Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 4
NOVEL CLARITHROMYCIN LOADED SELF EMULSIFYING DRUG DELIVERY SYSTEM FOR AMPLIFICATION OF SOLUBILITY AND ORAL BIOAVAILABILITY
Sundar Vankayala Devendiran1*, Vijayalakshmi Rajendran2 and Dhanaraju Magharla Dasaratha12Department of Pharmaceutical Analysis. GIET School of pharmacy, Rajahmundry, AP, India
Sundar Vankayala Devendiran, Department of Pharmaceutical Technology. GIET School of pharmacy, Rajahmundry, AP, India, Email: sundarvd@yahoo.co.in
Received: 29-Mar-2023, Manuscript No. dpc-23-94260; Editor assigned: 31-Mar-2023, Pre QC No. dpc-23-94260; Reviewed: 14-Apr-2023, QC No. dpc-23-94260; Revised: 17-Apr-2023, Manuscript No. dpc-23-94260; Published: 24-Apr-2023, DOI: 10.4172/0975-413X.15.4.5-13
Abstract
SEDDS (self-emulsifying drug delivery system) are of particularly relevant for the production of isotropic mixtures of oil, surfactant, co-surfactant, and drug in order to solve their physicochemical problems. Clarithromycin (CLA), a macrolide, has a 50 percent absolute bioavailability. The goal of this work was to create a liquid SEDDS system for CLA in order to increase solubility by increasing interfacial surface area and thereby boosting absorption. Clarithromycin SEDDS were produced with isopropyl myristate as the oily phase, Cremophor EL, Tween 80, Brij 58 as surfactants, and isopropyl alcohol, isobutanol, and transcutol EL as co-surfactants. Clarithromycin solubility was studied in a variety of vehicles, including isopropyl myristate, Cremophor EL, brij 58, tween 80, isopropyl alcohol, isobutanol, and transcutol RH, with isopropyl myristate yielding around 112.35 mg/ml. Formulations F9, which contain Brij 58 as a surfactant and transcutol RH as a co-surfactant, have a 120m minimum globule size, a 15-20 sec self-emulsification time, and maximal drug release. The ideal composition of formulation F9 SEDDS was resolute based on the findings of phase separation, self-emulsification, percentage transmittance, globule size, drug release, zeta potential, resilience to dilution, cloud point measurement, drug content, and dispersibility investigations.
PICTORIAL ABSTRACT
Keywords
Self-emulsifying drug delivery system; Clarithromycin; Cremophor EL; Self-emulsification’; Liquid SEDDS; Macrolide antibiotic
INTRODUCTION
SEDDS are one of the strategies for improving the oral bioavailability of poorly soluble pharmaceuticals by providing and retaining the drug in a dissolved state, in minute droplets of oil, during its transit through the gastrointestinal tract [1]. SEDDS has been demonstrated to be effective in increasing the oral bioavailability of medicines that are poorly water-soluble or lipophilic [2]. It has a number of benefits, including spontaneous production, thermodynamic stability, increased bioavailability, and preparation ease. SEDDS has a number of benefits, including increased solubility and bioavailability [3]. In order to achieve a necessary high bioavailability following mucosal administration, poorly soluble medications as well as hydrophobic ion pairs of drugs, such as ionic liquids, can be dissolved in oily droplets [4-6]. When SEDDS formulations are discharged into the gastrointestinal tract lumen, they come into contact with GI fluid and produce a fine emulsion (micro/ nano) known as in situ emulsification or self-emulsification, which leads to drug solubilization and bypassing the hepatic first-pass effect. A variety of in-vivo features of the lipid formulations have been linked to this bioavailability boosting function. Clarithromycin is a macrolide antibiotic that belongs to the class of antibiotics known as macrolides [7,8]. It has a 3hr elimination half-life that rises to 5-7 hr after administration. Clarithromycin has a 50 percent absolute bioavailability. Clarithromycin is a medication classified as BCS class II, meaning it has a poor solubility and good permeability profile [9]. Liquid self-emulsifying formulations have become more popular as medication carriers for lipophilic medicines in recent decades. As a result, the investigation's primary goal is to produce and test liquid SEDDS containing clarithromycin in order to increase the drug's solubility and hence improve its oral bioavailability [10]. We attempted to raise the solubility rate of clarithromycin as a result of this investigation in order to improve its efficacy and decrease variability.
MATERIALS AND METHODS
Materials
All of the constituents for the CLA-SEDDS composition were of good quality and came from reputable sources. Iso-propyl myristate, Iso-propyl alcohol, Iso-butanol (Riddhi Siddhi Chemicals, Thane), Cremophor EL, Tween 80, Brij 58, and Transcutol RH (AyonPharmaChem, Mumbai) (CDH fine Chemicals, New Delhi, India).
Methods
Solubility Study
The solubility was determined using the shake flask method. A solubility research is performed to discover the best oil and surfactant for the predicted medication. Clarithromycin solubility in various vehicles, such as oil (Iso-propyl myristate), surfactants (Cremophor, Tween 80, Brij), and co-surfactants (Iso-propyl alcohol, Iso-butanol, Transcutol) was tested by adding an excess quantity of clarithromycin to each cap vial containing 3ml of vehicles. After sealing, the mixture was vortexed for 10 min at maximum speed to ensure thorough mixing of clarithromycin with the vehicles. The mixtures were then agitated in a shaker that was kept at room temperature until they reached equilibrium (48hr). After 24 hr, the vial was examined for drug residue, and an extra amount of drug was given to the vial that showed no residue, and it was shaken for another 24 hr. After that, the mixture was centrifuged for 10 min at 3000rpm. The supernatant was collected into glass vials, and the drug concentration was determined using UV-visible spectroscopy at 210nm [11, 12].
Preliminary Study of Surfactant and Co-Surfactant and mutual miscibility study
The emulsification capacity of the surfactants and co-surfactants was tested using a method described in the literature. For the surfactant screening, two milliliters of co-surfactant, three milliliters of selected surfactant, and five milliliters of selected oil phase were produced and tested [13]. The determined amount of oil (Iso propyl myristate), surfactants (Cremophor, Tween 80, Brij), and co-surfactants (Iso propyl alcohol, Isobutanol, Transcutol) were placed in various test tubes and vigorously agitated before being set aside for a few hours to determine miscibility. Any signs of separation in the test tubes were visually checked.
Compatibility Study
The Fourier transform infrared (FT-IR) spectroscopy method was used to assess clarithromycin's drug excipient compatibility with oil and other excipients. The pure drug was triturated with KBr and pelleted by applying a pressure of 100 kg/cm2 for 2 min. Bruker FTIR Tensor Model, Germany, was used to evaluate the pellet. Prior to analyzing the test samples, the KBr background was acquired [14].
Construction of Pseudo Ternary Phase
The water titration method was used to create a ternary phase diagram in order to determine the component concentration range for the natural range of microemulsions. Ternary arrays were made using oil, surfactant, and co-surfactant with varying proportions of surfactant: co-surfactants, i.e. S/Co 1:1, 1:2, and 1:3. These surfactant blends: co-surfactants (S mix) were combined with the oily phase by adding tiny quantities and swirling constantly. Oil:S mix proportions ranged from 9:1, 8:2, 7:1, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9. The resulting blends were titrated with distilled water in 0.5 percent (w/w) increments, stirring well with each addition. The samples were visually evaluated for clarity once the systems had reached equilibrium. For each system of oil, surfactant, and cosurfactant, pseudo ternary phase diagrams were created. The point suggesting clear and isotropic mixes was taken into account [14].
Preparation of CLA loaded SEDDS formulations
With oil, surfactant, and co-surfactant, a number of SEDDS formulations were developed (Table 1). Each formulation included a single dosage of clarithromycin. The formulations were made by dissolving the medication in oil and adding surfactant and co-surfactant thereafter. To achieve a homogeneous isotropic mixture, the resultant mixtures were vortex mixed continuously and then sonicated for a few minutes. The SEDDS formulations were kept at room temperature until they were ready to be used [15].
| Formulation | Drug(mg) | Oil | Surfactant | Co-surfactant |
|---|---|---|---|---|
| Isopropyl myristate | Cremophor EL | Iso propyl alcohol | ||
| F1 | 250 | 10 | 30 | 60 |
| F2 | 250 | 20 | 26.5 | 53.5 |
| F3 | 250 | 30 | 23.3 | 46.7 |
| Isopropyl myristate | Tween 80 | Isobutanol | ||
| F4 | 250 | 15 | 28.3 | 56.7 |
| F5 | 250 | 25 | 25 | 50 |
| F6 | 250 | 35 | 21.6 | 43.4 |
| Isopropyl myristate | Brij 58 | Transcutol RH | ||
| F7 | 250 | 20 | 26.5 | 53.5 |
| F8 | 250 | 40 | 20 | 40 |
| F9 | 250 | 60 | 13.3 | 26.6 |
Characterization of CLA-SEDDS formulation
Clarity
Following aqueous treatment, the appearance and purity of CLA–SEDDS formulations were measured in percentage transmittance (percent T notation) [16]. Using a UV spectrophotometer and distilled water as a blank, the % transmittance of the system is determined at 210nm wavelength. Formulations are transparent if their % transmittance is more than 99 percent [17].
Dispersity and Emulsification speed
A standardized USP dissolution apparatus II is used to examine the effectiveness of self-emulsification of oral micro/nanoemulsions. At 37 degrees Celsius, one millilitre of each formulation is added to 500 millilitres of water. The mild agitation provided by a conventional stainless steel dissolving paddle moving at 100 rpm is typical [9]. SEDDS formulations were visually assessed for their self-emulsifying characteristics. In a nutshell, various compositions were classified based on the speed with which they emulsified the transparency of the resulting emulsion, and its apparent stability. The preconcentrate (SEDDS) was dropped into 250 ml of distilled water drop by drop for visual evaluation. After 24h, the resulting emulsion was examined visually to determine precipitation. Table 2 explains the self-emulsification time of the formulas [18] (Table 2).
| Physical State | Inference |
|---|---|
| Clear | Transparent or transparent with bluish tinge |
| Non clear | Turbid |
| Stable | No precipitation at the end of 24 hr |
| Unstable | Showing precipitation within 24 hr |
Robustness to Dilution
Dilution studies are critical in the creation and optimization of final SEDDS formulations. To create a stable emulsion, the right emulsifier blend is required. The dilution resistance of SEDDS formulations containing clarithromycin was investigated by diluting those 100 and 1000 times with 0.1N HCl, 6.8 phosphate buffer, and distilled water. The diluted emulsions were kept for 12 h and looked for evidence of drug precipitation and phase separation [9].
Droplet size and Zeta Potential Analysis
Optical microscopy was used to assess the globule size and size distribution of the produced SEDDS formulations. With the assistance of a stage micrometer, the eye piece micrometer was calibrated. The SEDDS sample was disseminated on a glass slide, and the size of 300 globules was measured with a microscope and a micrometer [19]. Zeta potential analysis was used to assess the charge of the droplets. In emulsion systems, the zeta potential aids in the prediction of flocculation and stability. A Zetasizer ZS 90 was used to assess droplet size and zeta potential of the produced emulsion (Malvern Instruments, UK). At 25°C and a 90° angle, light scattering was measured [14].
Determination of drug content
A little amount of acetone was used to dissolve self-emulsifying drug delivery system formulations containing clarithromycin. 0.1N HCl was used to make a 100ml volume. A 0.2ml sample was taken from the aforementioned stock solution and diluted in acetone to make a 10ml solution. A UV visible spectrophotometer was used to detect absorbance at 210nm in duplicate samples. As a reference blank, 0.1N HCl was utilized [11].
Cloud point measurement
In non-ionic surfactant-based SEDDS, the cloud point is an important consideration. When the temperature above the cloud point, an irreversible phase separation occurs, and the cloudiness of the mixture hampered medication absorption due to dehydration of the components. As a result, SEDDS' cloud point should be above 37°C to avoid phase separation [20, 21].
Microscopic observation of prepared SEDDS
The produced SEDDS were examined under a microscope after being transformed into O/w (oil in water) emulsions. The created SEDDS formulations were dropped into the distilled water one at a time, stirring constantly, resulting in the creation of a milky emulsion that was swirled for 5-10 mi. Under a microscope, the final emulsion was examined.
In-vitro drug release study
Quantitative in vitro dissolution tests using a USP type II dissolving device and 900 ml of pH 6.8 phosphate buffer solution at 100 rpm and 37°C are carried out to quantify drug release from the oil phase into the aqueous phase. 5ml of clarithromycin-containing SEDDS samples were put in baskets holding 900ml of 6.8 pH phosphate buffer, and samples were extracted at regular intervals and replaced with new medium. Using a UV spectrophotometer set at 210 nm, the samples were then examined [14].
Stability study
Freeze thawing is used to test the stability of a formulation. Three to four freeze-thaw cycles were performed on the SEDDS preconcentration of various formulations, including freezing at 40°C for 24 hr. After that, the varied formulations were centrifuged for 5 min at 3000 rpm. Phase separation and drug precipitation were detected visually in the formulations [22].
RESULTS
Solubility studies
Oil, surfactants, and co-surfactants were used in the self-emulsifying formulations, and the drug should be a clear, monophasic liquid when added to the aqueous phase at room temperature, with good solvent characteristics to allow the drug to be presented in the solution. Clarithromycin solubility was tested in several solvents, surfactants, and co-surfactants. Cremophor EL and Brij 58 had lower solubility than the others, with 47.21 mg/ml and 49.22 mg/ml, respectively, among the evaluated vehicles. Isopropyl alcohol, isobutanol, and Tween 80 have solubilities of 83.67 mg/ml, 72.49 mg/ml, and 64.72 mg/ml, respectively, indicating that they have a higher solubility than Cremophor and Brij (Table 3). Because of its higher hydrophilicity, superior drug solubility, and emulsion-forming capabilities, isopropyl myristate was chosen as the oily phase [23].
| Sl. No | Name of the vehicle | Solubility (mg/ml) |
|---|---|---|
| 1 | Iso propyl myristate | 112.35 |
| 2 | Cremophor EL | 47.21 |
| 3 | Tween 80 | 64.72 |
| 4 | Brij 58 | 49.22 |
| 5 | Iso propyl alcohol | 83.67 |
| 6 | Isobutanol | 72.49 |
| 7 | Transcutol | 62.86 |
Preliminary screening of surfactants and co-surfactants and Mutual miscibility study
Different non surfactants were tested for their capacity to emulsify the chosen oil phase in order to determine the appropriate amount of oil surfactant combination to utilise in SEDDS formulations. It was critical to see if the formulation could distribute well enough to produce a spontaneous emulsion. For the emulsification investigation, Cremophor RH, Tween 80, Brij 58, Iso propyl alcohol, Isobutanol, and Transcutol EL were chosen because they had high solubility potential for Clarithromycin. The chosen surfactants and co-surfactants showed no evidence of phase separation. At different amounts, the surfactant combination was entirely miscible with isopropyl myristate. As a result of the reciprocal miscibility of excipients, a method for stable liquid formulation can be found. Isopropyl myristate, the chosen vehicle, is a long-chain triglyceride (LCT) that is a good candidate for hydrophilic surfactants and co-solvents. Brij 58, a waxy co-surfactant, was melted to ensure that it was miscible with other components (Table 4).
| Screening of Surfactants and Co-surfactants | Mutual miscibility of oil/surfactant and co-surfactant | ||||
|---|---|---|---|---|---|
| Oil | Surfactant | Co-surfactant | Inference by visual observation | Oil/surfactant/co-surfactant | Miscibility |
| Iso propyl myristate | Cremophor EL | Iso propyl alcohol | Emulsion formed with milky appearance | Isopropyl myristate/Cremophor/Iso propyl alcohol | Completely miscible |
| Iso propyl myristate | Tween 80 | Isobutanol | Emulsion formed with milky appearance | Isopropyl myristate/Tween 80/Isobutanol | Completely miscible |
| Iso propyl myristate | Brij 58 | Transcutol | Emulsion formed with milky appearance | Isopropyl myristate/Brij/Transcutol | Completely miscible |
Construction of Ternary phase diagrams
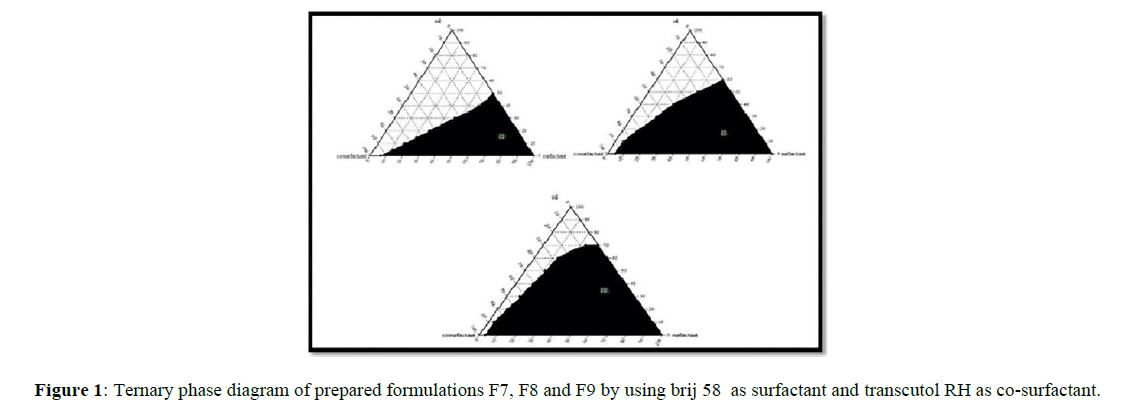
The Ternary phase diagrams were created to determine the optimal concentrations of Iso propyl myristate (oil), Cremophor RH, Tween 80, and Brij58 (surfactants), Iso propyl alcohol, Isobutanol, and Transcutol RH (co-surfactants) and their influence on emulsion formation. The ternary phase diagram's stable formulation zone was highlighted in black. The formulations with the best self-emulsifying characteristics were chosen for research based on the ternary phase diagrams. The mixture of surfactant and cosurfactant (Smix) ratio 1:2 of F9 demonstrated a larger self-emulsifying (micro emulsifying) area than the other formulations in the current investigation (Figure 1).
Drug excipients compatibility studies
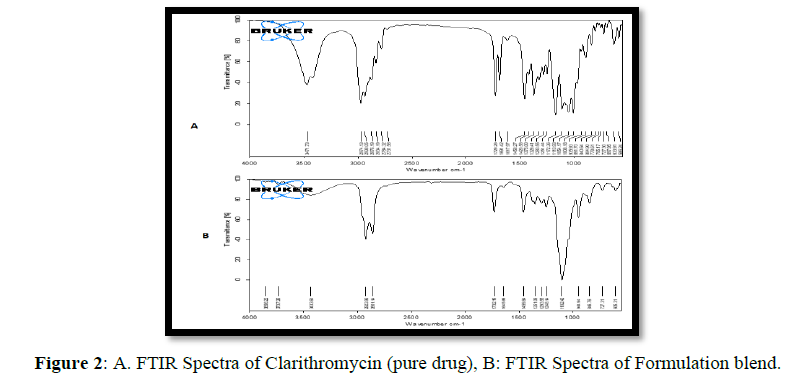
Clarithromycin's FTIR spectrum shows that the carboxyl group has a stretching peak at C=O 1619cm-1 and that the hydroxyl group has a distinctive absorption peak at -OH 3345 cm-1. The distinctive peaks of drug were noticed in the IR spectra of SEDDS formulations change at which the peak has been detected in the pure drug at 1729 cm-1, a characteristic stretching owing to C23H34O6, and there is a characteristic stretching of symmetrical ether group at 1100 cm-1 (Figure 2). This revealed that there was no chemical interaction between the medication, the drug and the excipients, and the SEDDS formulations.
Evaluation of Self-Emulsifying Drug Delivery System
Clarity
Using a UV spectrophotometer and distilled water as a blank, the % transmittance of the produced SEEDS was determined at 210nm. F1, F2, and F3 had percent transmittance values of 68.75, 70.66, and 80.54, respectively, indicating less clarity. The percent transmittance value of Formulas F7, F8, and F9 is more than 90%, indicating that they are clear. This might be because the emulsion is more transparent due to the reduced particle size.
Dispersibility and Emulsification speed
The formulations in-vitro performance is visually examined using the grading method. The visual observations revealed that all of the SEDDS formulations were of grade A and grade B. The aforementioned grading system was used to justify all of the formulations. A fast created clear micro emulsion and a rapidly formed less clear emulsion with a bluish white appearance can be seen in the formulations. The rate of emulsification might be used to determine SEDDS efficiency. According to the findings, all of the clarithromycin-loaded SEDDS formulations tested self-emulsified between 15-47 sec (Table 5). All of the examined systems had a short self-emulsification time, indicating their capacity to emulsify quickly and easily.
| Determination of dispersibility | Self-Emulsification | |||||
|---|---|---|---|---|---|---|
| Grade | Formation of microemulsion | Inference | Observations | Batch No. | Self-emulsification time | |
| Formulation exhibits rapid clear emulsion | Formulation exhibits rapid less clear emulsion | |||||
| A | Rapidly forming within 1 min | Clear appearance | F1 F3 F6 F8 F9 |
F2 F4 F5 F7 |
F1 | 40-45 |
| F2 | 40-47 | |||||
| B | Rapidly forming, slightly less clear | Bluish white appearance | F3 | 35-40 | ||
| F4 | 20-25 | |||||
| C | Emulsion formed within 2 min | Fine milky emulsion | F5 | 20-29 | ||
| D | oily appearance, emulsion formed longer than 2 min | Grayish white emulsion | F6 | 22-28 | ||
| F7 | 20-25 | |||||
| E | Exhibits poor emulsion | Large oil globules present on the surface | F8 | 17-25 | ||
| F9 | 15-20 | |||||
Robustness to Dilution
It means that the formulations F3, which contains Cremophor and isopropyl alcohol, and F7, which contains brij and transcutol, were less stable, since they precipitated within 30 min when compared to the other formulations.
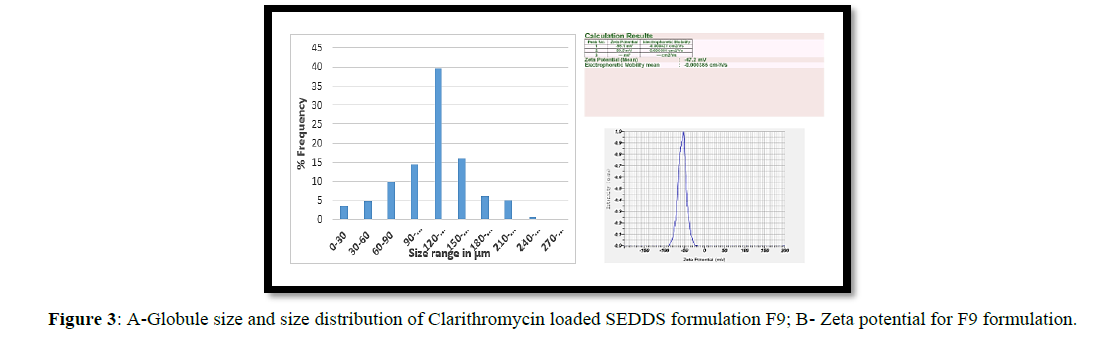
Determination of Globule size and Zeta potential
Optical microscopy with a calibrated eye piece micrometer was used to measure globule size. The average globule size of the SEDDS formulations F1 through F9 was discovered to be between 80 and 170 micrometers. Formulation F9 had the largest globule size of all the formulations tested, at 140m. Stable particles have zeta potentials of +30 or -30. The zeta potential of formulations F1-F3 produced using Cremophor as the surfactant and isopropyl alcohol as the cosurfactant is -19.5, -26.2, and -29.9 mV, respectively. The zeta potential of Formulations F4-F6 produced with tween 80 as a surfactant and isobutanol as a co-surfactant is -37.4, -31.5, -30.1. Formulations F7-F9 with brij 58 as surfactant and transcutol as co-surfactant had zeta potential values of -46.6, -44.8, and -47.2, respectively. Because of the higher concentrations of brij 58 and transcutol, F9 was more stable than the other formulations, having a zeta potential of -47.2 (Figure 3).
Determination of Drug Content
A U.V. spectrophotometer set to 210nm was used to determine the medication concentration of the clarithromycin-containing formulations. All SEDDS formulations had drug concentration ranging from 81.67 to 97.65%.
Cloud point measurement
The cloud point of clarithromycin SEDDS was found to be higher than 70°C in all formulations, indicating that it formed a stable emulsion at physiological temperature with no danger of phase separation.
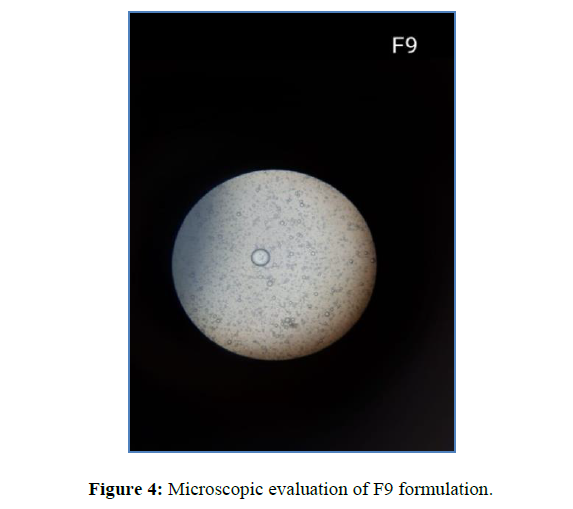
Microscopic observation
All of the emulsion globules recovered was spherical in structure, according to the microstructure examination (Figure 4).
In-vitro drug release
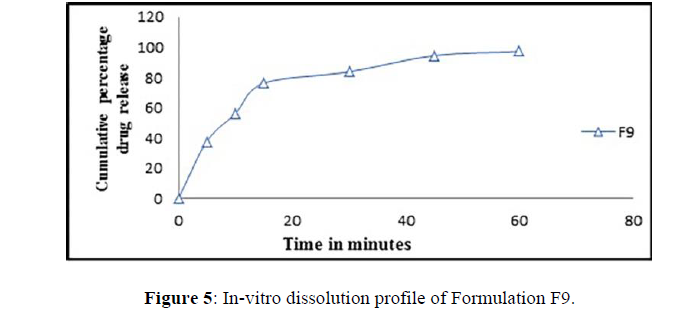
The clarithromycin SEDDS formulations that have been produced have a medication release range of 76.39–97.29. Formulation F9, which included Brij 58 and Transcutol RH, had the highest release after 60 min of all the batches (Figure 5). The improved formulation's maximal drug release was further aided by its self-emulsification time and globule size.
Because of the larger surface area, formulations with a small globule size improve medication solubility. When compared to previous created formulations, the optimized formulation F9 formed micro emulsion with smaller droplet size and improved clarithromycin solubility. The concentration of surfactant and the co surfactant ratio have an impact on droplet size reduction.
Stability Study
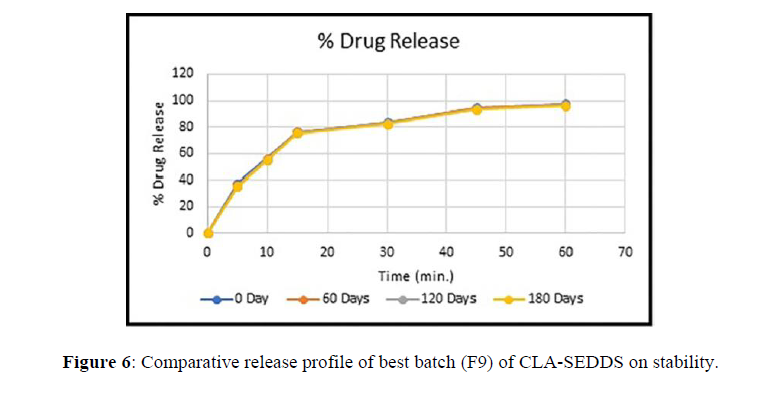
Stress tests were performed on the best formulation (F9). At intermediate and rapid circumstances, the formulation was shown to be stable for 6 months and at long-term conditions for 12 months. The drug release of the resulting formulation did not differ significantly (Figure 6).
DISCUSSION
The goal of this study was to increase the oral bioavailability and absorption of the weakly water-soluble antibiotic clarithromycin using a self-emulsifying drug delivery system. Clarithromycin SEDDS were produced with Isopropyl myristate as the oily phase, Cremophor EL, Tween 80, Brij 58 as surfactants, and isopropyl alcohol, isobutanol, and transcutol EL as co-surfactants.
Preliminary studies were performed for selection of oil, which was an important and critical requisite for formulation of SEDDS. Constructing a phase diagram is one of the primary steps and makes a backbone for the SEEDS, particularly when the aim is to accurately delineate a phase boundary. Phase diagrams were constructed separately for each ratio of Smix prepared so that self-emulsifying regions could be identified. The phase diagram (Figure 1) exhibits self-emulsion regions. The mixture of surfactant and cosurfactant (Smix) ratio 1:2 of F9 demonstrated a larger self-emulsifying (micro emulsifying) area. The cloud point of the CLA-SEDDS was found to be higher than 70°C in all formulations, indicating that it produced a stable emulsion at physiological temperatures without the possibility of phase separation.
The % drug content of all SEDDS formulations was found to be within the acceptable limits of drug content test. The globule size distribution of various formulations is given in Figure 3A. An increase in the ratio of the oil phase resulted in a proportional increase in globule size, because of the simultaneous decrease in the S/CoS proportion. Increasing the S/CoS ratio led to a decrease in mean droplet size. The size of the globules for the developed formulations was found to be in the range of 80m to 170m. Formulation F9 developed with higher concentration of brij 58 and transcutol was found to be more stable and had requisite zeta potential of -47 mV. The compatibility studies were performed using FTIR; no interactions were detected between excipients and the CLA. All the SEDDS formulations showed faster drug release than the API. The formulation F9 had shown the highest and quick drug release after 60mins.
CONCLUSION
On the basis of the findings of the pseudo ternary phase diagram, in vitro drug release, droplet size, and other criteria, the formulation was determined to be the best. The current study clearly showed the use of SEDDS in improving dissolving rate and, as a result, oral bioavailability of weakly water-soluble medicines such as Clarithromycin without causing incompatibility between the constituents.
SUMMARY
The goal of this project is to develop SEDDS of Clarithromycin to improve the solubility in water thereby improve the bioavailability. The oral delivery of water insoluble drugs like Clarithromycin can be made possible by SEDDS, which have been showed to be substantially improving oral bioavailability with future development of this technology. Self-emulsifying drug delivery system, consisting of Tween 80, Brij 58 as surfactants and isopropyl alcohol, isobutanol, and transcutol EL as co-surfactants was formulated. The mixture of surfactant and cosurfactant (Smix) ratio 1:2 of F9 demonstrated a larger self-emulsifying (micro emulsifying) area. Established SEDDS showed high solubilisation capacity. Among all the batches developed the formulation F9 comprising of Brij 58 and Transcutol RH, had the highest drug release after 60 min. various formulations were prepared with varying ratios of O/Smix, amongst the formulation F9 showed promising results. However, further research is needed in animal models to improve pharmacokinetic and pharmacodynamic effectiveness.
ACKNOWLEDGMENT
The authors are thankful to the management of GIET School of Pharmacy and Dr. MGR Educational and Research Institute for providing all necessary facilities to carry out this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Pouton CW. Eur J Pharm Sci. 2000, 11: p: S93-108.
- Gursoy RN, Benita S. Biomed Pharmacother. 2004, 58(3): p. 173-182.
- Siya D, Sinai K, Shilpa P. Int J Pharm Sci Res. 2017, 8(1): p. 191-196.
- Yasemin Sahbaz, Tri-Hung Nguyen, et al, Mol Pharm. 2017, 14(11): p. 3669-3683.
- Hywel D, Williams, Yasemin S, et al. Chem Comm. 2014, 50: p. 1688-1690.
- Nicholas B, Hongxia W, Young S, et al. J Control Release. 2016, 10: p. 106-119.
- Rajan BM and Sheth NS. Int J Pharm Sci. 2011, 3(2): p. 23-28.
- Kshitija K, Swati M. Int J Pharm Sci Res. 2013, 4(12): p. 4494-4507.
- Mrunalini RS, Manish SK. Der Pharma Scinica. 2014, 5(5): p. 27-35.
- Nair AB, Shah J, Al-Dhubiab BE, et al. Pharmaceutics. 2021, 13(4): p. 523.
- Shilpi R, Derle D. Int j pharm biol Sci. 2014, 4(3): p. 479-494.
- Yosra SRE, Magda AEM, Ossama YA. Int J Pharm. 2009, 380: p. 33-41.
- Ashok RP, Pradeep RV. The AAPS Journal. 2007, 9(3): p. E344-E352.
- Chintalapudi R, Murthy T et al. Int J Pharm Investig. 2015, 5(4): p. 205-213.
- Ahmed A, Sandra K, Karsten M. Eur J Pharm Sci. 2008, 35(5): p. 457-464.
- Bediako A, Kuntworbe N. Int J Pharm Sci Res. 2020, 11(11): p. 5619-5632.
- Vishesh KP. J Pharm Res Opin. 2011, 1: p. 80-84.
- Rajendra C, Harish P. Int J Pharm Sci. 2011, 3, p. 147-152.
- Rajesh BN, Pushpa BS, Amrapali BJ. Int J Pharm Sci Rev Res. 2015, 33(1): p. 102-108.
- Ozbej Z, Julia R. Drug Dev Ind Pharm. 2017, 43(10): p. 1694-1702.
- Danielle VS, Jeanetta DP, Joe V. Pharmaceutics. 2020, 12: p. 1-24.
- Anna CK, Marta S. Molecules. 2015, 20(12): p. 21010–21022.
- Prabagar B, Beom JL. Int J Pharm. 2009, 374(1-2): p. 66-72.
Indexed at, Google scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref