Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 10
Novel Pyirimidine Carboxamide Derivatives As Potential Anticancer Agents,snthesis And Characterisation
Shameem Sultana1* and Dr.shiva kumar22Gitam School of Pharmacy, GITAM Deemed to be University, Hyderabad, Telangana, India
Shameem Sultana, Gitam Institute of Pharmacy, GITAM Deemed to be University, Vishakapatnam, Andhra Pradesh, India, Email: shameem.syed82@gmail.com
Received: 25-Oct-2022, Manuscript No. dpc-22-78200; Editor assigned: 26-Oct-2022, Pre QC No. dpc-22-78200; Reviewed: 04-Nov-2022, QC No. dpc-22-78200; Revised: 05-Nov-2022, Manuscript No. dpc-22-78200; Published: 12-Nov-2022, DOI: 10.4172/0975-413X.14.10.34-42
Abstract
Background: Cancer was credited as the second leading cause to global death rate with estimated toll of deaths more than 18 million. Though many anticancer agents are available in the market, there is an essential need to explore novel drug candidates for cancer treatment due to the various adverse effects associated with the current anticancer agents. Pyrimidine scaffold is a ubiquitous heterocyclic system present in important anticancer agents. Current investigation aims to find the newer class of anticancer lead molecules with pyrimidine as the central pharmacophore. Objective: Primary objective of the current research is to evaluate the anti-cancer potential of the novel class of carboxamide functionality tethered pyrimidine derivatives against six different cancer cell lines.
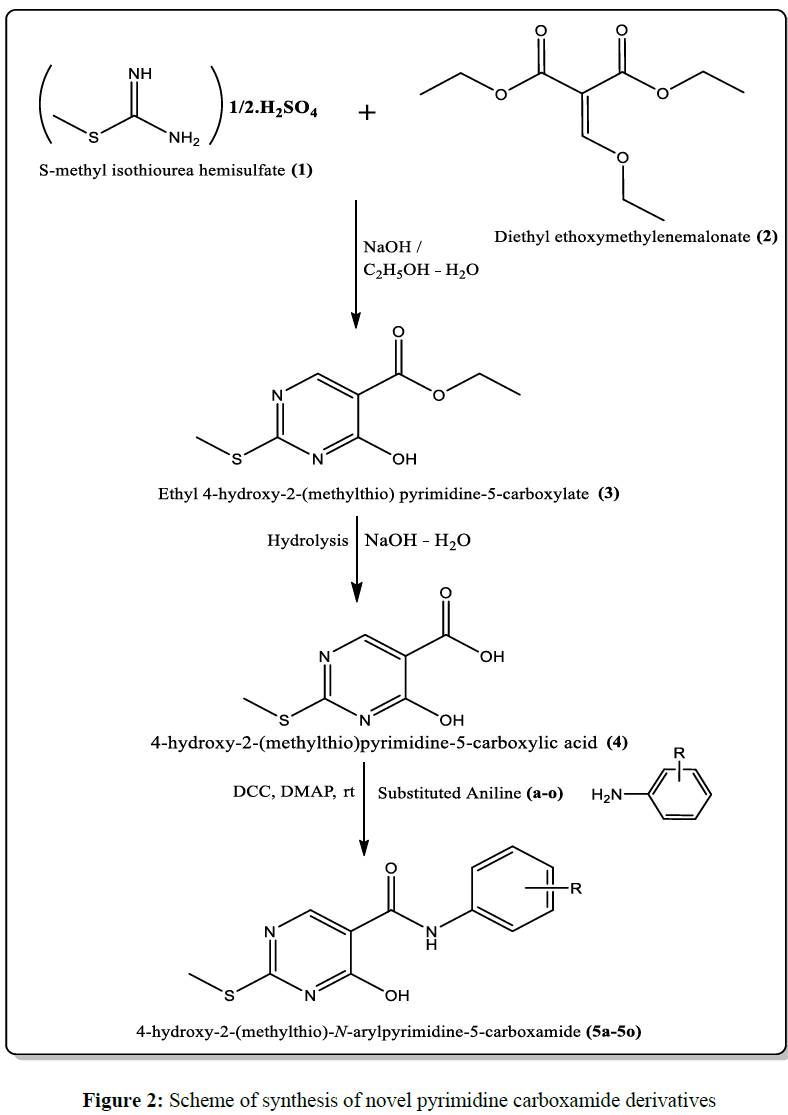
Method: Current research designed and synthesized a novel class of pyrimidine based molecular hybrids of aryl amines via carboxamide linkage. The targeted pyrimidine carboxamide derivative synthesized from a multistep process. Initially, Ethyl 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylate (3) was synthesized from the condensation of S -methylisothiourea hemisulfate (1) and diethyl ethoxymethylenemalonate (2) in the basic medium followed by the acid work up. The pyrimidine carboxylate synthesized hydrolyzed to its acidic form 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylic acid (4), that tethered with various aryl amines (a-o) through amide link with the help of coupling agent DCC/DMAP to yield final compounds 5a-5o. All the synthesized derivatives were characterized by IR, 1HNMR and MASS spectral methods and anti-cancer activity evaluated by employing MTT assay for six cancer cell lines and one normal human cell line.
