Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Physicochemical Properties of Chemurgic-Fenugreek (Trigonella foenumgraecum) Herb of Different Origin
Ahmad Dilshad
Abstract
Fenugreek is a well known herb for its medicinal and cosmetic qualities such as hypocholesterlaenic, antibiotic and antifertility activities. The purpose of the work was to study the influence of environmental conditions on physico chemical properties of herb grown in four different geographic locations. The values of moisture, oil and total phenolic contents were recorded higher in fenugreek seed grown in Yemen as 5.47 ± 0.66, 7.04 ± 0.21 and 18.52 ± 1.80 respectively, where as ash content was higher in Egyptian seed with sodium, iron and copper content. Calcium and magnesium were the most abundant mineral in all the samples followed by potassium and sodium. The polunsturated linoleic and linoleic fatty acid were the major fatty acids. The ratio of C18:2/ C18:3 was lowest (0.957) in Egypt followed by Yemen, India and Saudia. The amino acid composition revealed the presence of most essentail amino acids in Yeman seeds. These wide variations in compositions indicated the effects of environmental, cultiavars regions and and biotic factors.
Keywords
Fenugreek herb, Environmental variation, Proximate analysis, Oil content, Amino acid content
Introduction
Some seeds and leaves of herbs and shrubs are primarily known for their medicinal and cosmetic use and are present any time at home to use such as Tulsi (Ocimum sanctum), Aloe Vera (Aloe vera), Thyme (Thymus vulgaris), Rosemary (Rosmarinus officinalis), Lavender (Lavandula), Peppermint (Mentha × piperita), Ashwangandha (Withania somnifera), Bryophyllum (Bryophyllum pinnatum), mustard (Sinapis alba L.), black cumin (Nigella sativa L.) and fenugreek (Trigonella foenum-gracecum) which belongs to family Fabaceae. Fenugreek is native of Mediterranean region, southern Europe, western Asia and an area extending from Iran to parts of northern India. But nowadays widely cultivated in China, north and east Africa, Ukraine, and Greece [1] Fenugreek itself produces many effects like antidiabetic, antifertility, anticancer, antimicrobial, antiparasitic, and hypocholesterolaemic effects [2]. Its seeds are used in cooking to add aroma and flavor to food. Leaves of fenugreek are cooked in India as a vegetable, as it is the most important source of edible fatty acid but its chemical composition and contents may vary under different environmental conditions, Specially Fenugreek has been a source of medicinal treatment for thousands of years in most of the developing countries as found very easily. It has been reported that fenugreek seeds helps to lowers the blood glucose, which means that fenugreek seeds possesses hypoglycemic effect in diabetic patients [3,4]. It is supposed that hypoglycemic properties are possibly due to free amino acid of 4-hydroxyisoleucine in alloxan diabetic induced rats and diabetic patients [5,6]. Fenugreek is a well studied for its phytoestrogen properties, useful for patient of low estrogen level. It is used traditionally for physiological purpose such as hypocholesteremic, antiulcer, hypoglycemic, antoxidant agent along with neutraceutical purpose. The seeds of fenugreek contain 26% mucilage, 22% protein comprising of globulin, histidine, lecithin and albumin with a good amount of phosphorus, sulphur. It contains also handsome amount of minerals and soluble/insoluble fiber for good health [7]. The fenugreek seeds contain 7.1% oil by percentage with unacceptable and bitter taste. It mostly contains PUFA, as ω-3 and ω-6, fatty acids [8]. There are various researchers who analysed Egyptian origin fenugreek oil and reported as linoleic and linolenic acids, 33.7 and 13.8% respectively [9]. The presence of hexadecanoic acid was also reported by Badami and Sauvaire [10,11]. The effect of place and conditions of cultivation of plant on linoleic and linolenic acids is also an important factor and effects on composition. Two possible external factors namely climatic and edaphic play a role in chemical constituents in the seed. To deal with heavy demand of commercialized medicinal plants, soil quality (organic matter, depth and nutrient holding capacity) and pollutant represent a serious threat to quality of medicinal plant production [12]. It is reported that growth rate of fenugreek is seasonal and temperature-dependent [13]. Natural variations on physico Chemical properties have been studied limited. To carry froward, this work is aimed to determine some medicinal and nutraceutical properties including mineral and lipid composition of fenugreek herb from different environmental regions.
