Review Article - Der Pharma Chemica ( 2022) Volume 14, Issue 6
Plasma Transfusion Process for Covid-19 as an Alternative Treatment
Mohammad Asaduzzaman Chowdhury1, Md. Ruhul Amin1, Mohammod Abul Kashem2, Md. Abdus Shahid3 and Nayem Hossain4*2Department of Computer Science and Engineering, Dhaka University of Engineering and Technology (DUET), Gazipur-1707, Bangladesh
3Department of Textile Engineering, Dhaka University of Engineering and Technology (DUET), Gazipur-1707, Bangladesh
4Department of Mechanical Engineering, IUBAT-International University of Business Agriculture and Technology, Bangladesh
Nayem Hossain, Department of Mechanical Engineering, IUBAT-International University of Business Agriculture and Technology, Bangladesh, Email: nayem.hossain@iubat.edu
Received: 19-May-2022, Manuscript No. dpc-22-64345; Editor assigned: 23-May-2022, Pre QC No. dpc-22-64345; Reviewed: 06-Jun-2022, QC No. dpc-22-64345; Revised: 09-Jun-2022, Manuscript No. dpc-22-64345; Published: 16-Jun-2022, DOI: 10.4172/0975-413X.14.6.25-31
Abstract
Plasma is the fluid in the body's blood. It contains proteins and other substances that are important for overall health. Plasma transfers are used for patients with liver failure, serious infections, and severe burns. The blood contains proteins called antibodies that can be developed by the immune system of recovered COVID-19 patients to fight the SARS-CoV-2 virus. The application of convalescent plasma was successful in the Spanish flu outbreaks in 1918. Subsequently, the success of applying convalescent plasma has also been found during the SARS epidemic of 2003, the influenza pandemic of 2009, and the latest Ebola epidemic in 2014. Currently, convalescent plasma therapy is in use for the COVID-19 epidemic. The hundred-year-old convalescent plasma therapy has been reported to reduce the mortality rate of coronavirus infections in Iran by 40%. This article can be a source of useful information about plasma therapy being used successfully for the treatment of COVID-19 infected patients worldwide.
Keywords
Plasma transfusion; COVID-19; Recent observations; Clinical trials; Challenges
INTRODUCTION
Convalescent plasma is not a new concept, even though it has been considered an effective therapy against COVID-19 [1]. Donation of plasma or serum to patients from recovered patients, who have developed antibodies to a particular virus or bacterium, leads to the infected patient showing an enhanced resistance in their adaptive immune system, imparting acquired immunity [2]. The practice has been in use since the 1800s [3]. Classic adaptive immunotherapy convalescent plasma therapy has been applied to prevent and treat infectious diseases for more than a century [4]. During the Spanish influenza epidemic of 1918, it was used as a potential therapy with mixed results. Over the past two decades, classic therapy has been used successfully in the treatment of the SARS, MERS, and 2009 H1N1 epidemics with satisfactory efficacy and protection [5-8]. Convalescent plasma might provide short-term humoral insusceptibility against the SARS-CoV-2 coronavirus [9]. Most patients who recover from COVID-19 develop antibodies against various SARS-CoV-2 proteins 2–3 weeks after infection, which is detectable by ELISA, or other quantitative tests. Exchange of plasma from patients with antibodies various SARS-CoV-2 proteins would neutralize infection, stem replication, and limit the progression of tissue harm. This approach is anticipated to work best in patients with less extreme contamination, before the infection course, or prophylactically, in exceedingly vulnerable people such as unprotected healthcare workers or family caregivers of COVID-19 patients [10]. Convalescent plasma treatments appear to have a potential restorative effect in the treatment of severe COVID-19 cases. A single dosage of convalescent plasma with a high concentration of neutralizing antibodies can quickly diminish the viral stack and gradually produce clinical results.
