Research Article - Der Pharma Chemica ( 2018) Volume 0, Issue 0
Microwave Assisted Synthesis, Physicochemical & Spectral Characterization of Common Molecules
Ambatkar MP1*, Rathi LG2, Rokde VV1, Danao KR1 and Mahajan UN1
1Dadasaheb Balpande College of Pharmacy, Besa, Nagpur (MS), India
2Institute of Pharmaceutical Education and Research, Borgaon (Meghe), Wardha (MS), India
Abstract
Some common molecules like antipyrine and toluene-p-sulphonamide have been synthesized by Microwave Assisted method i.e. “Green Synthesis”. The reaction yields were compared with reported conventional method. The purpose of this study was increasing practical yield, lowering the reaction time and reducing pollution. The products were characterized by various techniques like melting point, Thin Layer Chromatography (TLC), partition coefficients, dissociation constants, % ionization, Fourier Transform Infrared (FTIR) spectroscopy, Mass and Proton Nuclear Magnetic Resonance (1H-NMR) spectroscopy.
Keywords
Synthesis, Antipyrine, Toluene-p-sulphonamide, Microwave
Introduction
Synthesis of organic and pharmaceutical compounds require more than 2-3 h heating for completion of reaction. So, there is no sufficient time for purification, drying and evaluation of the synthesized product. Thus, new technique was developed for synthesis i.e. microwave assisted organic synthesis which completed the reaction in minutes only. Microwaves are electromagnetic waves containing electric and magnetic field components. The electric field applies a force on charged particles as a result of which the charged particles start to migrate or rotate. Due to movement of charged particles further polarization of polar particles takes place. This method is beneficial because of several advantages including high percentage practical yields, reduced reaction time and reduced pollution [1-3]. Keeping this in view, the newly developed microwave assisted method is applied for synthesis of antipyrine and toluene-p-sulphonamide. In this paper, synthesized products were characterized by the physico-chemical studies like melting point, TLC, partition coefficients, dissociation constants and % ionization. The structures of synthesized compounds were confirmed by Ultraviolet (UV), Fourier Transform Infrared (FTIR) spectroscopy, Mass and Nuclear magnetic resonance (NMR) spectroscopy [4-9].
Materials and Methods
All the reagents were of analytical purity, and were used without further purification. CATA’s Scientific Microwave Synthesis System was used in our experiments. A refluxing system was connected with the microwave oven. DBK programmed melting point apparatus was used for determination of melting point. The purification of synthesized products was done by thin layer chromatography. Shimadzu FT-IR - 8400 S using KBr pellets in the region of 4000-500 cm-1 was used for recording IR spectrum. Bruker Avaze II (400 MHz) and QP- 2010 PLUS GC-MS were used for NMR and Mass spectrum respectively.
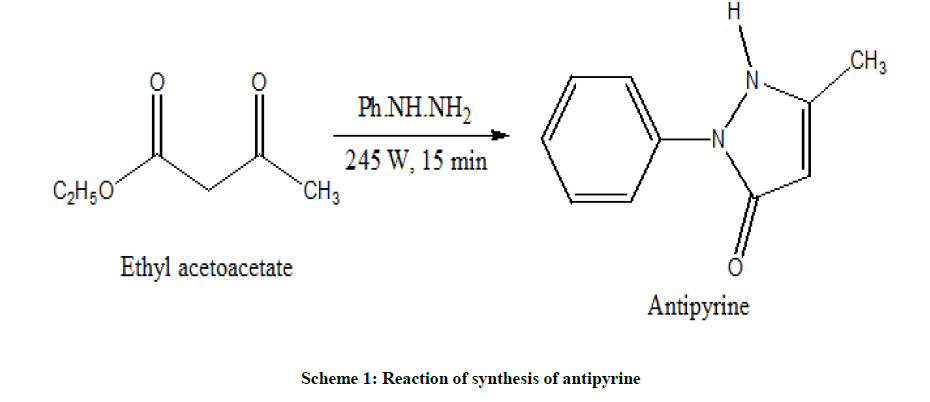
Synthesis of antipyrine
The mixture of 0.5 ml of ethyl acetoacetate and 0.3 ml of phenylhydrazine was refluxed for 15 min in microwave oven (power input: 245 W, 3 P). The reddish syrup was cooled and 0.9 ml ether was added. The syrup was solidified within 15 min. The product was filtered off and washed with ether to remove coloured impurities. The product was recrystallized from a mixture of ethanol and water [10]. The product was purified by thin layer chromatography using chloroform: ethyl acetate (8:2) (Scheme 1).
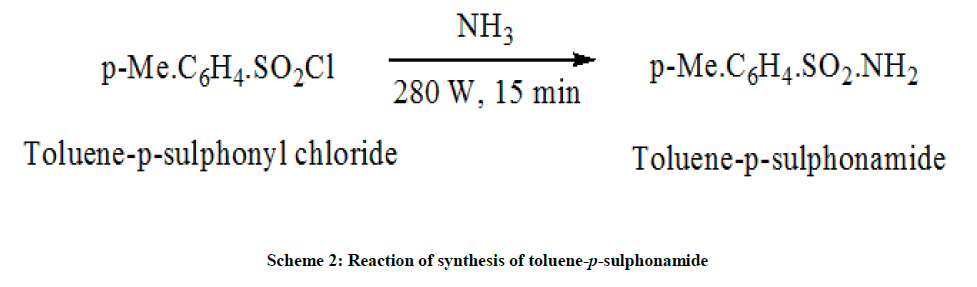
Synthesis of toluene-p-sulphonamide
The mixture of fine uniform powder of toluene-p-sulphonyl chloride (0.632 g) and 1.27 g of ammonium carbonate was refluxed by microwave irradiation in oven for 15 min (Power input: 280 W, 4 P). The mixture was cooled and extracted with a little cold water to remove the excess of ammonium salts. The recrystallization was done from hot water [10]. The purity of product was checked by thin layer chromatography using Chloroform: Ethyl acetate (7:3) (Scheme 2).
Results and Discussion
Table 1 shows reaction time taken by microwave irradiation and conventional method, the compounds were obtained in good yields, also the compounds were compared for spectral characterization.
| Synthesized compound | Conventional method [10] | Microwave method | |||
|---|---|---|---|---|---|
| Time (min) | Yield (%) | Time (min) | Output (Watt) | Yield (%) | |
| Antipyrine | 120 | 80 | 15 | 245 | 87.29 |
| Toluene-p-sulphonamide | 120 | 88 | 25 | 280 | 95.78 |
Table 1: Reaction time and yield (%) of products by conventional and MWI method
Characterization
The structure of antipyrine and toluene-p-sulphonamide have been established on the basis of IR, NMR and Mass spectral data. The spectral characterization of the compounds has been given as below:
Antipyrine: IR (KBr) (cm-1): 3100 cm-1 (SP2 C-H stretching), 1785.96 cm-1 (C=O stretching of cyclic ketone), 2900 cm-1 (aliphatic C-H stretching), 3400 (w) & 1525.59 cm-1 (N-H stretching & def. respectively). GC-MS (m/z)=174 (M+); 1H-NMR (400 MHz, CDCl3): δ=2.20 (s, 3H), 3.43 (s, 1H), 7.26 (s, 1H), 7.37-7.41 (m, Ar-H), 7.84-7.86 (m, Ar-H).
Toluene-p-sulphonamide: IR (KBr) (cm-1): 3326.98 & 3240.19 cm-1 (asymmetric & symmetric N-H stretch), 3050 & 1596.95 cm-1 (aromatic C-H & C=C stretching respectively), 2950-2900 cm-1 (Aliphatic C-H stretch), 1325.01 & 1151 cm-1 (Anti & sym S=O stretching). GC-MS (m/z)=171 (M+); 1H-NMR (400 MHz, CDCl3): δ=7.45-7.47 (m, J=1.96, Ar-H), 7.34-7.36 (m, J=3.00, Ar-H), 7.25 (s, 2H of NH2), 1.25 (s, 3H of CH3).
Table 2 shows melting point, Rf value [11-13] and physicochemical parameter of antipyrine and toluene-p-sulphonamide.
| Compound | Molecular formula (Mol. Wt.) | Nature | Melting point (°C) | Rf value | Partition coefficient [12] (P) |
Dissociation constant [13] (pKa) |
% Ionization |
|---|---|---|---|---|---|---|---|
| Antipyrine | C10H10N2O (174) | Yellow crystals | 126-129 | 0.50 | 4.63 | 1.61 | 20.45% |
| Toluene-p-sulphonamide | C7H9NO2S (171) | White crystals | 137-140 | 0.63 | 3.81 | 5.65 | 12.36% |
Table 2: Physicochemical data of synthesized products
Conclusion
In summary, a microwave assisted organic synthesis was developed which was beneficial because of several advantages including high percentage practical yields, reduced reaction time and reduced pollution. antipyrine and toluene-p-sulphonamide have successfully synthesized with this hydrothermal procedure. All the synthesized compounds were subjected to physico-chemical and spectral analysis. In conclusion, microwave technique was successfully developed for routine practical classes for UG students in chemistry laboratories.
References
- E.J. Corey, X.M. Cheng, The Logic of Chemical Synthesis, Wiley Interscience Publication, New York, 1995, 361.
- G. Thomas, Medicinal Chemistry-An Introduction, John Wiley and Sons Ltd, Chichester, England, 2004, 69.
- A. Martin, Int. Res. J. Pharm., 2014, 11(4), 64-66.
- A.G. Nerkar, D. Pawale, M.R. Ghante, S.D. Sawant, Int. J. Pharm. Pharm. Sci., 2013, 564-566.
- R.B. Silverman, The Organic Chemistry of Drug Design and Drug Action, Elsevier Academic Press, Berlington, USA; 2001, 78-85.
- R.P. Gore, Der Pharma Chemica.,2014, 6(6), 35.
- B.S. Sekhon, Int. J. Pharm. Res., 2010, 2(1), 827-833.
- M. Lindell. A Master’s thesis- Modeling of a system for microwave assisted organic synthesis. Royal Institute of Technology, Department of Signals, Sensors and Systems: Stockholm, 2003, 7-9.
- S. Badami, A. Minimathew, S. Thomas, B. Suresh, Ind. J. Pharm. Edu. Res., 2003, 37(4), 199-203.
- B.S. Furniss, A.J. Hannaford, P.W.G. Smith, A.R. Tatchell, Vogel’s Textbook of Practical Organic Chemistry, Pearson education, 2003, 882, 897, 912, 1046, 1068, 1150.
- G.R. Chatwal, S.K. Anand, Instrumental Methods of Chemical Analysis, 2004, 2.599-2.600.
- A. Pyka, M. Babuska, M. Zachariasz, Acta Poloniae Pharmaceutica- Drug Research., 2006, 63(3), 159-167.
- D. Biplab, J.K. Gupta, Ind. J. Pharm. Edu. Res., 2003, 37(2), 100-104.