Material and Methods
Collection of sample
Fenugreek herb were obtain from Riyadh region of Saudi Arabia which was imported from India, Yemen, Egypt and Saudi Arabia region. Samples were randomly collected from 3 places of each region after confirmation of grown in differnr regions. The samples were sun dried before grinding to fine powder and stored in polythene before analysis.
Proximate analysis
Moisture, pH, ash, mineral content, crude fiber, protein, amino acids and In vitro digestibilty were determined by the methods reported by the Association of official Analytical Chemists [14]. Total phenolic were measured following the protocol developed by Kaur and Kapoor [15]. Briefly, 200 L of crude extract (1 mg/mL) were made up to 3 mL with distilled water, mixed thoroughly with 0.5 mL of Folin-Ciocalteu reagent for 3 min, followed by the addition of 2 mL of 20% (w/v) sodium carbonate. The absorbance was measured at 650 nm, after keeping sample for a one an hr in the dark. The total phenolic content was calculated from the calibration curve, and the results were expressed as mg of gallic acid equivalent per g dry weight.
Color analysis
Fenugreek dried herbs were analyzed using Hunter Lab colorimeter (Hunter Lab D65 Spectrocolorimeter Ultrascan PRO, Reston, VA) [16]. The results were expressed as Hunter Lab L (whiteness/darkness), a (red/green), and b (yellow/blue) values on the screen of colorimeter and recorded. Each reading gave three different coordinates L, a and b.
Fatty acid analysis
The fatty acids were determined by Gas Chromatography (GC) according to the procedure described by Metcalf et al. and AOAC [17,18]. Fatty Acids Methyl Esters (FAME) and Elements like calcium, iron, sodium, potassium, copper, zinc and magnesium were determined according to as described in our previous work [19].
Amino acids
To find the amino acids present in seeds taken from different conditions, well homogenized, vacuum dried and fat free samples were hydrolyzed in 6 N HCl under vacuum at 110ºC for 24 hr. The hydrolyzed samples were then injected into Shimadzu LC-10AD HPLC connected to Shimadzu RF-10A Spectrofluorometric detector.
In vitro digestibility
50 ml aqueous suspension of sample were adjusted to pH 8.0 with 0.1 HCl or 0.1 NaOH. After that slurry sample incubated at 37ºC for few minuets in a contolled water bath. Separately an enzyme mixture of 1.6 mg trypsin+3.1 mg α Chemotrpsin+1.3 mg peptidase/ml was maintained in ice bath an adjusted to pH 8.0 with the help of Hcl or NaOH. During shaking at 37ºC, 5 ml of enzyme was added to the suspension sample. The pH of the suspension after incubation for 10 min at the same temperature was recorded and the In Vitrodigestibility was calculated. According to the regression equation [20].
Y=210.464–18.103 X (Y=In Vitrodigestibility (%): X=pH of the sample suspension after 10 min. digestion with mixture of enzymes). Analysis of pH it was analysed to know the nature of acidity of the collected sample.
Statistical analysis all chemical determinations were calculated as means of triplicate ± Standard Deviation (SD). Data were subjected to statistically analysis using SAS, system using of analysis of variance [21]. Value of probability at 5% was used to indicate significance.
Result and Discussions
The proximate analyses results as obtained from Fenugreek seeds collected from four different origins or countries were summarized in Table 1. Moisture content ranged from 4.2 ± 0.09 -5.5 ± 0.06 gm per 100 gm sample recorded from different origin. The difference in percentage of moisture content taken from different sample vary very little. Although the moisture content varies as India <Egypt<Saudi Arabia<Yemen. The low percentage of moisture in Indian seeds shows that their shelf life is more compared to other Fenugreek seeds taken from other origins. This helps in less bacterial, fungal and contamination effects.
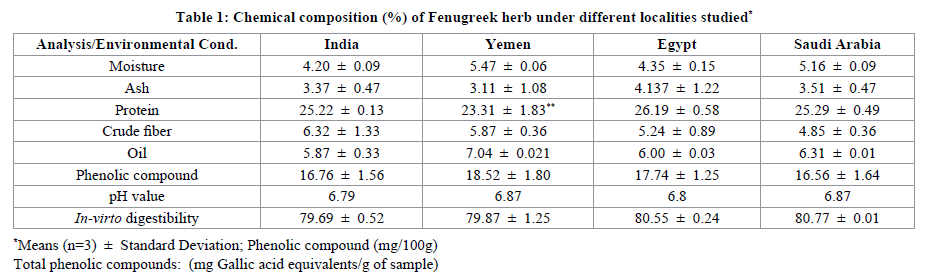
The ash content varies from 3.11 ± 6.4-4.14 ± 1.22, which is nearly same as reported for Fenugreek earlier [22]. The high percentage of protein present in Fenugreek seed (Table 1) among all other parameters taken under consideration showed that it help to lower plasma cholesterol and glucose levels in Type 2 diabetic patients [23]. Highest protein content was found from the seed taken from Egypt (26.19 ± 0.58) and least from Yemen (23.31 ± 1.83), which shows the Egyptian seeds are having high medicinal activity compared to other grown in different environmental condition or soil.
Another parameter i.e., crude fiber, which is also responsible for lowering plasma cholestrol and keeping digetion fit showed significantly different range, which is found least for Saudi Arabian Fenugreek seeds (4.85 ± 0.36) and highest for Indian Fenugreek seeds (6.32 ± 1.33). The percentage of protein and crude fiber found in our finding were similar and comparable as reported earlier [24]. Fenugreek seeds contain approximately 7.5% oil as also reported by early researchers [25]. Fenugreek oil has a golden-yellow colour, although oil has a unacceptable and a bad taste used as edible oil and very effective in treatment of various skin diseases like abscesses, boils, burns, eczema, and gout Oil content which varied as in descending order, 7.04 ± 0.021 (Yemen), 6.31 ± 0.01 (Saudi Arabia), 6.00 ± 0.03 (Egypt) and 5.87 ± 0.33 (India) (Table 1). Also the pH determined for the seeds taken into consideration is in the range of 6-7, which shown slight acidic character. Total phenolic compounds extracted with Methanol had high content in Seed grown in Yemen and less Saudi Arabia, which is an indication of higher radical scavenging activity.
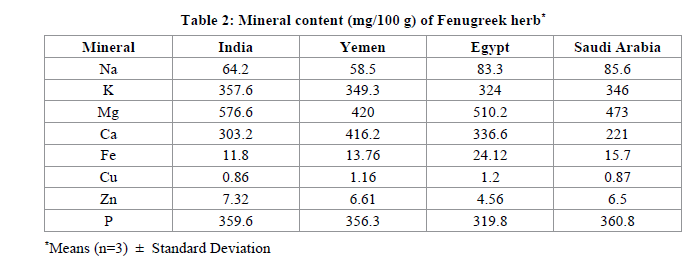
Minerals are very important constituents of the body. These helps to control various regulations in the body like heart related blood pressure, digetion, nousea, bronchtis and tension etc. Results of mineral conetens are presented in Table 2. It is intresting to note that magnesium level was highest ranges (420.0 -576.6) in all seeds followed by potassium (324.0-357.36), Calcium (221.0-416.2) and Sodium (64.2-85.6 mg/100 g). potassium in Fenugreek seeds were comparatively lower than the values reported earlier [26- 28], Whereas Mg, P and Ca are similar as declared by Slema [28]. Iron and copper levels were noted similar with Jasser and Ziwar [26,24], whereas metal Zinc was little higher (ranged 4.56-7.32) in all seeds by Selma and Pandey [28]. The variatins in amount could the factors of soil profiles of different regions.
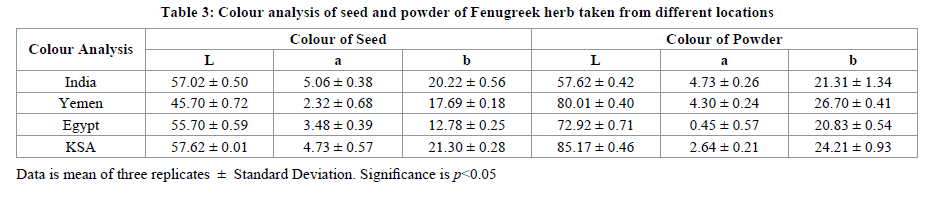
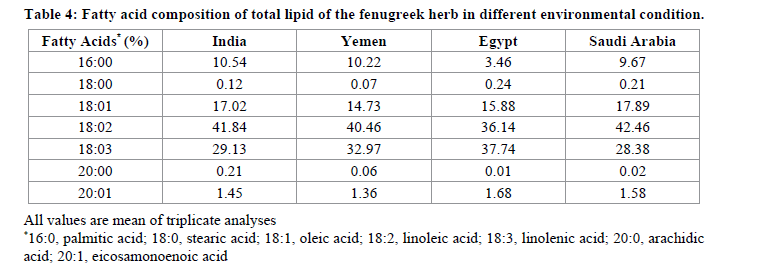
Colour is also an important quality parameter affecting commercially. Measurements of colour are performed to determine the influence of temperature on thermally processed products [29-32]. The results of color measurement of Fenugreek in terms of Hunter L, a, and b values, are presented in Table 3. Colour assessment of both seed and powder of Fenugreek herb was done by evaluating the values of L (whiteness/darkness), a (red/green), and b (yellow/blue) were investigated. Measuring seed colour helps in quality control in the international trade market. In seed, Hunter L values varied in the range of 45.70 to 57.62 among the cultivars. Higher L values indicate “whiteness” or “lighter” color; thus Saudi Arabia Fenugreek seeds have light colour, whereas Yemen seed have dark colour. The statistical analysis recorded showed a significant difference (≤ 0.05) in L values of seed. The Hunter colour Lvalues for Fenugreek seed powder showed a large difference (57.62 to 85.17) except Indian origin, which shows similar result of L in seed and powder form with difference in decimal place only. The Hunter color a values, of the seeds ranged from 2.32 to 5.06. The statistical analysis showed a significant (≤ 0.05) difference of “a” value in seeds. While Hunter values (a) of powder varied from 0.45 to 4.73. Out of all recorded data, none of the seeds or powder having Hunter values were below zero or in the negative range, which reflected the absence of any greenish shades in seed or powder. Also, Hunter b values varied in between 12.78 to 21.30 in seed and from 20.83 to 26.70 in powder form of Fenugreek. The Hunter b value of seed were observed to be high for Saudian Arabian seed and low for Egyptian seeds. Table 4 presents the fatty acid composition obtained. The major fatty acids present in each seeds obtained from different origin is linoleic acid who’s highest concentration was found in Saudi Arabia seeds (42.46%) followed by linolenic acid in all seeds. Hitditch and Willam [33] observed that atmospheric conditions is the principal factor for the varaions in linolenic acid. Oleic acid, arachidic acid and eicosamonoenoic acid were present in traces.
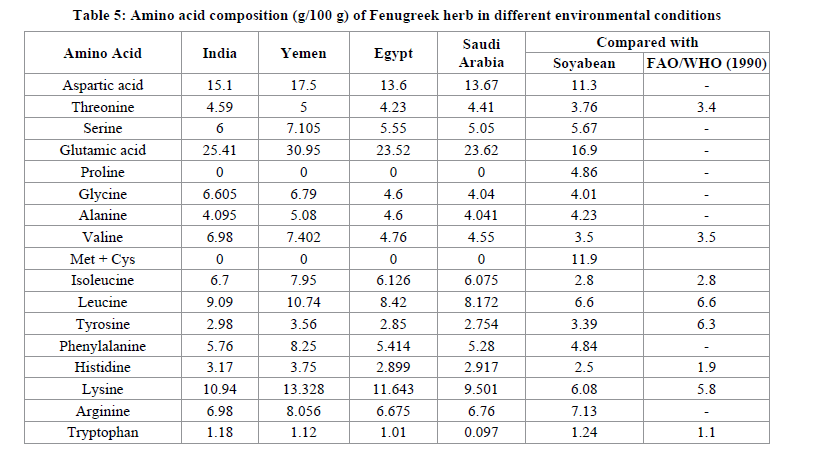
The results obtained were nearly same as obtained earlier by Sulieman et al. [34]. Essential amino acids, valine, isoleucine, leucine, lysine, methionine, phenylalanine, hreonine, tryptophan and histidine of all four cultivars along with all amino acids requirement pattern suggested by FAO/WHO are shown in Table 5 [35]. The amino acid profile of all sample contained adequate are compared with FAO/WHO and soya bean except for limiting amino acid cystine + methonine which is only present in soyabean [35,36]. In the studied seeds of Yemen Fenugreek, the percentage of essential amino acids like lysine, phenylalanine, leucine, isoleucine, valine and threonine are greater comparable to other found in different environmental conditions. So the Fenigreek herb of Yemen is more nutritive. While remaining non essential amino acids are also present in greater amount grown in Yemen region.
In vitrodigestibilty of fenugreek of four cultivars as an important functional properties (Table 1). Significant difference of invitro change was observed in Indian cultivars followed by Saudia Egypt, Yemani and Indian respectively, which is quite comaparable with reported [26]. Invitordigestibility properties is directly related to with profile of amino acids (Table 5). Crude Fiber (CU) and In vitro Digestibility (IVD) is more in Indian cultivars which is comparable with Pandey and Awasti [37] whereas low as described some where Shalini and Sudesh [22].
Conclusion
In conclusion, we observed that most of the variance were independent of the environmental conditions. The moisture content is in between 4.20-5.47 g/100 g sample, while ash varies from 3.11-4.14 taken from different origin. The protein content was high in seed taken from Egypt and least from Yemen. Crude fiber, is found minimum in Saudi Arabian fenugreek seeds and maximum for Indian Fenugreek seeds. The Oil content is in order are given as in descending order, Yemen>Saudi Arabia>Egypt>India. Minerals like magnesium, calcium, phosphorus and potassium are present at macro level in all varieties of herb taken. Fatty acids like linoleic acid was found at highest concentration in Saudi Arabian seeds followed by India, Yemen and Egypt. Whereas linolenic acid was high in Eygpt seeds and low in Indian seed. Yemen herb is more beneficial for body as all essential amino acids are present in it. Under the favorable condition, the corresponding predicted response values of L, a and b showed that Saudi Arabia Fenugreek seeds have light colour, whereas Yemen seed have dark colour, also Saudi Arabian powder form has lightest colour, while Indian at darkest side. Total phenolic compounds shows an indication as a source of antioxidantss along with medicial active components. Based on well propotioned amount obtained of essential fatty acids, essential amino acids and minerals can be justifed the therapeutic use of the herb and also can be expolited as for their beneficial nutraceutical properties.
References
[1] G.A. Petropoulos, 2002, UK, PP, 255.
[2] M. Al-Habori, A. Raman, Taylor & Francis, London, UK, 1st edn., 2002, 10, 163-182.
[3] J. Zafar, F. Health Benefits, Natural Health Products., 2008.
[4] R.D. Sharma, T.C. Raghuram, Eur. J. Clin. Nutr., 1990, 44(4), 301-306.
[5] Y. Sauvaire, P. Petit, C. Broca, M. Manteghetti, Y. Baissac, J. Fernandez-Alvarez, R. Gross, M. Roye, A. Leconte, R. Gomis,
G. Ribes, Diabetes., 1998, 47(2), 206-210.
[6] M.R. Haeri, M. Izaddoos, M.R. Ardekani, M.R. Nobar, K.N. White, Phytother, Res., 2009, 23(1), 61-64.
[7] C. Gopalan, B.V. Ramshastri, S.C. Balasubramanian, India, 1989.
[8] http://www.ormed.edu/newsletters/fenugreek.html
[9] M. Shahat, Appl. Chem., London, 1947, 569-575
[10] R.C. Badami, G.S. Kalburgi, Karnatak. Univ. J. Sci., 1969, 24, 16-19.
[11] Y. Sauvaire, G. Ribes, J.C. Baccou, M.M. Loubatieres-Mariani, Lipids., 1991, 26(3), 191-197.
[12] M.I. Qureshi, M. Israr, M.Z. Abdin, M. Iqbal, Environ. Exp. Botany., 2005, 53, 185-193.
[13] K. McCormick, R. Norton, H.A. Eagles, 2006, 639.
[14] A.O.A.C., 1995, (16th edn.), Washington, DC, USA.
[15] C. Kaur, H.C. Kapoor, Int. J. Food Sci. Technol., 2002, 37, 153-161.
[16] A.N.Yousif, T.D. Durance, C.H.Scaman, B. Girard, J. Food Sci., 2002, 65(6), 926-930.
[17] L.D. Metcalfe, A.A. Schuitz, J.R. Pelka, Anal. Chem., 1966, 38, 514-515.
[18] A.O.A.C., Official Method of Analysis (15th Edn.) 1990, Association of Official Analytical Chemists Washington, DC, USA.
[19] M.A. Salah, D. Ahmad, Food Chem., 2002, 437-441.
[20] H.W. Hsu, D.L. Vavak, L.D. Satterelee, G.A. Miller, J. Food. Sci., 1977, 42, 1269.
[21] R.G.D. Steel, J.H. Torrie, 2nd ed., 1980, New York: McGraw-Hill.
[22] H. Shalini, J. Sudesh, J. Food. Biochem., 2003, 27,165-176.
[23] Z. Mada and I. Shomer, J. Agric. Food. Chem., 1990, 38, 1535-1539.
[24] J.B. Ziwar, Tikrit. Univ., Iraq, 2010,15(3),15-20.
[25] L.A. El-Sebaiy, A.R. El-Mahdy, Food. Chem., 1983, 10(4), 309-319.
[26] F.M. Al-Jasass, M.S. Al-Jasser, The Scientific World Journal., 2012.
[27] A.R. El-Mahdy, L.A. El-Sebaiy, Food. Chem., 1982, 9(3), 149-158.
[28] S. Selma, U. Güngör, S. Guzel, A. Iilçim, G. Kökdil, Turk. J. Pharm. Sci., 2014, 11(3), 255-262.
[29] M. Aamir, M. Ovissipour, B. Rasco, T.J. Juming, S. Sablani, Int. J. Food. Prop., 2014, 17, 2012-2024.
[30] C.H. Chong, C.L. Law, F. Figiel, A. Wojdyło, M. Oziembłowski, Food Chem., 2013, 141, 3889-3896.
[31] H.S.F. Erge, Karadeniz, N. Koca, Y. Soyer, GIDA, 2008, 33(5), 225-233.
[32] M. Zielin´ska, P. Zapotoczny, O. Alves-Filho, T.M. Eikevik, W. Błaszczak, Dry. Technol., 2013, 31, 633-642.
[33] T.P. Hilditch, P.N. Willam, 4th edn., 614, London Champan and Hall., 1964, England.
[34] E. Sulieman, A.M. Ali, O.J. Hemavathy, Int. J. Food Sci. Technol., 2008, 43, 380-382.
[35] FAO/WHO, Nutrition Report Series, 1990, 724.
[36] I.M. Vasconcelos, E.A. Siebra, A.A.B. Maia, R.A. Moreira, A.F. Neto, G.J.A. Campelo, J.T.A. Oliveira, J. Sci. Food. Agri., 1997, 75, 419-426.
[37] H. Pandey, P. Awasti, J. Food. Sci. Technol., 2015, 52(2), 1054-1060.