Plasma Transfusion Process
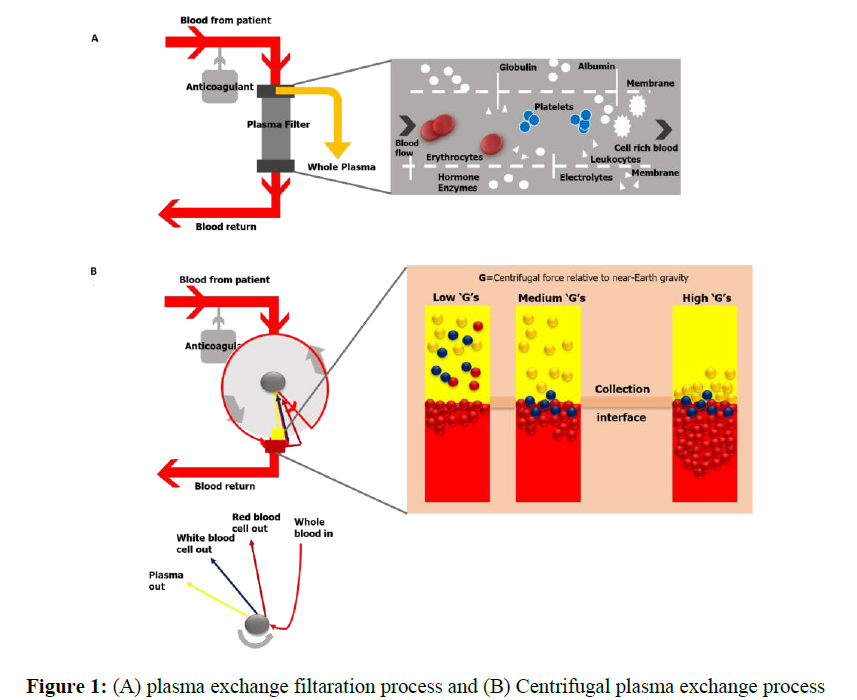
Therapeutic plasma exchange (TPE) is a technique that separates a large amount of the patient's plasma from the cellular components of blood and replaces it with appropriate fluid instead [11]. TPE can be performed in several ways, manually or using mechanized frameworks. In manual TPE, blood is extracted in repeated cycles and centrifuged to separate the blood cells. The supernatant plasma is disposed of at that point, and the leftover portion of the blood is returned to the patient along with a suitable substitution fluid. TPE, which utilizes robotized gadgets, can be categorized into two types: centrifugal TPE (cTPE) and membrane filtration TPE (mTPE). During cTPE, entire blood is extracted through the same access points and centrifuged to separate the plasma from cellular components. The supernatant plasma is then expelled, and a substitution liquid is blended with the remaining blood and returned to the patient to anticipate hypovolemia [12]. During mTPE, the patient’s blood is pumped through a parallel-plate or hollow-fiber filter. The pores of the filter membranes are of an adequate size to permit the passing of a part of the blood plasma, but not the cellular components. Plasma evacuation is shown in Figure 1. The concept is also discussed in previous study [11] (Figure 1). Figure 1:
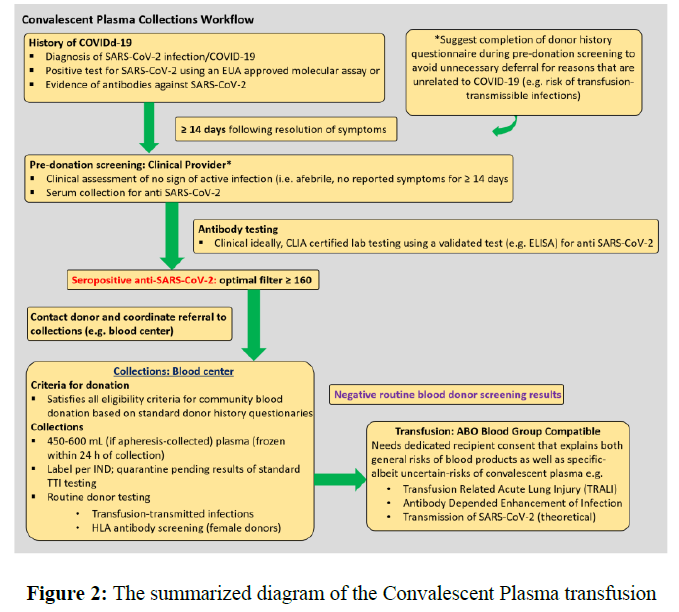
Following the standardization of treatment in accordance with China's experience and the rest of the world, the summarized therapeutic schedule of the convalescent plasma is as follows (Figure 2). The explanation of the idea is also available in literature [13].
Mechanism of Plasma Transfusion Process to deactivate COVID-19
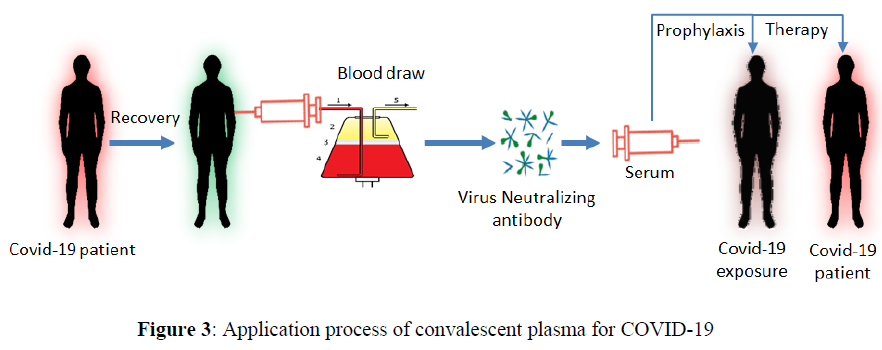
COVID-19 convalescent plasma is usually collected from patients who have recovered from COVID-19 if they are qualified to donate blood and have undergo required testing. They need to have demonstrated COVID-19 illness and appear to be fully recovered. COVID-19 survivors must start a broad titer of antibodies after approximately 28 days. Hence, donors are not chosen unless they have a recorded COVID-19 infection within 28 days [14]. To be eligible for donation, there ought to be confirmation of COVID-19 infection by a laboratory test with the total disappearance of symptoms for at least 14 days before donation. Female donors must be tested negative for HLA antibodies. Donors should be tested negative for COVID-19 by nasopharyngeal swab or molecular diagnostic test of blood. Donors develop characteristic SARS COV-2 neutralizing antibody titers from a titer more prominent than 1/320. Eligible patients must have serious life-threatening infections with laboratory-confirmed COVID-19. One of the ways to avoid complications related to plasma transfusion is to use filter antibodies obtained from the serum of patients who have recently recovered from COVID-19 (Figure 3). The plasma transfusion process is studied recently for COVID-19 treartment as alternative solution [4].
The antibodies present in convalescent plasma provide their restorative impact through the assortment of components. The antibody can bind to a given pathogen (e.g., infection), in this manner, specifically neutralizing its infectiousness; however, other antibodies, with interceded pathways like complement activation, phagocytosis may contribute to its restorative effect, and antibody-dependent cellular cytotoxicity. Non-neutralizing antibodies bind to the pathogen but do not meddle with its capacity to replicate in vitro frameworks; however, they contribute to prophylaxis and upgrade recuperation [15, 16]. Critically inactive antibody administration offers something as it is a short-term method to bestow quick immunity to susceptible people. Usually, the case is within the setting of a novel, rising infection such as SARS-CoV-2/COVID-19.
Recent Observations from COVID-19 Treatment
Coronavirus infection (COVID-19), which was first recorded in December 2019, presents a worldwide challenge, especially with the growth of elderly patients with pneumonia and in the absence of authoritative treatment [17,18]. The development of a new vaccine or drug is a time-consuming process, and administering common drugs/medications can serve as an effective alternative to combat the disease [19]. The treatment strategy targeting TLR5 may be an essential means of treating COVID-19 [20]. During the current pandemic, it has been reported that the number of deaths due to coronavirus in Iran have decreased by 40% using plasma therapy [21]. Convalescent plasma has been used to treat COVID-19 patients in China [22]. A series of five critically ill patients in China on mechanical ventilation, reported improved oxygenation, reduced viral load, clinical stability and improved clinical status when convalescent plasma transfusion was used [23].
In an experimental study of ten patients with severe COVID-19, convalescent plasma was collected with neutralizing antibody titers at or exceeding a 1:640 dilution [24]. All ten patients showed improvement in symptoms (e.g., cough, fever, chest pain, and shortness of breath) within 1-3 days of plasma transfusion. They also confirmed radiological improvement in pulmonary lesions. Another study found that plasma therapy was well tolerated and could progress clinical outcomes by neutralizing the virus in severe COVID-19 cases [25]. A study was performed on some critically ill patients with SARS-CoV-2 infection who received convalescent plasma and supportive care. It was found that all patients eventually recuperated from the SARS-CoV-2 virus. No serious adverse reactions related to convalescent plasma infection have been reported [26].
The first pilot plasma treatment against COVID-19 was tested at Houston Methodist Hospital, USA. It later received FDA approval for plasma transfusion for critically ill patients [27]. According to the FDA, doctors need to treat COVID-19 patients with hydroxychloroquine, a drug that is regularly utilized to treat lupus and malaria; it has been shown in limited cases to speed up recuperation. However, hydroxychloroquine has bigger safety concerns than convalescent plasma. Plasma treatment is analogous to a blood transfusion and carries similar risks, such as acute lung damage caused by liquid overload or allergic reactions [28].
Texas doctors have been utilizing plasma from recuperated COVID-19 patients to treat others. However, they are not sure whether it will be effective. In the absence of a vaccine, doctors and researchers are looking to so-called convalescent plasma since they consider it low risk and as it has been an effective treatment amid past epidemics [28]. The experimental trial will utilize plasma donations from the University of Chicago patients who had tested positive for COVID-19 and have since recuperated. Other persons who have recuperated from the infection are also recruited [29]. While supporting data protection and the potential efficacy of convalescent plasma, randomized experiments are needed [30]. Also, high-dose intravenous immunoglobulin (IVIG) has been recommended as a possible therapy for COVID-19 [31]. The detailed dosages and classification of convalescent plasma (concerning antibody titers) are exceedingly variable (Table 1).
| Disease | Location | Dose of CP | Titer | Summary finding | Reference |
|---|---|---|---|---|---|
| >COVID-19 | Shenzhen, China | Two consecutive transfusions of 200-250 mL (400mL total) | • ELISA Anti-SARS-CoV-2–antibody titer >1:1000 • Neutralizing antibody titer 1 >40 |
• Uncontrolled case series of 5 critically ill patients • Administration of CP 10-22d post-admission • All had steroids and anti-virals • Improvement in clinical status of all patients |
[23] |
| COVID-19 | Wuhan, China | 200 mL | • Neutralizing Anti-SARS-CoV-2–antibody titer >1:640 | • Uncontrolled case series of 10 severely ill patients • Other therapies included steroids, antimicrobials, anti-virals • Median onset of symptoms to CP in 16.5 days (IQR11.0–19.3 days) • Improvement in clinical status of all patients • No significant adverse effect |
[24] |
There is no direct immunization or any particular anti-viral medication/vaccine available for the treatment of critically ill patients. Most healthcare centers provide only supportive care such as ventilation, oxygenation, and fluid management [32]. Combined treatment with low-dose corticosteroids and anti-viral inhalation of targeted treatment has served as the treatment regimens for critical COVID-19 cases. Other detailed, helpful protocols that are used for the treatment of seriously sick patients are presented in Table 2.
| Classes | Potential treatment options | Reference |
|---|---|---|
| >Anti-viral | > 85% of patients received anti-viral specialists. Remdesivir is right now under trials at more than ten medical institutions in Wuhan and has been known to prevent MERS-CoV. | [33] |
| Anti-malarial | An old anti-malarial, chloroquine phosphate, has been effective in repressing the exacerbation of pneumonia due to its anti-viral and anti-inflammatory actions | [34] |
| Herbal treatments | There was widespread use of Conventional Chinese Medicationamid the last SARS-COV outbreak, and it is right now being used in China. The most commonly utilized five herbs were Astragali Radix (Huangqi), Glycyrrhizae Radix Et Rhizoma (Gancao), Saposhnikoviae Radix (Fangfeng), Atractylodis Macrocephalae Rhizoma (Baizhu), and Lonicerae Japonicae Flo. | [35] |
Circadian rhythms are self-sustaining, endogenous oscillation, exhibiting periodicities of about one day or 24 hours. Normally, circadian rhythms are synchronized according to the bodys pacemaker clock, located in the suprachiasmic nucleus of the hypothalamus.
The physiology and biochemistry of human being is not constant during the 24 hours, but variable in a predictable manner as defined by the timing of the peak and through of each of the bodys circadian processes and functions. The peak in the rhythms of basal gastric and secretion, white blood cells (WBC), lymphocytes, prolactin, melatonin, eosinophils, adrenal corticotrophic hormone (ACTH), follicle stimulating hormone (FSH), and leuteinizing hormone (LH), is manifested at specific times during the nocturnal sleep span [32-41]. The peak in serum cortisol, aldosterone, testosterone plus platelet adhesiveness and blood viscosity follows later during the initial hours of diurnal activity.
Clinical Data: Case Study [24]
The standard characteristics of the patients did not show any significant difference between the convalescent plasma treatment group and the control group, while the clinical results of these two groups were different. The general characteristics of patients in the trial of different ages, sex, principal symptoms, and comorbidity are listed in Table 3. Details of the additional treatments are given to the ten patients who received convalescent plasma transfusions are shown in Table 4. Table 5 shows the comparison of laboratory parameters before and after convalescent plasma transfusion.
| No. | Sex | Age | Clinical classification |
Days of Admission from symptom onset |
Days of convalescent plasma therapy from symptom onset |
Clustering Infection |
Principal symptoms | Comorbidity |
|---|---|---|---|---|---|---|---|---|
| >1 | M | 46 | Severe | 8 | 11 | No | Fever, cough, sputum production, shortness of breath, chest pain |
Hypertension |
| 2 | F | 34 | Severe | 0 | 11 | Yes | Cough, shortness of breath, chest pain, nausea and vomiting |
None |
| 3 | M | 42 | Severe | 8 | 19 | Yes | Fever, cough, sputum production, shortness of breath, sore throat, diarrhea |
Hypertension |
| 4 | F | 55 | Severe | 10 | 19 | No | Fever, cough, sputum production, shortness of breath |
None |
| 5 | M | 57 | Severe | 4 | 14 | No | Fever, shortness of breath |
None |
| 6 | F | 78 | Severe | 8 | 17 | Yes | Fever, cough, sputum production, shortness of breath, muscle ache |
None |
| 7 | M | 56 | Severe | 4 | 16 | No | Fever, cough, sputum production, arthralgia |
None |
| 8 | M | 67 | Severe | 10 | 20 | No | Fever, cough, headache, diarrhea, vomiting |
Cardiovascular and cerebrovasculardiseases |
| 9 | F | 49 | Severe | 1 | 10 | No | Cough, shortness of breath |
None |
| 10 | M | 50 | Severe | 3 | 20 | No | Shortness of breath | Hypertension |
| M=male. F=female | ||||||||
| No. | Drugs administered | Oxygen support | |||
|---|---|---|---|---|---|
| Anti-viral treatment | Antibiotic or antifungal treatment |
Corticosteroid treatment |
Before convalescent plasma therapy | After convalescent plasma therapy | |
| >1 | Arbidol0.2g q8h po. Ribavirin 0.5g qdi.v. |
Cefoperazone Sodium i.v. | None | High-flow nasal cannula, Mechanical ventilation |
Mechanical ventilation |
| 2 | Arbidol0.2g q8hpo. | Cefoperazone Sodium i.v. | None | None | None |
| 3 | Arbidol0.2g q8hpo. | Moxifloxacini.v. | Methylprednisolone i.v. | High-flow nasal cannula, Mechanical ventilation |
High-flow nasal cannula |
| 4 | Ribavirin 0.5g qdi.v. | Linezolid i.v.Imipenem -Sitastatin Sodium i.v. | Methylprednisolone i.v. | Mechanical ventilation | High-flow nasal cannula |
| 5 | Arbidol0.2g q8hpo.
Remdesivir0.2gqdi.v.
Interferon-ɑ 500MIUqdinh. |
Moxifloxacini.v.Cefoperazone Sodium and Tazobactam Sodium i.v. |
Methylprednisolone i.v. | Low-flow nasal cannula | Low-flow nasal cannula |
| 6 | Arbidol0.2g q8h po. | Cefoperazone Sodium i.v. Levofloxacin i.v. |
Methylprednisolone i.v. | High-flow nasal cannula | High-flow nasal cannula |
| 7 | Arbidol0.2g q8h po. | Cefoperazone Sodium and Tazobactam Sodium i.v. Fluconazole i.v. |
Methylprednisolone i.v. | High-flow nasal cannula | None |
| 8 | Arbidol0.2g q8h po. Ribavirin 0.5g qdi.v. |
None | None | None | None |
| 9 | Arbidol0.2g q8h po. Oseltamivir75mg q12h po. Peramivir0.3gqdi.v. |
None | None | Low-flow nasal cannula | Low-flow nasal cannula |
| 10 | Arbidol0.2g q8h po.
Interferon-ɑ 500MIU qdinh. |
Cefoperazone Sodium i.v.Caspofungini.v. |
Methylprednisolone i.v. | High-flow nasal cannula | High-flow nasal cannula |
| po.=peros. i.v.=intravenous injection. inh.=inhalation. | |||||
| Clinical Factors | Before CP transfusion | After CP transfusion |
|---|---|---|
| >C-reactive protein (mg/L, normal range 0-6) | 55.98 (15.57-66.67) |
18.13 (10.92-71.44) |
| Lymphocyte (109 per L, normal range 1.1-3.2) | 0.65 (0.53-0.90) |
0.76 (0.52-1.43) |
| Alanine aminotransferase (U/L, normal range 9-50) | 42.00 (28.25-61.85) |
34.30 (25.75-53.90) |
| Aspartate aminotransferase (U/L, normal range 15-40) | 38.10 (28.50-44.00) |
30.30 (17.30-38.10) |
| Total bilirubin (μmol/L, normal range 0-26) | 12.40 (11.71-22.05) |
13.98 (12.20-20.80) |
| SaO2 (%, normal range ≥95) | 93.00 (89.00-96.50) |
96.00 (95.00-96.50) |
| SaO2= oxyhemoglobin saturation.CP=convalescent plasma | ||
Potential Challenges Facing Plasma Transfusions
The risks of transfer recipients may not be different from that of standard plasma. There are also non-infectious risks of transfusion, such as allergic transfusion responses, transfusion-related circulatory over-burden (TACO), and transfusion-related acute injury [36]. It should be noted that treatment with human immunoglobulin was related to an expanded same-day thrombotic occasion hazard (0.04 to 14.9%) [37]. other issues to be considered include the introduction of high-quality screening and the requirement for proper selection of donors having tall neutralizing antibody titers [38]. Moreover, it is also important to ensure the production and use of convalescent plasma following specific ethics and regulated conditions for the potential role of these products in human origin [39].To conclude, it is not known to what extent the convalescent plasma treatment can accelerate the growth of a natural immune response, especially when administered for prophylaxis.
Some Summarized Studies
Date collected from January 1, 2020 – January 16, 2021 emphasized [40] that corona effected patients treated by plasma transfusion process has small level of mortality rate in comparison to the other available treatments. Moreover, the transfusion applied initially within 3 days of hospitalization indicated the upper level of titer plasma causes little patient mortality. These data can be used as sources for confirming the efficacy of person’s convalescent plasma as a medicinal element for patients who were admitted in clinic.
14 corona affected patients of average age 65 years among them seven are man and seven are woman who are immunosuppressed at the initial stage of viral infection and no inspectionable anti-viral IgG are influential factors for recovering by the plasma transfusion, and the IgG titre after this process can be used as an effective predicting variables for recovery from this disease [41]. The patients who have no opportunity to take mechanical ventilation, The IgG antibody levels in relation to plasma transfusion was engaged a smaller level of risk of death in comparison of less antibody levels [42].
Some immunomodulatory strategies [43] can used to mitigate the risks of corona viral patients during use of plasma transfusion [44]. Beside meditations, optimized, randomed and controllable plasma related clinical trials are important more for treating the corona effected people alternatively.
CONCLUSIONS
This review of convalescent plasma treatment has a potential therapeutic impact on the treatment of severe COVID-19 patients. The use of convalescent plasma is an interim approach, such as immunization, until any successful and effective medication or treatment is developed. Even though the concept is simple and various steps are involved, each step requires collaboration among different organizations, including benefactors, blood centers, or other plasma collection centers, to treat healthcare staff, doctors, and patients as well as ensuring the safety of healthcare administrators and controllers. Other caveats are that there are no large long-term trials that have demonstrated consistent benefits of using plasma. However, this article provides information about plasma transfusion for the treatment of SARS-CoV-2 infections.
REFERENCES
- Carter CE, Benador NM. Pediatr Nephrol. 2014, 29: p. 35-50.
- Evan M. Bloch, Shmuel Shoham, et al., J Clin Invest. 2014, 130(6): p. 2757-2765.
- Luke TC, Kilbane EM, Jackson JL, et al., Ann Intern Med. 2006, 145(8): p. 599-609.
- Casadevall A, Pirofski LA. J Clin Invest. 2020, 130(4): p. 1545-1548.
- Cheng Y. Eur J Clin Microbiol Infect Dis. 2005, 24: p. 44-46.
- Zhou B, Zhong N, Guan Y. N Engl J Med. 2007, 357: p. 1450-1451.
- Hung IF. Clin Infect Dis. 2011, 52: p. 447-456.
- Ko JH. Antivir Ther. 2018, 23: p. 617- 622.
- Yeh KM, Chiueh TS, Siu LK, et al., J Antimicrob Chemother. 2005, 56(5): p. 919-22.
- Yuen, Kit-San. Cell & bioscience. 2020, 10: p. 40.
- Michiel Etienne Janssens, Stuart Wakelin. Eur Oncol Haematol. 2018, 14(2): p. 105-109
- Pusey CD, Levy JB. Blood Purif. 2012, 33: p. 190-198.
- Evan M. Bloch EM, Shoham S, et al. J Clin Invest. 2020, 130(6): p. 2757-2765.
- FDA. Emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/donate-COVID-19-plasma
- van Erp EA, Luytjes W, Ferwerda G, et al., Front Immunol. 2019, 10: p. 548.
- BM, Yu WH, Karim MM, et al., Cell Host Microbe. 2018, 24(2): p. 221-33.
- Chakraborty C, Sharma AR, Bhattacharya M, et al. Asian Pac J Trop Med. 2020, 13(6): p. 242-246
- Chakraborty C, Sharma A, Sharma G, et al., Eur Rev Med Pharmaco. 2020, 24: p. 4016e4026.
- Saha A, Sharma AR, Bhattacharya M, et al., Arch Med Res. 2020, 51(6): p. 585-586.
- Chakraborty C, Sharma AR, Bhattacharya M, et al., J Med Virol. 2020, 25997.
- Plasma therapy reduces coronavirus deaths in Iran by 40%.
- Xinhua. China puts 245 COVID-19 patients on convalescent plasma therapy.
- Shen C, Wang Z, Zhao F, et al., JAMA. 2020.
- Duan K, Liu B, Li C, et al. medRxiv. 2020, 20036145.
- Kai D, Bende L, Li C, Zhang H, et al. Proceedings of the National Academy of Sciences. 2020, 117 (17): p. 9490-9496.
- Zhang B, Liu S, Tan T, et al., Chest. 2020, 158(1): p. e9-e13.
- Fda Approves First Plasma Therapy for Houston Methodist Covid-19 Patient
- Texas doctors are using plasma from recovered COVID-19 patients to treat others. They’re not yet sure if it will work.
- University of Chicago Researchers to Study Plasma Transfusions for COVID-19 Patients.
- Chen L, Xiong J, Bao L, et al., Lancet Infect Dis. 2020, 211(1): p. 80-90.
- Cao W, Liu X, Bai T, et al. Open Forum Infect Dis. 2020, 7(3).
- Chen Z, Hu J, Zhang Z, et al., medRxiv. 2020, 20040758.
- Lai CC, Shi TP, Ko WC, et al., Int J Antimicrob Agents. 2020, 55(3): p. 105924.
- Gao J, Tian Z, Yang X. Biosci Trends. 2020, 14(1): p. 72-73.
- Luo H, Tang Q-L, Shang Y-X, et al., Chin J Integr Med. 2020, p. 32065348.
- Hendrickson JE, Hillyer CD. Anesth Analg. 2009, 108(3): p. 759-69.
- Menis M, Sridhar G, Selvam N, et al., Am J Hematol. 2013, 88: p. 1035-1040.
- Kai Duan, Bende Liu, Cesheng Li et al., PNAS. 2020, 117(17): p. 9490-9496.
- Marano G, Vaglio S, Pupella S, et al., Blood Transfus. 2016, 14: p. 152-157.
- Klassen Stephen A, Jonathon W Senefeld, Patrick W Johnson, et al. Mayo Clin Proc. 2021, 96(5): p. 1262-1275.
- Rodionov Roman N, Anne Biener, Peter Spieth, et al. The Lancet Microbe. 2021, 2(4): p. e138.
- Joyner Michael J, Rickey E Carter, Jonathon W Senefeld, et al., N Engl J Med. 2021, 384(11): p. 1015-1027.
- Deokar Kunal. J Anaesthesiol Clin Pharmacol. 2020, 36(3): p. 419-423.
- Valeria Selvi. BioMed Research International. 2020, p.1-8.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref