Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 10
Molecular Interaction of Characteristic of Glyzifer at 308 K of Various Concentration by Ultra-Sonication Method
Balakrishnan J1*, Prakash N1 and Ekambaram S2
1Department of Chemistry, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, Tamil Nadu 602105, India
2Department of Chemistry, RKM Vivekananda College, Chennai, Tamil Nadu 600004, India
- *Corresponding Author:
- Balakrishnan J
Department of Chemistry
Saveetha School of Engineering
Saveetha Institute of Medical and Technical Sciences (SIMATS)
Chennai, Tamil Nadu 602105, India
Abstract
Acoustic parameters depiction of Glyzifer–Water system was carried out using ultra sonication, on different concentrations by molar mass by volume percentage [0.2%, 0.4%, 0.6%, 0.8% and 1.0%] at definite temperature of 308 K using ultrasonic interferometer. Limitations like sound property, flow property, mass-water ratio, adiabatic compressibility, Acoustic Impedance, maximum distance length, free bound time, Adsorption factor and molar compressibility are accounted and it is supportive for association of molecular interaction of binary mixture glyzifer–water system.
Keywords
Glyzifer, Ultra-sonication’s acoustic impedance, Adiabatic compressibility, Adsorption coefficient, Free length, Binary mixture
Introduction
Intermolecular contact plays a vital role in 20 and 30 liquid combination [1,2]. They impact the organization, alignment and conformation of molecule in solution. Sonication of liquid mixture have been used for qualitative solvation e of ranking of bonding link in fluid mixtures [3,4]. The everyday application of using mixed solvent rather than single solvent in engineering and biological processes in such a way that it provides a inclusive special of solvent-solvent mixture with necessary belongings [5-7]. The rates of reaction and stability of the intermediates formed, depends on the molecular contact of the medium. This usage of medicine glyzifer treats or prevents low plasma levels of for anemia or during gestation. Iron is an important mineral that the body needs to produce red blood cells and keep you in good health. Side effects of glyzifer primes abdominal related problems like pains, irregular stool or stomach upset may occur. Glyzifer is oral diabetic medicine which helps to control blood plasma, over doses leads may to hemochromatosis, hemosiderosis. Water, solvent with the combination of glyzifer with water at various concentration for the connection of molecular relations [8-15]. From this ultrasonic characterization comparable adiabatic compressibility, Intermolecular free length, acoustic impedance, relaxation time, wada constant and adsorption coefficient was intended. The values are plotted against concentration. The graph obtained is enlightened on the basis of various molecular interactions [16] contemporary in the system and how the relations are affected by the change of concentration at temperature 308 K.
Materials and Methods
Experimental part
Solvent water was purified by double distillation. The solution of glyzifer-water of altered concentration by weight by volume percentage is prepared at room temperature (308 K). All the concentrations of mixture are allowed to attain constant temperature in constant temperature bath, before carrying out the experiments. The masses scheming of solution were carried out using a pycnometer of bulb capacity of 10 ml. The pycnometer was standardized using double distilled water. The accuracy of masses was found to be +0.001 g/cc. The flow rate of binary mixture were determines using an Oswald’s Viscometer (sigma chemical instruments). The ultrasonic velocities of pure solvent and binary mixture were measured using a single crystal path interferometer at 2 MHz (Mittal Enterprises, New Delhi). The accuracy in ultrasonic velocity was found to be +0.05%. The temperature controlled bath of test liquid was maintained at an accuracy of +0.02 in an electrical controlled thermostat.
The solvent–solute binary mixtures are prepared by using weight by volume percentage by using jobs variation method. From the measured mass (density), Flow rate (viscosity) and sonication (ultrasonic velocity), the adiabatic compressibility, Intermolecular free length (Maximum free distance), relaxation time, Acoustic impedance, adsorption coefficient and Wada constant (Table 1).
| Concentration of Solution | Adiabatic Compressibility (β) × 10-7 | Acoustic Impedance | Free Length Lf × 10-10 | Relaxation time × 10-9 | Absorption Co-efficient × 10-11 | Wada constant |
|---|---|---|---|---|---|---|
| 0.2 | 8.1 | 1169 | 3.1 | 8.92 | 1.49 | 2941 |
| 0.4 | 6.1 | 1271 | 2.97 | 7.73 | 1.19 | 2942 |
| 0.6 | 4.5 | 1482 | 2.75 | 5.81 | 0.77 | 2931 |
| 0.8 | 3.6 | 1666 | 2.8 | 4.12 | 0.56 | 2922 |
| 1 | 2.8 | 1882 | 2.44 | 6.78 | 0.71 | 2911 |
Table 1: Determination of the different parameters for the association of molecular interaction of binary mixture glyzifer-water system
Results and Discussion
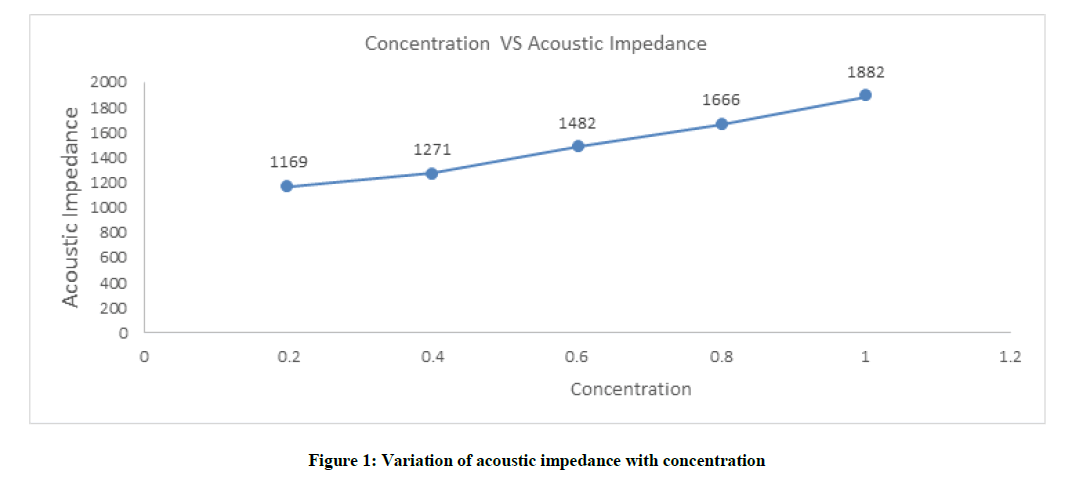
Acoustic impedance (Z)
Acoustic impedance increases with increases concentration of solutions the point at the low concentrations the values are gradual level, nonetheless increase sharply subsequently at higher concentrations, which is shown in Figure 1 and Table 1.
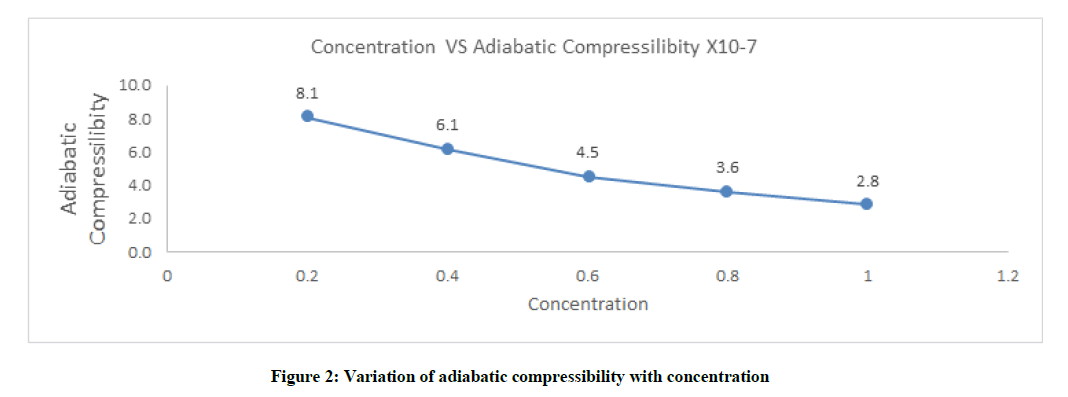
Adiabatic compressibility (β)
Adiabatic compressibility decreases with increases in concentration. This is due to the molecule occupies quantified the volume area. The decrease inclines with plodding as concentration increases which is shown in Figure 2 and Table 1.
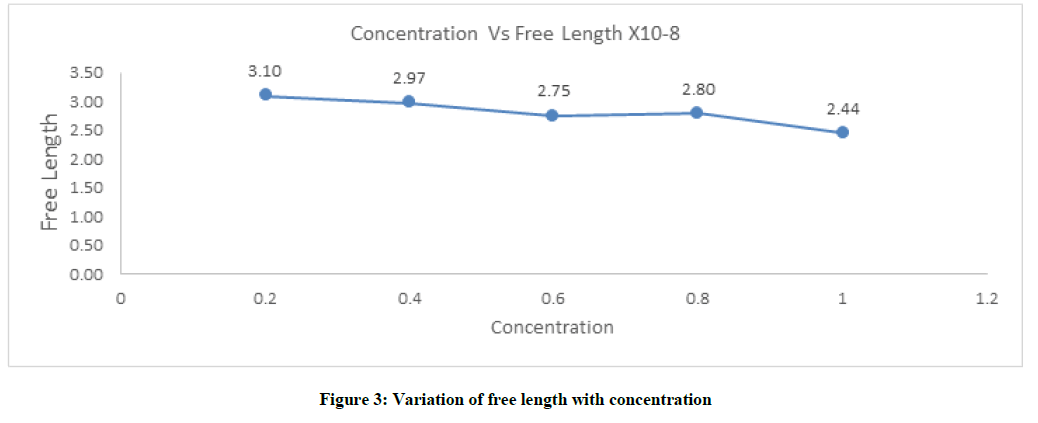
Intermolecular free length (Lf)
The intermolecular free length decreases and increase, then decrease as concentration of solution increases. The concentration at 0.8 section show notable increases due to available maximum distance between the solvent–solute concentrations which is shown in Figure 3 and Table 1.
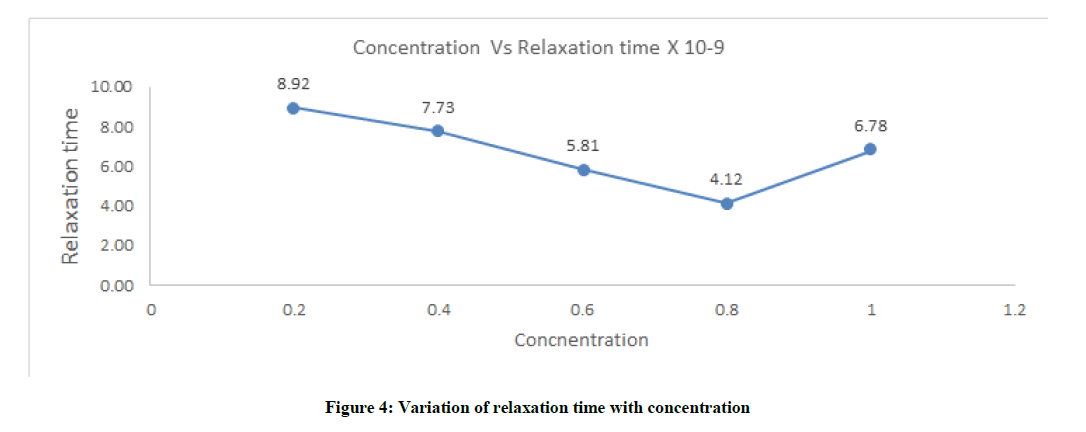
Relaxation time (τ)
Relaxation time decreases with increases with increases in concentration. It decreases sharply till 0.8 concentration segment and again increase very quickly, this is due the fact that the solvent-solute interaction is zero. The molecule has no oscillation at this 0.8 segment which is shown in Figure 4 and Table 1.
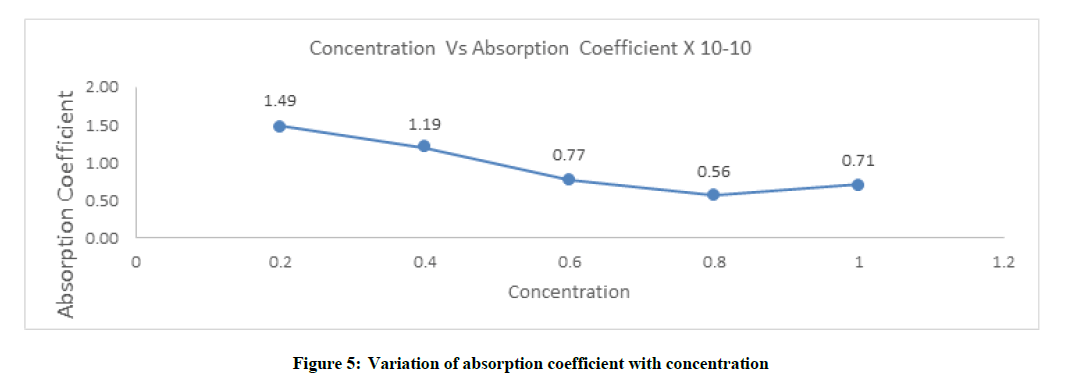
Absorption coefficient (α/f2)
Absorption coefficient also has same effect as relaxation time. It decreases with increases in concentration of solution which is shown in Figure 5 and Table 1.
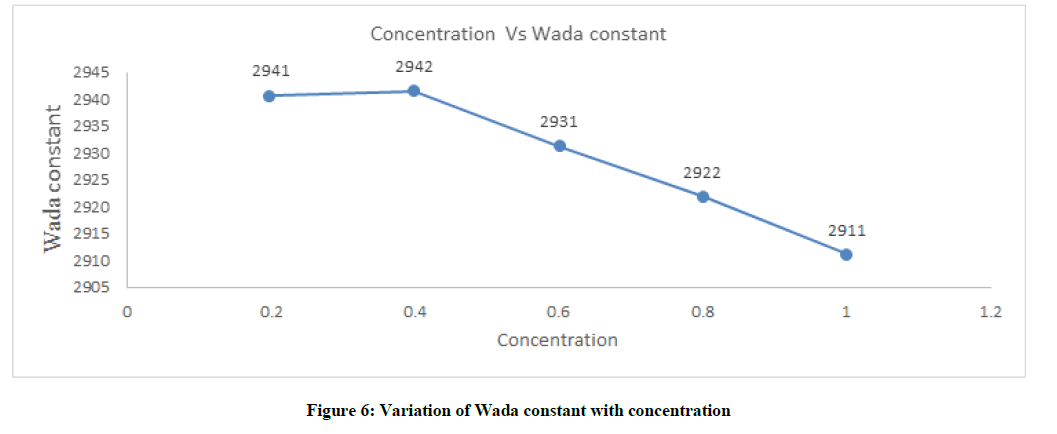
Molar compressibility or Wada’s constant (B)
The molecular compressibility or wada constant increases from 0.2 to 0.4 and decrease sharply towards the higher concentration. Molecules are free in lower concentration when concentration increases the molecule become closer the volume so the values are decreasing towards higher concentration which is shown in Figure 6 and Table 1.
Conclusion
The concentration plays a vital role in solvent-solute interaction between the glyzifer water system and it was found clearly in the parameter of Molar compressibility or Wada’s constant (B), Absorption coefficient (α/f2), Relaxation Time (τ) and Intermolecular Free Length (Lf). A strong molecular interaction seen in the glyzifer-water system.
Acknowledgement
I thank department of Chemistry, Management of Saveetha institute of Medical and Technical Sciences (SIMATS) for given me the opportunity, constant encouragement, support to do the work and make this article to be published.
References
- J. Balakrishnan, V. Balasubramanian, J. Chemical Pharmaceut. Res., 2012, 4(8).
- J. Balakrishnan, V. Balasubramanian, J. Chemical Pharmaceut. Res., 2012, 4(9).
- C.C. Kanagam, J. Balakrishnan, R. Thamarai selvan, Ind. J. Phys. chem., 2011, 6(1).
- J. Balakrishnan, G. Ramachandran, R. Rajeswari, V. Balasubramanian, J. Elixir App. Chem., 2013, 65, 19719-19723.
- J. Balakrishnan, S. Ekambaram, S. Rajesh, V. Balasubramanian, J. Elixir App. Chem., 2013, 65, 20063-20067.
- P.S. Nikam, T.R. Mahale, M. Hasan, J. Acoust. Acta., 1998, 84, 579.
- P.S. Nikam, M.C. Jadhav, M.J. Hasan, J. Molec. Liq., 1998, 76, 1.
- P.S. Nikam, T.R. Mahale, M. Hasan, Ind. J. Pure App. Phys., 1999, 37, 92.
- V. Kannappan, R. Jayasanthi, E.J.P. Malar, J. Phys.Chem. Liq., 2000, 40 133.
- O.P. Chimankar, R. Shiriwas, V.A. Tabhane, J. Chemical Pharmaceut. Res., 2011, 3(3), 587-596.
- S. Thirumaran, P. Anbuselvi, Asian J. Chem., 2009, 21(9), 7261.
- S. Thirumaran, D. George, J. Eng. App. Sci., 2008, 27(2), 281.
- S. Thirumaran, K. Ramya, Asian J. Chem., 2009, 21(8), 6359.
- S. Thirumaran, J.E. Jayakumar, Ind. J. Pure App. Phys., 2009, 47, 265.
- S.R. Aswale, S.S. Aswale, A.B. Dhote, J. Chemical Pharmaceut. Res., 2011, 3(6), 233-237.
- K. Sreekanth, D. Sravanakumar, M. Kondaiah, J. Chemical Pharmaceut. Res., 2011, 3, 229-241.