MangaonkarFateh V Singh, Chemistry Division, School of Advanced Science, VIT University, Chennai Campus, Chennai-600127, Tamilnadu, India,
Abstract
Indole scaffolds are important chemical entity found in biologically important synthetic and naturally active compounds. Various synthetic approaches are available for the construction of functionalized indoles including several classical and non-classical approaches. In classical approaches, numerous metal catalysts such as palladium, copper, rhodium, ruthenium and iron have been used successfully in recent few years. In this review, various aspects of classical and non-classical approaches used to construct functionalized indoles are discussed.
Keywords
Indoles, Cyclization reactions, Fischer Indole synthesis, Classical and non-classical reactions
Introduction
The indole ring system represents a key structural component that is widely distributed in nature [1]. Functionalized indoles are known as ubiquitous heterocycle in nature as it displays vast number of application in pharmaceuticals, chemical and agricultural industries [2,3]. Various indole-cored architectures are existing in nature and considered as important scaffolds in synthetic and medicinal chemistry [4]. Several synthetic compounds having indole functionality are known for various biological activities.
Indomethacin 1 andsumatriptan 2 are known as potent non-steroidal anti-inflammatory agents [5,6]. Serotonin 3 is mainly found in animals and plays an important role in controlling the central nervous system [7]. Several naturally occurring compounds containing indole scaffold have clinical importance. Mersicarpine 4 is a tetracyclic dihydroindole derivativethat was isolated from the bark of Malayan Kopsiafruticosa and Karborea [8-12]. Cryptotackiene 5 is an antiplasmodial agent that was isolated from the West African shrub Cryptolepis Sanguinolenta [13-15]. Scytonine 6 is a pigment that was isolated from the organic extract of plant species Scytonema 10 [16]. Thienodolin 7 was found in the culture broth of Streptomyces Albogriseolus and it exhibits both growthpromoting and growth-inhibiting activities in rice seedling [17]. Bombyxbatryticatus (BB-3) 8 is an analgesic drug obtained from dried larvae of the silkworm Bombyx Mori [18]. Caulersin 9 is a bisindole-based naturally occurring compound which is isolated from algae Caulerpaserrulata. It is exhibits antitumor activity [19].
In past few decades, various synthetic methods have been used for the construction functionalized indoles. Functionalized indoles have been synthesized by both classical and non-classical approaches. In this review, we have covered various classical and nonclassical approaches appeared in past five years for the construction of functionalized indoles
Classical approach for the synthesis of functionalized indole
Pd-catalyzed synthesis of functionalized indoles
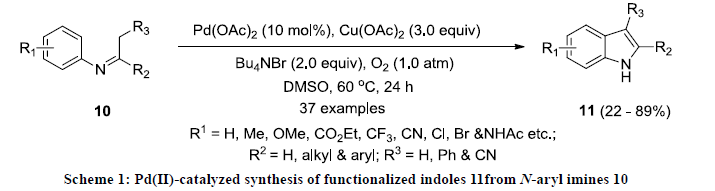
In 2012, Yoshikai and co-workers reported the synthesis of 2,3-disubstitutedindoles 11 via Pd(II)-catalyzed oxidative cyclization of N-aryl imines 10 (Scheme 1) [20]. All the reactions was carried out in DMSO at 60 oC using Pd(OAc)2 as catalyst, Cu(OAc)2 as additive in the presence of atmospheric O2. The reaction products were isolated in useful yields and functional groups were tolerated successfully in this reaction. In addition, fused indoles were also synthesized in useful yields using similar approach.
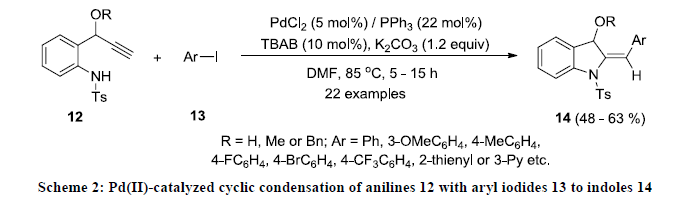
Chowdhury and co-workers developed the synthesis of functionalized indole derivatives 14 in moderate yields via Pd(II)-catalyzed cyclic condensation of N-protected-anilines 12 with aryl iodides 13 [21]. All the reactions were carried out in DMF at 85 oC under argon atmosphere using PdCl2/PPh3 catalytic system in the presence of K2CO3 base and phase transfer catalyst Bu4NBr (TBAB)
(Scheme 2). Using this approach wide range of functionalize indoles were prepared as this catalytic system display excellent functional group tolerance. In addition, the similar catalytic approach was successfully applied for the construction of 2-substituted quinolines.
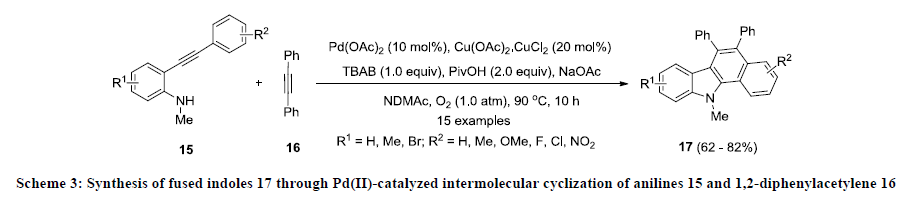
Furthermore, Liang and co-workers developed an efficient Pd(II)-catalyzed approach for the construction of functionalized fused indoles 17 [22]. This approach allows the preparation of structurally diverse fused indole systems from simple substrate. In this methodology, anilines 15 were treated with 1,2-phenyl acetylene 16 in dimethylacetamide (DMAc) using palladium acetate as catalyst, Cu(OAc)2.CuCl2 as an oxidant and NaOAc as base (Scheme 3). Additionally, additives such as pivalic acid (PivOH) and tetrabutylammonium bromide (TBAB) were also used to improve the overall yield of the reaction. Finally, fused indoles 17 were isolated in good to excellent yields and various electron-donating and -withdrawing functional groups were tolerated in this reaction.
After this report, Takemoto and co-workers reported a new method for the synthesis of functionalized indole [23]. This methodology involves Pd(II)-catalyzed cascade reaction of o-methylphenyl isocyanides 18 with aryl halides 19 which gave 2-arylindoles 20 as reaction product (Scheme 4) in moderate yields. In this reaction, methyl phenyl isocyanide is coupled with aryl iodide in presence of palladium acetate and bulkier ligand such as Ad2PnBu. Additionally, the same catalytic approach was successfully applied for the construction of fused indole systems.
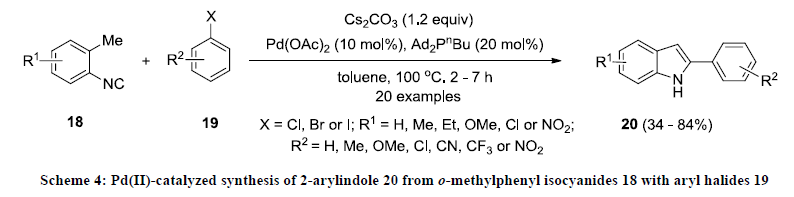
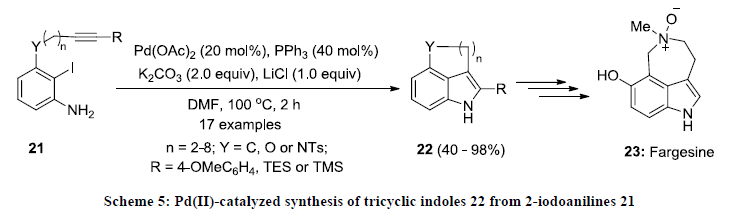
In 2013, Jia and co-workers reported a direct method for the synthesis of polycyclic indoles 22 via intramolecular Larock indole synthesis [24]. In this report, 2-iodoaniline derivatives 21 were cyclized into tricyclic indoles 22 in good to excellent yields using Pd(OAc)2/PPh3 catalytic system in DMF (Scheme 5). Furthermore, the reactions products 22 were found as useful substrates for synthesis of bioactive compound fargesine 23.
In 2014, Ghorai and co-workers developed an efficient strategy for the synthesis of 2,3-disubstituted indole derivatives 25 by Pd(II)- catalyzed aerobic oxidative cycloisomerization of o-allylanilines 24 using Pd(OAc)2/PPh3 catalytic system in DMF under oxygen atmosphere (Scheme 6) [19,25]. All the reactions were working smoothly and reaction products were isolated in good to excellent yields. Various electron-donating and –withdrawing functionalities were tolerated under this reaction condition.
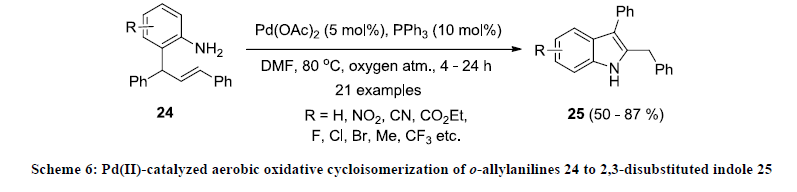
Recently Yu and co-workers reported the synthesis of functionalized indole by Pd-catalyzed oxidative annulation of functionalized pyrroles 26 with ketones 27 using Pd(OAc)2 as catalyst, Cu(OAc)2.H2O as oxidant in the presence of sodium acetate (Scheme 7) [26]. Tetrabutylammonium bromide (TBAB) and pivalic acid (PivOH) were used as additive to increase the reaction yields. All the reactions were carried out in the mixture of DMSO and DMF (1:9) and reaction products 28 were isolated in moderate yields.