Results: All the synthesized compounds were screened for anti-cancer activity against six cancer cell lines including A 549 (lung), DU 145 (prostate), HT 29 (colon), MCF-7 (breast), SiHA (cervical), B16F10 (mouse skin melanoma) and one normal human fibroblast cell lines. All the compounds displayed decent cytotoxicity profile when compared with the standard drug doxorubicin. Among the synthesized compounds (5a to 5o) tested, four compounds, 5f, 5d, and 5l have demonstrated excellent cytotoxicity against selected cancer cell lines.
Conclusion: Comparatively, most of the compounds displayed decent cytotoxic potential relative to the standard drug doxorubicin. Further investigations are needed to establish the detailed mechanism of action of the developed novel pyrimidine carboxamide derivatives.
Keywords
Pyrimidine; Carboxamide; Antibacterial; Antifungal; Antitubercular
INTRODUCTION
Around 50% of current research in organic chemistry fundamentally composed of heterocycles [1,2]. This predominance in the heterocycles research is mainly attributed to the emergence of heterocycle-based compounds that possess wide range of applications in material science, and medical science especially drugs [3]. In the context of drug discovery and development pyrimidine nucleus is one of the major pharmacophores targeted by medicinal and pharmaceutical chemistry research groups owing to their versatile and remarkable pharmacological profiles [4]. From a chemical point of view, the study of pyrimidines began in 1884 with Pinner [5], who synthesized pyrimidine derivatives by condensing ethyl acetoacetate with amidines [6].
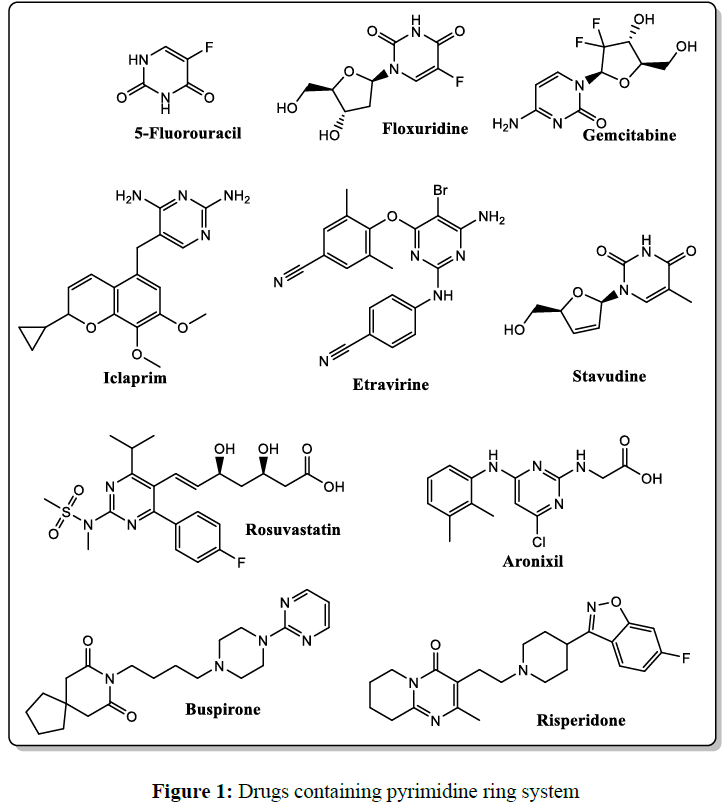
Medicinal chemists developed and utilized many pyrimidine scaffolds to design novel therapeutics with a broad range of pharmacological activities, including anti-proliferative [8-10], antitumor [11, 12], antiviral [13, 14], anti-inflammatory [15-18], antibacterial [19, 20], and antifungal [21, 22], anti-Alzheimer [23], and anti-tubercular [24] properties. Also, the pyrimidine ring is a principal pharmacophore in many major classes of therapeutically effective drugs (Figure 1), such as anticancer (5-Fluorouracil, Floxuridine, Gemcitabine), antipsychotic (Buspirone, Risperidone), antihyperlipidemic (Rosuvastatin, Aronixil), antiviral (Etravirine, Stavudine), and antibiotic (Iclaprim) [25].