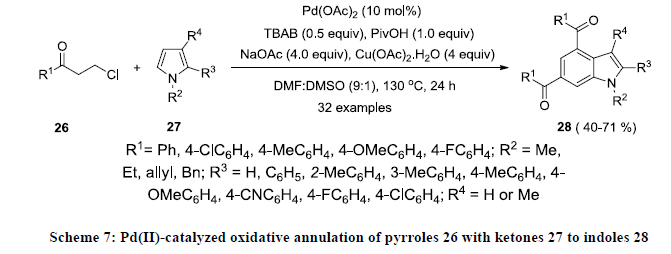
Cu-catalyzed synthesis of functionalized indoles
Like palladium, Cu is another transition metal which is more commonly used as catalyst during the construction of indoles. In 2005, Cusack and co-workers developed Cu-catalyzed approach for the synthesis of functionalized indoles 31 by intramolecular cyclization of ene-carbamates 29 using CuI as catalyst, L-proline 30 as ligand in the presence of K2CO3 in 1,4-dioxane (Scheme 8) [27]. The reactions were found quite slow and reaction products 31 were isolated in poor yields.
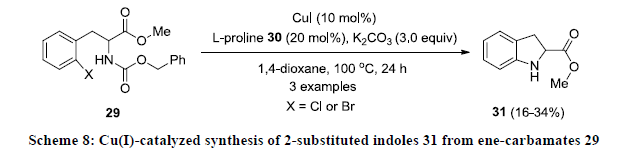
In 2011, Wang and co-workers reported another Cu-catalyzed approach for the construction of indole systems. In this report, alkynes 33 were directly coupled with N-tosylhydrazones 32 using catalytic amount of CuBr (Scheme 9) [28]. All the reactions were carried out in acetonitrile at 100 oC and reaction products 34 were isolated in 38-74% yields. Various electron-donating and electronwithdrawing functionalities were successfully tolerated under mentioned reaction condition.
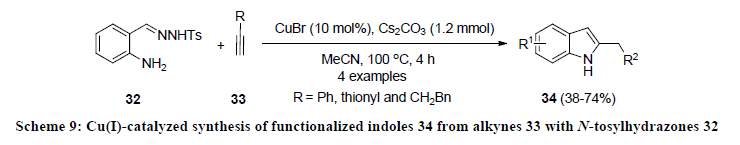
In 2015, Hwang and co-authors developed the synthesis of functionalized indoles 37 through photo-induced copper-catalyzed threecomponent coupling of anilines 35, aryl acetylenes 33, and substituted benzoquinones 36 using catalytic amount of CuCl (Scheme 10) [29]. All the reactions were carried out in methanol at room temperature under visible light. Various electron-donating and withdrawing functionalities on all the precursors were successfully tolerated and reaction products 37 were isolated in good to excellent yields.
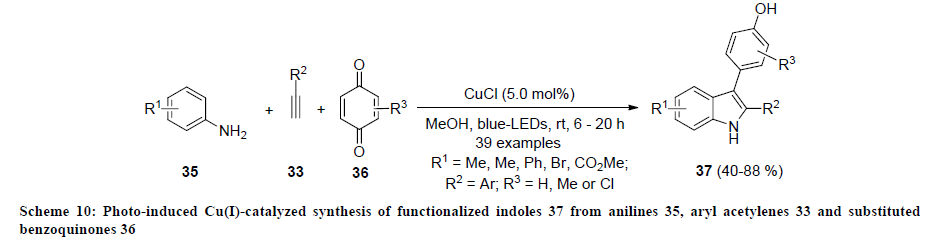
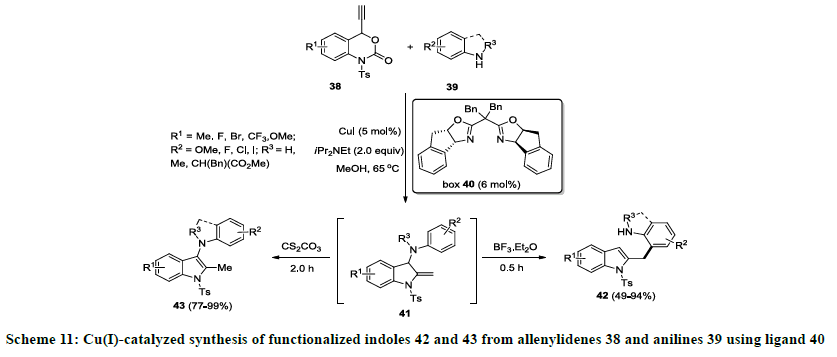
Recently, Lu and co-workers developed an efficient strategy for the synthesis of functionalized indole derivatives using copper catalyzed sequential reaction [30]. In this report, allenylidenes 38 were coupled with functionalized anilines 39 using CuI as catalyst, bisoxazoline (box) 40 as ligand in the presence of iPr2Net (DIPEA) in methanol. Initially, pivotal 3-aminoindoline intermediate 41 was observed that undergo aza-Cope rearrangement on treatment with Lewis acid (TFA or BF3.Et2O) and 3-aminoindolines 42 were isolated in good to excellent yields. In addition, 1,3-proton shift was observed when intermediate 41 was treated with base (CS2CO3) and different indole derivatives 43 were isolated in moderate to excellent yields (Schemes 11 and 12). Furthermore, same catalytic approach was successfully applied for the synthesis of natural product 10H-indolo[3,2-b]quinolone.
Rh-catalyzed synthesis of functionalized indoles
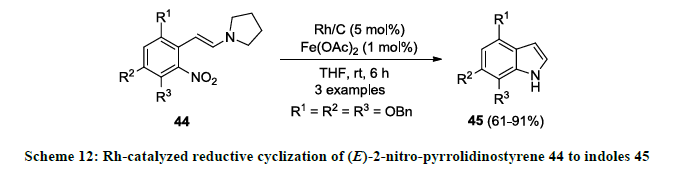
Rhodium metal is also used successfully in the synthesis of indole scaffolds. In 2006, synthesis of functionalized indole 45 was reported by Rh-catalyzed reductive cyclization of (E)-2-nitro-pyrrolidinostyrene 44 using Rh/C as catalyst, Fe(OAc)2 as an additive in THF (Scheme 12) [31]. Functionalized indoles 45 were isolated as reaction products in good to excellent yields. It was noted that reaction product 45 was isolated in slightly poor yield when OBn group was attached at ortho-position to nitro group in 44 (R3=OBn).
In 2008, Fagnou and co-workers developed a different strategy for the synthesis of functionalized indoles in presence of rhodium catalyst 48 [32]. In this methodology, functionalized acetanilides 46 were treated with internal alkyne 47 using catalytic amount of [Rh(Cp*)Cl2]2 48 in the presence of Cu(OAc)2.H2O (oxidant) in tert-amyl alcohol (1,1-dimethylpropanol) (Scheme 13). Additionally, silver salt was used as additive to improve the reaction yields. All the reactions were completed in short reaction time and 2,3-disubstituted indoles 49 were isolated in good to excellent yields. Various acetanilides bearing electron-donating and -withdrawing functionalities were used as precursors but reaction products 49 were obtained in higher yields with electron-donating functionalities. Interestingly, both aliphatic and aromatic alkynes were successfully employed as substrates in this reaction.
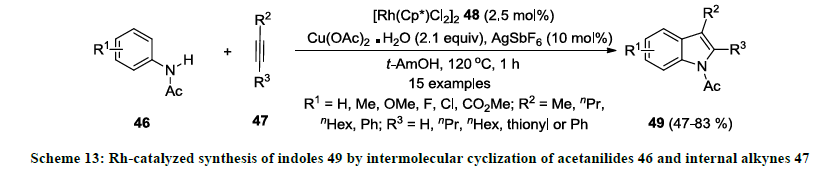
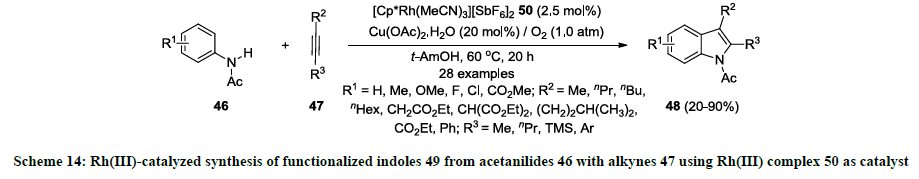
Furthermore, same research group reported a new rhodium(III)-complex [Cp*Rh(MeCN)3][SbF6]2 50 and used as catalyst for the synthesis of similar indoles 49 [33]. According this report, similar 2,3-distituted indoles 49 were synthesized from acetanilides 46 and internal alkynes 47 using catalytic amount of Rh(III)-complex 50 in tert-amyl alcohol (1,1-dimethylpropanol) without using any additive (Scheme 14). Additionally, Cu(OAc)2.H2O was used in catalytic amount (20 mol%) while atmospheric oxygen was used mainly as an oxidant. Various functionalities were successfully tolerated both precursors acetanilides 46 and alkynes 47 and reaction products 49 were isolated in good to excellent yields. It was also noted that TMS group on external alkyne 47 (R3=TMS) could not work well and corresponding indole product 49 (R3=TMS) was isolated in 20% yield.
Fe-catalyzed synthesis of functionalized indoles
In a literature, a solitary report on Fe-catalyzed synthesis of functionalized indoles is available. In the report, the synthesis of functionalized indoles was achieved by intramolecular cyclization of N-substituted anilines 51 using FeCl3 as catalyst, Cu(OAc)2. CuCl2 as oxidant in the presence of K2CO3 (Scheme 15) [34]. All the reactions were carried out in DMF at 120 oC and reaction products 52 were isolated in 32-72% yields. Cyclization reactions were working with arenes bearing an electron-donating group at both the para- and ortho-position in substrates 51 but reaction products 52 were obtained in better yields in the substituent at paraposition than ortho-position. Interestingly, opposite reaction pattern was observed in case of arenes bearing electron-withdrawing functionalities at both para-position than ortho-position 51.
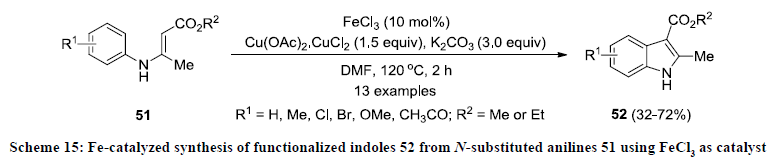
Ru-catalyzed synthesis of functionalized indoles
Like other transition metals (Pd, Cu, Rh and Fe), ruthenium has been also used as catalyst to synthesize functionalized indoles. In 2012, Lygin and Ackermann developed a Ru-catalyzed approach for the synthesis of functionalized indoles 54 by oxidative annulation of N-2-pyrimidyl anilines 52 with internal alkyne 47 using RuCl2 (p-cymene)]2 54 as catalyst and KPF6 as co-catalyst (scheme 16) [35]. Water was used as green solvent that makes this approach simpler and environmentally friendly. The reaction products 55 were isolated in moderate to excellent yields and various functional groups were tolerated successfully on both substrates under mentioned reaction conditions.
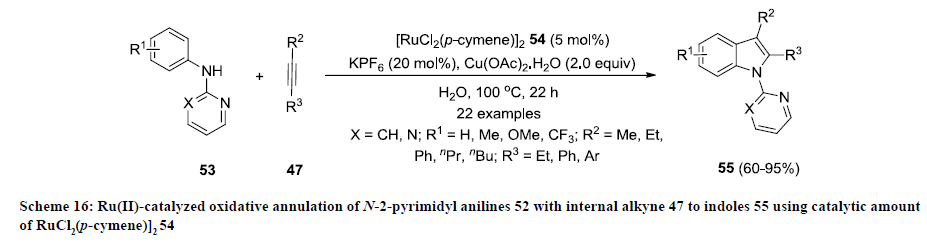
Non-classical approaches for the synthesis of functionalized indoles
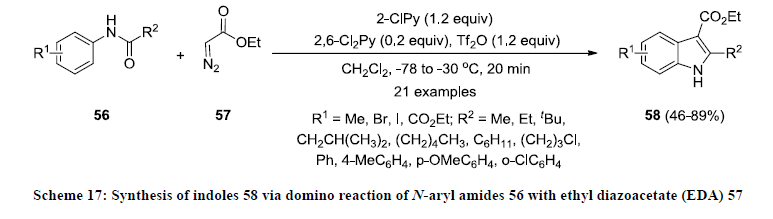
There are few non-classical approaches are available in the literature for the synthesis of indoles. In 1991, Tobinaga and co-workers achieved the synthesis of functionalized indole derivatives by Diels-Alder cycloaddition of 3-thioacetylpyrrol with substituted alkyl dienophile [36]. In 1998, Barluenga and co-workers reported the synthesis of polycyclic indoles via intramolecular cycloaddtion reaction. This was the first intramolecular carbometalation approach of lithiated double bonds [31,37]. In 2004, O’Shea and co-workers developed a non-classical approach for the synthesis of functionalized indoles 55 by intramolecular carbolithiation of ortho-aminostyrenes 54 with organolithium reagents [38]. After this report, Wang and co-workers developed a direct method for the synthesis of functionalized indoles 58 by domino reaction of N-aryl amides 56 with ethyl diazoacetate (EDA) 57 (Scheme 17) [39]. All the reactions were carried out in dichloromethane in presence of 2-chloropyridine (2-ClPy) as nucleophile and 2,6-dichloropyridine (2,6-Cl2Py) as additive. The reaction products 58 were isolated in good to excellent yields.
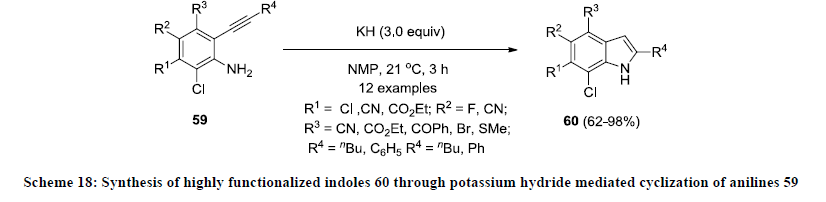
Furthermore, Knochel and Stoll reported an efficient strategy for the synthesis of 2,4,5,6,7-pentasubstituted indoles 60 by direct potassium hydride mediated cyclization of polyfunctionalized anilines 59 (Scheme 18) [40]. Fully functionalized indoles 60 were isolated in good to excellent yields. Various functional groups such as esters, nitriles and halogenides were successfully tolerated in mentioned conditions and it is one of the unique approaches that provide an easy access for the synthesis highly functionalized indoles in high yields.
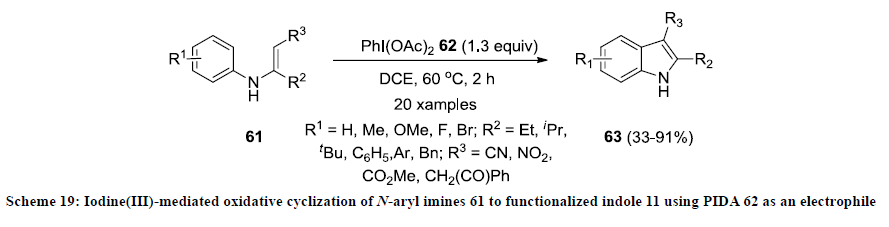
In 2009, Zhao and co-workers reported the construction 2,3-disubstitutedindoles 63 by iodine(III) mediated oxidative cyclization of N-aryl imines 61 using PIDA 62 as an electrophile in 1,2-dichloroethane (DCE) (Scheme 19) [41]. In most of the reactions, functionalized indoles 63 were isolated in excellent yields except imines bearing OMe or F groups on aryl ring. This approach is quite efficient due to easy availability and environmental friendly nature of PIDA 62 as an electrophile [42].
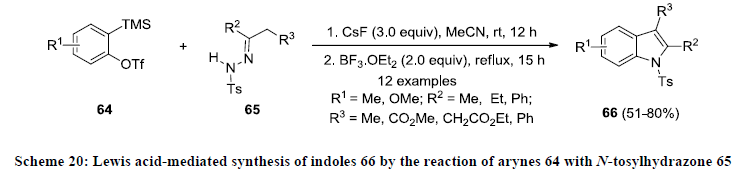
Furthermore, Greaney and co-workers employed a traditional route for the construction of functionalized indoles 66 via Fischer- Indole cyclization. In this approach, functionalized indoles 66 were synthesized by the reaction of aryne precursor 64 with tosylhydrazone 65 in presence of CsF followed by the addition of BF3.OEt2 (Scheme 20) [43]. All the reactions were carried out in acetonitrile and reaction products 66 were isolated in moderate to high yields.
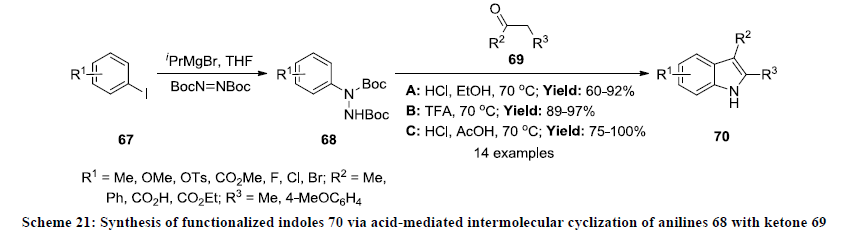
In 2011, Moody and Inman developed the synthesis of functionalized indole derivatives in moderate to excellent yields [44]. Initially the starting substrates N-protected aryl hydrazines 68 were prepared from corresponding aryl iodides 67 by halogenmagnesium exchange reaction and then condensed with ketones 69 under acidic reaction conditions. The cyclization reactions were carried out in different reaction conditions A, B and C and reaction products were isolated in useful yields in all the three conditions (Scheme 21). The wide range of functionalized indoles 70 were synthesized in this reaction as reaction system display excellent functional group tolerance.
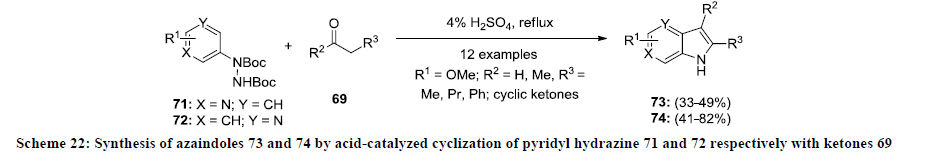
Furthermore, the same group extended their work for the synthesis of functionalized azaindoles 72. In this report, the synthesis of 4- and 6-azaindoles 73 and 74 was achieved by acid-mediated condensation of N-protected pyridyl hydrazines 71 and 72 with ketone 69 in 4% H2SO4 at reflux temperature (Scheme 22) [45]. It was observed that 6-azaindoles 74 were isolated in better yields than 4-azaindoles 73 under similar reaction and conditions. In addition, the same approach was applied for the synthesis of functionalized 6H-thieno[2,3-b]pyrroles.
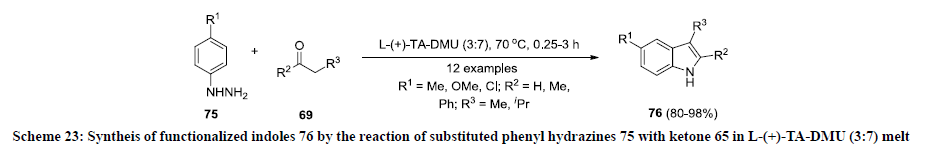
In 2012, Konig and co-workers reported a new approach for the synthesis of functionalized indoles using a reaction medium consisting of low melting L-(+)-tartaric acid and dimethyl urea (DMU) mixture in 3:7 ratio [46]. In this report, phenyl hydrazines 75 were treated with ketone 69 in the mixture of L-(+)-tartaric acid and dimethyl urea (DMU) mixture in 3:7 ratio at 70 oC. The reaction products 76 were obtained in excellent yield (Scheme 23). Furthermore, the reaction product 70 was found as useful substrate for synthesis of bioactive compounds pyridoindolobenzodiazepiene and Dimebon [47-49].
Conclusion
This review article summarizes possible synthetic routes for the construction of functionalized indoles. During the preparation of this manuscript, our main goal was to discuss various aspects of both classical and non-classical approaches for the synthesis of indoles. Various indole systems are important synthetic intermediates for the construction of biologically active synthetic and naturally occurring scaffolds. This review would be quite informative for the organic chemists specially working in chemistry of nitrogen containing heterocyclic scaffolds.
Acknowledgements
Fateh V Singh and is thankful to Science and Engineering Research Board (SERB), New Delhi for providing financial support (Start-UP Research Grant: SB/FT/CS-068/2014). Fateh V Singh and Saeesh R. Mangaonkar are thankful to VIT University, Chennai Campus, Chennai.
References
[1] M. Shiri, J. Chem. Rev., 2012, 3508-3549.
[2] J. Barluenga, F. Rodreguez, F. J. Fanans, Chem. Asian J., 2009, 1036-1048.
[3] M. Somei, F. Yamada, Nat. Prod. Rep., 2004, 278-311.
[4] T. Kawasaki, K. Higuchi, Nat. Prod. Rep., 2005, 761-793.
[5] G. Fronza, A Mele, E. Redenti, P. Ventura, J. Org. Chem., 1996, 909-914.
[6] Z. Wojnarowska, J. Knapik, M. Rams-Baron, A. Jedrzejowska, M. Paczkowska, A. Krause, J Cielecka-Piontek, M. Jaworska, P.
Lodowski, M. Paluch, Mol. Pharmaceutics., 2016, 1111-1122.
[7] E. H. P. Young, J. Chem. Soc., 1958, 3493-3496.
[8] T. S. Kam, G Subramaniam, K. H. Lim, Y. M. Choo, Tetrahedron. Lett., 2004, 5995-5998.
[9] K. Cimanga, T. De Bruyne, L. Pieters, M. Claeys, A. Vlietinck, Tetrahedron Lett., 1996, 1703-1706
[10] M. H. M. Sharaf, P. L. Schiff, A. N. Tackie, C. H. Phoebe, G. E. Martin, J. Heterocycl. Chem., 1996, 239-243
[11] J. L. Pousset, M. T. Martin, A. Jossang, B. Bodo, Phytochemistry., 1995, 735-736
[12] K. Cimanga, T. De Bruyne, L. Pieters, M. laeys, A. Vlietinck, J. Nat. Prod., 1997, 688-691.
[13] V. Bultel-Ponce, F. Felix-Theodose, C. Sarthou, J. F. Ponge, B. Bodo, J. Nat. Prod., 2004, 678-681.
[14] K. Kanbe, H. Nakagawa, M. Okamura, Y. Okami, T. Takeuchi, S. Hattori, M. Hamada, Biosci. Biotechnol. Biochem., 1993,
632-635.
[15] K. Kanbe, H. Nakagawa, T. Nakamura, Y. Okami, T. Takeuchi, Biosci. Biotechnol. Biochem., 1993, 636-637.
[16] H. Kikuchi, N. Takahashi, and Y. Oshima, Tetrahedron Lett., 2004, 367-370.
[17] J. Y. Su, Y. Zhu, L. M. Zeng, and X. H. Xu, J. Nat. Prod., 1997, 1043-1044.
[18] Y. Wei, I Deb, and N. J. Yoshikai, Am. Chem. Soc., 2012, 9098-9101.
[19] C. Chowdhury, B. Das, S. Mukherjee, and B. Achari, J. Org. Chem., 2012, 5108-5119.
[20] X. Xia, N. Wang, L. Zhang, X. Song, X. Liu, and Y. Liang, J. Org. Chem., 2012, 9163-9170.
[21] T. Nanjo, C. Tsukano, and Y. Takemoto, Org. Lett., 2012, 4270-4273.
[22] D. Shan, Y. Gao, and Y. Jia, Angew. Chem. Int. Ed., 2013, 4902-4905.
[23] R. Nallagonda, M. Rehan, and P. Ghorai., Org. Lett., 2014, 4786-4789.
[24] T. Guo, Q. Jiang, Z. Yu, Org. Chem. Front., 2015, 1361-1365.
[25] C. Barberis, T. D. Gordon, C. Thomas, X. Zhang, K. P. Cusack, Tetrahedron Lett., 2005, 46, 8877-8880.
[26] L. Zhou, Y. Shi, Q. Xiao, Y. Liu, F. Ye, Y. Zhang, and J. Wang, Org. Lett., 2011, 13, 968-971.
[27] A. Sagadevan, A. Ragupathi, and K. C. Hwang, Angew. Chem. Int., Ed. 2015, 13896-13901.
[28] T. Li, B. Cheng, Y. Wang, M. Zhang, L. Lu, W. Xiao, Angew. Chem. Int. Ed., 2016, 12422-12426.
[29] A. Akao, K. Sato, N. Nonoyama, T. Mase, N. Yasuda, Tetrahedron Lett., 2006, 969-972.
[30] D. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess, K. Fagnou, J. Am. Chem. Soc., 2008, 16474-16475.
[31] D. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc., 2010, 18326-18339.
[32] Z. H. Guan, Z. Y. Yan, Z. Ren, X. Y. Liu, Y. M. Liang, Chem. Commun., 2010, 46, 2823-2825.
[33] L. Ackemann, A. V. Lygin, Org. Lett., 2012, 764-767.
[34] M. Murase, S. Yoshida, T. Hosaka, S. Tobinaga, Chem. Pharm. Bull., 1991, 489-492.
[35] J. Barluenga, R. Sanz, A. Granados, F. J. Fananas, J. Am. Chem. Soc., 1998, 4865-4866.
[36] A. Kessler, C. M. Coleman, P. Charoenying, D. F. O’Shea, J. Org. Chem., 2004, 7836-7846.
[37] S. L. Cui, J. Wang, Y. G. Wang, J. Am. Chem. Soc., 2008, 13526-13527.
[38] A. H. Stoll, P. Knochel, Org. Lett., 2008, 113-116.
[39] W. Yu, Y. Du, K. Zhao, Org. Lett., 2009, 2417-2420.
[41] F. V. Singh, W. Wirth, Synthesis., 2012, 1171-1177
[42] F. V. Singh, J. Rehbein, W. Wirth, ChemistryOpen., 2012, 245-250
[43] F. V. Singh, T. Wirth, Comprehensive Organic Synthesis II., (Ed: Knochel, P and Molander, G), Elsevier, 2014, 880-933
[44] F. V. Singh, W. Wirth, Synthesis., 2013, 2499-2511
[45] F. V. Singh, W. Wirth, Chem. Asian J., 2014, 950-971.
[46] D. McAusland, S. Seo, D. G. Pintori, J Finlayson, M. F. Greeney, Org. Lett., 2011, 3667-3669.
[47] M. Inman, C. J. Moody, Chem. Commun., 2011, 788-790.
[48] M. Inman, A. Carbone, C. J. Moody, J. Org. Chem., 2012, 1217-1232.
[49] S. Gore, S. Baskaran, B. Konig, Org. Lett., 2012, 4568-4575.