The global cancer burden is estimated to have risen to 18.1 million new cases and 10.1 million deaths in 2020 [26]. Despite major advancements and sophisticated technology developments in the cancer treatment strategies globally, cancer continues as a major disorder threatening human health [27, 28]. Among the major strategies employed for the mitigation of cancer, chemotherapy is considered as key feasible method owing to its simplicity relative to other complex strategies such as surgical procedures and radiation therapies etc. [29, 30]. Despite the success of many anticancer chemotherapeutic agents in the treatment of various types of cancers, the side-effects and long-term sequelae of anti-cancer drugs remain a foremost source of apprehension for both patients and clinicians. In most cases, the current approaches to counter the anti-cancer drug-induced side effects are often effective to some extent and are hardly address the potential longer-term sequelae [31]. Hence, target specific, better-tolerated and effective new anticancer agents are needed to address and overcome this challenge. Hence, target specific, better-tolerated and effective new anticancer agents are needed to address and overcome this challenge. Various research groups and pharmaceutical industries are continuously making efforts to develop the newer anticancer agents that are target specific, efficiently on the cancer cells which can produce selective action. Furthermore, the prodigious similarity between normal and tumor cells and the diverse nature of tumors are the main hurdles foiling the development of definitive chemotherapeutic agents [32]. Thus, discovery and development of novel anti-cancer agents is an essential and neverending process in medicinal and pharmaceutical industry. As Pyrimidine derivatives are the major lead molecule that directly inhibits major cancer pathways, so an effort was made to develop novel organic molecules with pyrimidine ring system as a central part and evaluated their invitro cytotoxicity against six selected cancer cell lines along with one normal human cell line (Figure 1).
MATERIALS AND METHODS
Synthetic grade chemicals used for the synthesis were purchased from sigma Aldrich, Bangalore, India. Merck-precoated aluminium TLC plates of silica gel 60 F254 were employed for the reaction monitoring and the spots visualized with iodine vapours and in UV chamber. Column chromatography was used for the purification and isolation of the pure compounds. Melting points were determined by Remi electronic melting point apparatus. IR spectra were recorded on Agilent FTIR by KBr pellet method. 1H NMR recorded on BRUKER DRX – 500 MHz. Chemical shift values (δ) articulated in ppm with reference to internal standard tetra methyl silane (TMS). The splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. MASS recorded on BRUKER ESI-IT MS.
General Procedure for the synthesis of Pyrimidine carboxamide derivatives
General scheme of synthesis designed for novel pyrimidine carboxamide derivatives were given in Figure 2.
Procedure for the synthesis of Ethyl 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylate (3) [33]
In a 100ml round bottomed flask (RBF) charged with 20ml ethanol was added with 12 mmol of S -methylisothiourea hemisulfate (1) and 10 mmol of diethyl ethoxymethylenemalonate (2). To this mixture 3ml of freshly prepared sodium hydroxide (10% solution) was added drop wise with continuous stirring. After the complete addition of sodium hydroxide solution, the reaction mixture further stirred for 4hours and the continuously monitored via Thin Layer Chromatography. After completion of the reaction, the contents of the RBF were worked up with dilute hydrochloric acid solution and the final pH adjusted to 3. The aqueous layer was extracted with three equal proportions (3*10ml) of ethyl acetate, washed with brine solution then dried over anhydrous sodium sulfate. The crude product of Ethyl 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylate (3) obtained from the rotary evaporation of the ethyl acetate portions and it was further purified by recrystallization from ethyl acetate. Compound 3 obtained as white crystalline solid with melting point of 133-135ºC.
Procedure for the synthesis of 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylic acid (4) [34]
Ethyl 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylate (3) was added to 10ml of 1N Sodium hydroxide solution in 50ml RBF, stirred at room temperature for 2hours. After completion, reaction mass was worked up with 0.5N HCl solution and the pH was adjusted to 2. A white coloured precipitate of 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylic acid (4) obtained in acidic pH was filtered, dried under suction and recrystallized from ethanol.
Procedure for the synthesis of 4-hydroxy-2-(methylthio)-N-arylpyrimidine-5-carboxamide (5a-5o)
To the 20ml dichloromethane solution of 10mmol of DCC and 0.5mmol of DMAP in a 100ml RBF, 10mmol of 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylic acid (4) was added and stirred. To this mixture various substituted aryl amines (5a-5o) were added slowly, stirred continuously for 18hours under room temperature and filtered to remove the dicyclohexylurea formed in the reaction. Then the filtrate was evaporated under vacuum and the crude product of pyrimidine carboxamide (5a-5o) was further purified using column chromatography with 10% ethyl acetate - hexane mobile phase.
Anticancer activity
Six cancer cell lines including A 549 (lung), DU 145 (prostate), HT 29 (colon), MCF-7 (breast), SiHA (cervical), B16F10 (mouse skin melanoma) and one normal fibroblast (L929) which were obtained from American Type Culture Collection (ATCC).
All the cells were cultured in DMEM Medium (Gibco, Life Technologies) except HT-29 which is cultured in RPMI 1640 (Gibco, Life Technologies) supplemented with 10% foetal bovine serum (Gibco, Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator at 370C with 5% CO2. MTT [3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide], Para formaldehyde, Phosphate Saline Buffer (pH 7.4), DMSO, Crystal violet (0.5% w/v in ethanol), Hoechst stain were purchased from Sigma-Aldrich India. The MTT assay procedure was adopted from the standard protocol described by Carmichael et al., 1987 [35].
RESULTS AND DISCUSSION
Chemistry
All the designed novel pyrimidine carboxamides were successfully synthesized from the designed synthetic scheme (Figure 2). Initially, Ethyl 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylate (3) was synthesized from the condensation of S -methylisothiourea hemisulfate (1) and diethyl ethoxymethylenemalonate (2) in the basic medium followed by the acid work up. The pyrimidine carboxylate synthesized hydrolysed to its acidic form 4-hydroxy-2-(methylthio) pyrimidine-5-carboxylic acid (4), that tethered with various aryl amines (a-o) through amide link with the help of coupling agent DCC/DMAP. 4-hydroxy-2-(methylthio)-N-arylpyrimidine-5-carboxamide (5a-5o) derivatives were synthesized in moderate to good yields.
Spectral data
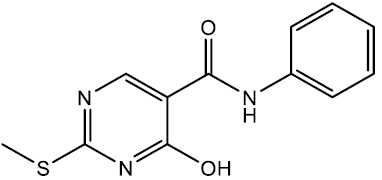
4-hydroxy-2-(methylthio)-N-phenylpyrimidine-5-carboxamide (5a)
Off white crystals, IR (KBr): νmax in cm-1: 1610 (C=N), 3284 (N-H), 3058 (=C–H), 1292 (C-N), 1541 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 9.01 (s, 1H), 8.40 (s, 1H), 7.71 – 7.65 (m, 2H), 7.36 – 7.29 (m, 2H), 7.09 (tt, J = 6.8, 1.2 Hz, 1H), 2.53 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H11N3O2S ([M + H]+): 261.30, found 262.25.
N-(3-chlorophenyl)-4-hydroxy-2-(methylthio) pyrimidine-5-carboxamide (5b)
Off white crystals, IR (KBr): νmax in cm-1: 1608 (C=N), 3282 (N-H), 3061 (=C–H), 1290 (C-N),1542 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.76 (s, 1H), 8.40 (s, 1H), 7.96 (t, J = 2.2 Hz, 1H), 7.70 (ddd, J = 8.3, 2.3, 1.3 Hz, 1H), 7.38 (t, J = 8.0 Hz, 1H), 7.17 (ddd, J = 7.8, 2.2, 1.2 Hz, 1H), 2.53 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10ClN3O2S ([M + H]+): 295.70, found 296.65.
N-(4-chlorophenyl)-4-hydroxy-2-(methylthio) pyrimidine-5-carboxamide (5c)
Off white crystals, IR (KBr): νmax in cm-1: 1608 (C=N), 3283 (N-H), 3064 (=C–H), 1291 (C-N),1544 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.99 (s, 1H), 8.40 (s, 1H), 7.74 – 7.68 (m, 2H), 7.42 – 7.36 (m, 2H), 2.53 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10ClN3O2S ([M + H]+): 295.70, found 296.65.
4-hydroxy-2-(methylthio)-N-(3-nitrophenyl) pyrimidine-5-carboxamide (5d)
Off white crystals, IR (KBr): νmax in cm-1: 1603 (C=N), 3274 (N-H), 3065 (=C–H), 1292 (C-N),1539 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 9.38 (s, 1H), 8.61 (t, J = 2.3 Hz, 1H), 8.40 (s, 1H), 8.05 (ddd, J = 8.0, 1.9, 0.9 Hz, 1H), 7.83 (ddd, J = 8.0, 2.5, 1.0 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 2.53 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10N4O4S ([M + H]+): 306.30, found 307.20.
4-hydroxy-2-(methylthio)-N-(4-nitrophenyl) pyrimidine-5-carboxamide (5e)
Off white crystals, IR (KBr): νmax in cm-1: 1607 (C=N), 3279 (N-H), 3053 (=C–H), 1288 (C-N),1537 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 9.86 (s, 1H), 8.40 (s, 1H), 8.29 – 8.23 (m, 2H), 8.04 – 7.98 (m, 2H), 2.53 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10N4O4S ([M + H]+): 306.30, found 307.20.
N-(4-bromophenyl)-4-hydroxy-2-(methylthio)pyrimidine-5-carboxamide (5f)
Off white crystals, IR (KBr): νmax in cm-1: 1604 (C=N), 3275 (N-H), 3059 (=C–H), 1284 (C-N),1540 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 9.10 (s, 1H), 8.40 (s, 1H), 7.67 – 7.61 (m, 2H), 7.52 – 7.46 (m, 2H), 2.61 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10BrN3O2S ([M + H]+): 340.20, found 341.10.
4-hydroxy-2-(methylthio)-N-(m-tolyl)pyrimidine-5-carboxamide (5g)
Off white crystals, IR (KBr): νmax in cm-1: 1605 (C=N), 3280 (N-H), 3055 (=C–H), 1286 (C-N),1542 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.84 (s, 1H), 8.40 (s, 1H), 7.53 (t, J = 2.1 Hz, 1H), 7.45 (ddd, J = 7.8, 2.1, 1.0 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.94 (ddd, J = 7.1, 2.6, 1.4 Hz, 1H), 2.59 (s, 3H), 2.29 (s, 3H). ESI-MS: m/z Anal. Calcd. For C13H13N3O2S ([M + H]+): 275.35, found 276.25.
N-(2,6-dimethylphenyl)-4-hydroxy-2-(methylthio)pyrimidine-5-carboxamide (5h)
Off white crystals, IR (KBr): νmax in cm-1: 1610 (C=N), 3265 (N-H), 3055 (=C–H), 1289(C-N),1544 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 13.93 (s, 1H), 10.29 (s, 1H), 8.40 (s, 1H), 7.09 (s, 3H), 2.56 (s, 3H), 2.19 (s, 6H). ESI-MS: m/z Anal. Calcd. For C14H15N3O2S ([M + H]+): 289.35, found 290.30.
N-(3-chloro-2-methylphenyl)-4-hydroxy-2-(methylthio)pyrimidine-5-carboxamide (5i)
Off white crystals, IR (KBr): νmax in cm-1: 1604 (C=N), 3269 (N-H), 3064 (=C–H), 1288 (C-N),1535 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 10.40 (s, 1H), 8.40 (s, 1H), 7.45 (dd, J = 8.2, 1.2 Hz, 1H), 7.31 (dd, J = 7.9, 1.2 Hz, 1H), 7.18 (t, J = 8.0 Hz, 1H), 2.56 (s, 3H), 2.24 (s, 3H). ESI-MS: m/z Anal. Calcd. For C13H12ClN3O2S ([M + H]+): 309.75, found 310.65.
N-(3,4-dichlorophenyl)-4-hydroxy-2-(methylthio)pyrimidine-5-carboxamide (5j)
Off white crystals, IR (KBr): νmax in cm-1: 1610 (C=N), 3285 (N-H), 3063 (=C–H), 1292 (C-N),1545 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.96 (s, 1H), 8.40 (s, 1H), 8.00 (d, J = 2.2 Hz, 1H), 7.58 (dd, J = 8.0, 2.1 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 2.65 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H9Cl2N3O2S ([M + H]+): 330.20, found 331.15.
4-hydroxy-N-(3-methoxyphenyl)-2-(methylthio)pyrimidine-5-carboxamide (5k)
Off white crystals, IR (KBr): νmax in cm-1: 1606 (C=N), 3279 (N-H), 3058 (=C–H), 1284 (C-N),1539 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 9.18 (s, 1H), 8.40 (s, 1H), 7.38 (ddd, J = 8.0, 2.2, 1.3 Hz, 1H), 7.24 – 7.15 (m, 2H), 6.75 (ddd, J = 7.8, 2.2, 1.2 Hz, 1H), 3.78 (s, 3H), 2.49 (s, 3H). ESI-MS: m/z Anal. Calcd. For C13H13N3O3S ([M + H]+): 291.35, found 292.30.
4-hydroxy-N-(4-methoxyphenyl)-2-(methylthio)pyrimidine-5-carboxamide (5l)
Off white crystals, IR (KBr): νmax in cm-1: 1612 (C=N), 3289 (N-H), 3069 (=C–H), 1298 (C-N),1550 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.93 (s, 1H), 8.40 (s, 1H), 7.61 – 7.55 (m, 2H), 6.96 – 6.90 (m, 2H), 3.78 (s, 3H), 2.47 (s, 3H). ESI-MS: m/z Anal. Calcd. For C13H13N3O3S ([M + H]+): 291.35, found 292.30.
N-(3-fluorophenyl)-4-hydroxy-2-(methylthio) pyrimidine-5-carboxamide (5m)
Off white crystals, IR (KBr): νmax in cm-1: 1606 (C=N), 3280 (N-H), 3060 (=C–H), 1295 (C-N),1538 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.01 (s, 1H), 8.90 (s, 1H), 8.40 (s, 1H), 7.70 (dt, J = 12.1, 2.2 Hz, 1H), 7.53 (ddd, J = 7.9, 2.4, 1.3 Hz, 1H), 7.39 (td, J = 7.6, 5.0 Hz, 1H), 6.94 (dddd, J = 10.0, 7.5, 2.1, 1.0 Hz, 1H), 2.65 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10FN3O2S ([M + H]+): 279.30, found 280.20.
N-(4-fluorophenyl)-4-hydroxy-2-(methylthio) pyrimidine-5-carboxamide (5n)
Off white crystals, IR (KBr): νmax in cm-1: 1610 (C=N), 3290 (N-H), 3065 (=C–H), 1289 (C-N),1539 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 14.13 (s, 1H), 8.86 (s, 1H), 8.39 (s, 1H), 7.55 – 7.49 (m, 2H), 7.18 – 7.10 (m, 2H), 2.61 (s, 3H). ESI-MS: m/z Anal. Calcd. For C12H10FN3O2S ([M + H]+): 279.30, found 280.20.
4-hydroxy-2-(methylthio)-N-(3-(trifluoromethyl) phenyl)pyrimidine-5-carboxamide (5o)
Off white crystals, IR (KBr): νmax in cm-1: 1606 (C=N), 3286 (N-H), 3066 (=C–H), 1290 (C-N),1540 (C=C); 1H NMR (500 MHz, DMSO-d6) δ 13.93 (s, 1H), 8.88 (s, 1H), 8.34 (s, 1H), 8.08 (t, J = 2.2 Hz, 1H), 7.94 (ddd, J = 7.5, 2.2, 1.2 Hz, 1H), 7.49 (dd, J = 10.4, 7.6 Hz, 1H), 7.29 (ddd, J = 10.3, 2.2, 1.2 Hz, 1H), 2.65 (s, 3H). ESI-MS: m/z Anal. Calcd. For C13H10F3N3O2S ([M + H]+): 329.30, found 330.20.
Structural and physical data of the pyrimidine carboxamide derivatives were enumerated in Table 1.
| Compound No | R | Structure | Melting Point (°C) | % Yield |
|---|---|---|---|---|
| 5a | -H |
|
209-211 | 63 |
| 5b | 3-Cl | 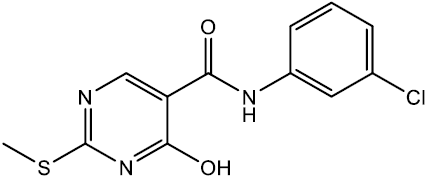 |
233-234 | 74 |
| 5c | 4-Cl | 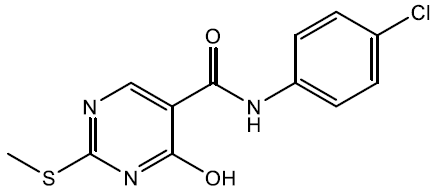 |
241-243 | 71 |
| 5d | 3-NO2 | 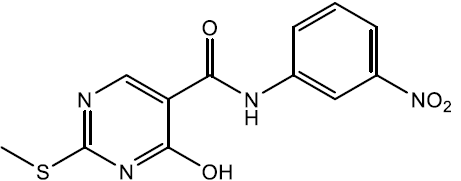 |
226-228 | 79 |
| 5e | 4-NO2 | 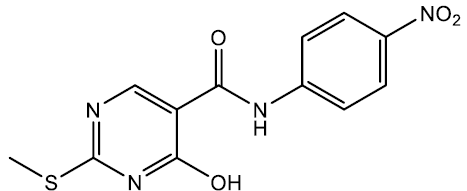 |
238-239 | 74 |
| 5f | 4-Br | 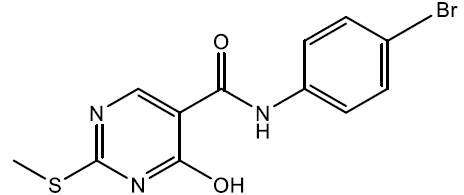 |
248-250 | 69 |
| 5g | 3-CH3 | 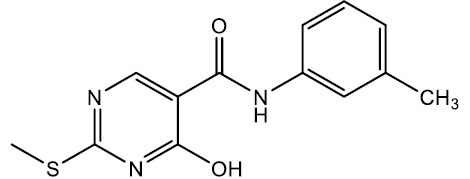 |
213-215 | 65 |
| 5h | 2, 6-CH3 | 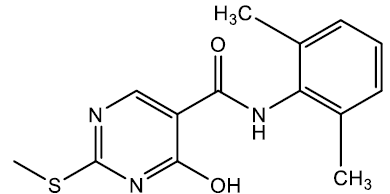 |
204-205 | 62 |
| 5i | 2-CH3, 3-Cl | 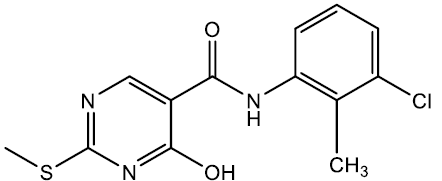 |
227-229 | 61 |
| 5j | 3, 4-Cl | 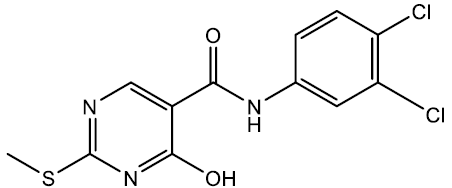 |
239-240 | 73 |
| 5k | 3-OCH3 | 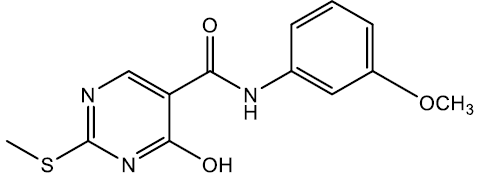 |
222-223 | 68 |
| 5l | 4-OCH3 | 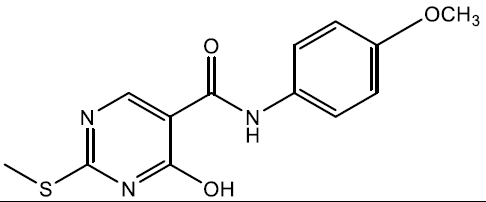 |
235-236 | 70 |
| 5m | 3-F | 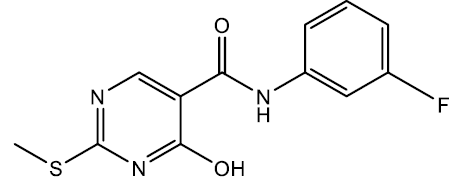 |
189-191 | 81 |
| 5n | 4-F | 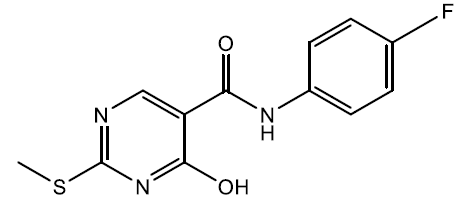 |
198-199 | 79 |
| 5o | 3-CF3 | 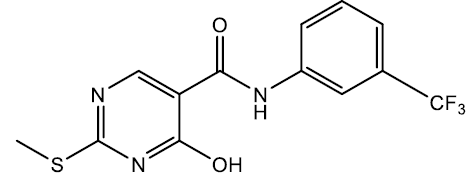 |
184-185 | 83 |
Table 1: Structural and physical data of Pyrimidine Carboxamides
Anticancer activity
Results of MTT Assay were displayed in Table 2 and the assay results displayed the cytotoxic potential of all the synthesized compounds in comparison to the standard drug Doxorubicin. The results displayed the cytotoxicity potential of the synthesized derivatives. Except, the compound 5e, most of the compounds displayed good to moderate cytotoxicity against five cancer cell lines (A 549, DU 145, MCF-7, SiHA, and B16F10), among the tested six cell lines. Majority of the compounds displayed high cytotoxicity spectrum towards SiHA and MCF-7 followed by B16F10, DU 145 and A 549 cell lines. All the compounds displayed moderate to low cytotoxicity values against the HT 29 cell lines (Table 2).
| Sample No. | IC50 (μM) a | ||||||
|---|---|---|---|---|---|---|---|
| A 549 | DU 145 | HT 29 | MCF-7 | SiHA | B16F10 | L929 | |
| 5a | >100 | >100 | >100 | 21.8±3.36 | 16.64±1.14 | 21.11±1.88 | >100 |
| 5b | 22.44±1.58 | 31.05±3.39 | 24.72±1.3 | 28.7±6.1 | 21.2±2.47 | 20.15±3.17 | 40.25±1.4 |
| 5c | >100 | >100 | 44.89±1.95 | 55.16±2.25 | 41.15±1.12 | >100 | 44.17±5.2 |
| 5d | 10.2±2.11 | 13.14±1.13 | 14.33±1.14 | 19.33±1.14 | 13.72±2.25 | 16.72±2.25 | >100 |
| 5e | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 5f | 13.75±1.23 | 13.02±2.23 | 14.21±2.16 | 17.54±1.17 | 8.15±2.12 | 19.35±1.34 | 38.87±5.1 |
| 5g | 23.51±2.4 | 20.3±4.7 | 36.72±1.3 | 21.7±3.6 | 22.16±1.25 | 21.8±1.5 | 36.68±2.5 |
| 5h | >100 | 56.71±1.36 | 28.42±2.18 | >100 | 17.68±0.14 | 28.11±1.48 | >100 |
| 5i | >100 | 43.15±2.39 | 41.72±3.83 | >100 | 19.16±2.25 | 38.38±1.3 | >100 |
| 5j | 21.72±2.18 | >100 | >100 | 20.8±3.37 | 15.64±1.14 | >100 | 44.6±2.3 |
| 5k | >100 | >100 | >100 | 25.8±1.46 | 32.16±2.25 | 28.38±1.7 | 39.25±2.8 |
| 5l | 10.01±1.12 | 14.3±1.25 | 19.40±1.3 | 6.44±2.12 | 6.22±3.1 | 15.2±1.5 | 41.6±3.7 |
| 5m | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 5n | >100 | >100 | >100 | >100 | 13.64±1.12 | 21.11±1.19 | >100 |
| 5o | 21.54±1.61 | 22.05±2.3 | 48.72±3.3 | 34.7±1.87 | 19.11±1.25 | 23.9±1.12 | 41.15±2.98 |
| Doxorubicin b | 1.02±2.7 | 0.94±3.4 | 1.18±1.1 | 1.23±1.8 | 0.98±1.46 | 1.4±0.54 | 1.12±0.65 |
| All values are given as means ± standard deviation. aIC50 is the drug concentration effective in inhibiting 50% of the cell growth measured by MTT method. bDoxorubicin was used as positive control in this study. |
|||||||
Table 2: Anti-proliferative activity (IC50 values) of compounds 5a-5o
Results also disclose that among all the cell lines employed for the study, SiHA, B16F10, MCF-7 cell lines were inhibited by majority of the compounds. Comparatively all the compounds displayed low toxicity towards the normal human cell lines than the cancer cell line that implies the less cytotoxicity of the pyrimidine carboxamides.
CONCLUSION
In conclusion, synthesis of a novel series of pyrimidine carboxamide hybrids (5a-5o) were synthesized using commercially available starting materials and economically feasible method. The structure elucidation was done with the help of their physical, analytical, and spectral data. All the synthesized compounds were screened for anti-cancer activity against six cancer cell lines including A 549 (lung), DU 145 (prostate), HT 29 (colon), MCF-7 (breast), SiHA (cervical), B16F10 (mouse skin melanoma) and one normal human fibroblast cell lines. All the compounds displayed decent cytotoxicity profile when compared with the standard drug doxorubicin. Among the synthesized compounds (5a to 5o) tested, four compounds, 5f, 5d, and 5l have demonstrated excellent cytotoxicity against selected cancer cell lines. Further studies are needed to establish the possible mechanism of anticancer action can helpful in the future development of pyrimidine nucleus based carboxamide hybrids as novel anticancer agents.
Conflict of Interest
Authors disclose no conflict of interest
References
- Monier M, El-Mekabaty A, Elattar KM. Synth Commun. 2018, 48: p. 839-875.
- Mert BD, Elattar KM. Curr Org Chem. 2018, 22: p. 386-410.
- Sunderhaus JD, Dockendorff C, Martin SF. Tetrahedron. 2009, 65: p. 6454-6469.
- Sasada T, Kobayashi F, Sakai N, et al., Org Lett. 2009, 11: p. 2161-2164.
- Pinner A. Chemistry Europe. 1884, 17: p. 2519-2520.
- Pinner A. Chemistry Europe. 1885, 18: p. 759-763.
- Joule JA, Mills K. Heterocyclic Chemistry. John Wiley & Sons, 2008.
- Sridhar S, Prasad YR, Dinda S. Int J Pharm Sci Res. 2011, 2: p. 2562.
- Shanmugasundaram P, Harikrishnan N, Aanandini MV, NIScPR. 2011.
- Álvarez P, Marchal JA, Boulaiz H, et al., Expert Opin Ther Pat. 2012, 22: p. 107-123.
- Cai TB, Tang X, Nagorski J, et al., Bioorg Med Chem. 2003, 11: p. 4971-4975.
- Ye T, Han Y, Wang R, et al., Bioorg Chem. 2020, p. 103796.
- Jin K, Sang Y, De Clercq E, et al., Bioorg Med Chem Lett. 2018, 28: p. 3491-3495.
- Huang B, Kang D, Tian Y, et al., Chem Biol Drug Des. 2020, 97: p. 67-76
- Kalcic F, Kolman V, Ajani H, et al., Chem Med Chem 2020, 15: p. 1398-1407
- Tageldin GN, Fahmy SM, Ashour HM, et al., Bioorg Chem. 2018, 78: p. 358-371.
- Atatreh N, Youssef AM, Ghattas MA, Bioorganic Chemistry Volume. 2019, 86: p. 393-400.
- Bakr RB, Ghoneim AA, Azouz AA. Bioorganic chemistry. 2019, 88: p. 102964.
- Bruno O, Schenone S, Ranise A, et al., Farmaco. 1999, 54: p. 95-100.
- Mokale SN, Shinde SS, Elgire RD, et al., Bioorg Med Chem Lett. 2010, 20: p. 424-426.
- El-Sharkawy KA, AlBratty MM, Alhazmi HA. J Pharmaceut Sci. 2018, 54.
- Foroughi HO, Kargar M, Erjaee Z, et al., Monatshefte für Chemie - Chemical Monthly, 2020.
- Iraji A, Khoshneviszadeh M, Firuzi O, et al., Bioorg Chem. 2020, 97: p. 103649.
- Sui YF, Li D, Wang J, et al., Bioorg Med Chem Lett. 2020, 30: p. 126982.
- Sahu, Meeta & Siddiqui, Nadeem. 2016, 8: p. 8-21.
- Global cancer report-2020, International Agency for Research on Cancer, WHO report September 2020.
- Alafeefy AM, Ceruso M, Al-Tamimi AMS, et al., Bioorg Med Chem. 2014, 22: p. 5133–5140.
- Alafeefy AM, Ceruso M, Al-Jaber NA, et al., J Enzyme Inhib Med Chem. 2015, 30: p. 581–585.
- De Martino JK, Boger DL. Drug Fut. 2008, 33: p. 969-979.
- Curran WJ. Oncology. 2002, p. 63: 29-38.
- Nurgali K, Jagoe RT, Abalo R. Front Pharmacol. 2018, 9: p. 245-247.
- El-Messery SM, Hassan GS, Nagi MN, et al., Bioorg Med Chem Lett. 2016, 26: p. 4815-4823.
- Wang H, Wang H, Yang L, et.,2015, 32: 412-415
- Yan S, Ivanov, Alexander & Dar'in, et al., Chemistry of Heterocyclic Compounds. 2007, 43: p. 1479-1480.
- Carmichael J, DeGraff WG, Gazdar AF, et al., Cancer Res. 1987, 47: p. 936-942.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref