Short Communication - Der Pharma Chemica ( 2022) Volume 14, Issue 2
Microwave Assisted, Fly Ash Catalyzed Synthesis of Coumarin Derivatives: Green Approach
Madhav J Hebade, Ravindra P Shimpi and Omprakash S Chavan*Omprakash S Chavan, Department of Chemistry, Badrinarayan Barwale College, Jalna-431213, Maharashtra, India, Email: omprakashschavan@gmail.com
Received: 31-Jan-2022, Manuscript No. dpc-22-52916; Accepted Date: Feb 02, 2022 ; Editor assigned: 02-Feb-2022, Pre QC No. dpc-22-52916; Reviewed: 18-Feb-2022, QC No. dpc-22-52916; Revised: 24-Feb-2022, Manuscript No. dpc-22-52916; Published: 03-Mar-2022
Abstract
Solvent free synthesis of substituted Coumarin by Von Pechmann condensation of phenols with β-ketoesters catalyzed by fly ash as a byproduct from thermal power station by microwave irradiation method. Our present protocol is economical and clean comprise of green reagent, solvent and catalyst.
Keywords
Pechmann Condensation; Coumarin; MWI; Green Synthesis
Introduction
Coumarins [1] and their various derivatives has attracted considerable attention of medicinal and organic chemist from many years, due to its large number of pharmaceutical activities like anti-bacterial [2], anti-cancer [3], anti-coagulant, anthelmintic, hypnotic, optical brighteners [4], antiinflammatory and anti-HIV activities [5].
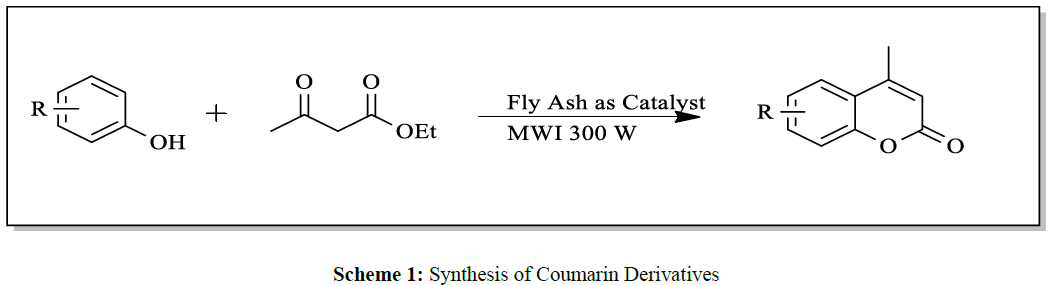
The representative synthetic routes of Coumarin and its derivatives include Pechmann [6], knoevenagel [7], Perkin [8], Reformatsky [9] and Wittig [10] condensation reactions. Among these, Pechmann condensation is one of the most widely used method for synthesis of Coumarin. Acid catalyst have been used in the Pechmann [6] reaction including use of simple starting materials that is phenol and β-ketoesters in the presence of variety of acidic agents, such as chlorosulfonic acid [11], Sulfuric acid [6], melamine formaldehyde resin supported H+ ion catalyzed [12], ionic liquid catalyzed [13], oxalic acid catalyzed [14], silica triflate catalyzed, heterogeneous catalyst, zirconia supported catalyst etc (Scheme 1).
Fly ash is mainly consist of the components of vary considerably, but all fly ash includes substantial amounts of silicon dioxide (SiO2) (both amorphous and crystalline), aluminium oxide (Al2O3) and calcium oxide (CaO), the main mineral compounds in coal-bearing rock. It has found very vast applications in many reactions such as, rearrangement reaction, condensation reaction, usually acts as strong Lewis acid catalyst and dehydrating agent [15-18] (Table 1 and 2).
| Substrate | Product | Time in Sec. | M. P. in °C | Yielda (%) | |
| Obs. | Lit | ||||
|
 |
60 | 184-86 | 185[14] | 98 |
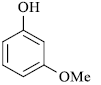 |
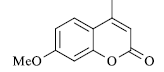 |
60 | 158-60 | 161[14] | 95 |
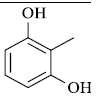 |
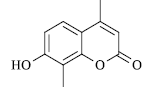 |
60 | 138-39 | 138[14] | 90 |
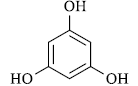 |
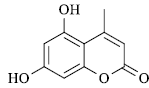 |
60 | 285-86 | 285[14] | 90 |
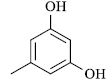 |
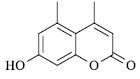 |
60 | 257-58 | 258[14] | 92 |
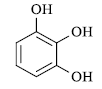 |
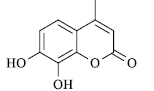 |
80 | 235-36 | 237[14] | 89 |
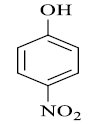 |
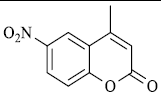 |
110 | 147-49 | 150[14] | 72 |
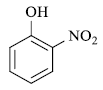 |
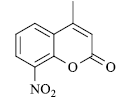 |
100 | 183-184 | 185[14] | 79 |
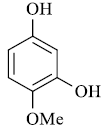 |
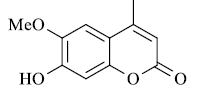 |
90 | 164-165 | 165[14] | 91 |
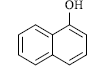 |
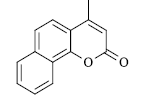 |
120 | 156-158 | 155[14] | 87 |
| aIsolated Yield | |||||
| Entry | Catalyst | Mol % | Yielda |
| 1 | Fly Ash | 0 | -- |
| 2 | Fly Ash | 5 | stress |
| 3 | Fly Ash | 10 | 40% |
| 4 | Fly Ash | 15 | 59% |
| 5 | Fly Ash | 20 | 98% |
| 6 | Fly Ash | 25 | 93% |
| 7 | Fly Ash | 30 | 88% |
| bReaction Condition: Resorcinol (10mmol), Ethyl acetoacetate (10mmol) and fly ash (20 mol %), MWI 300W aIsolated Yield | |||
Experimental
General experimental procedure for synthesis of 7-hydroxy-4-methylcoumarins
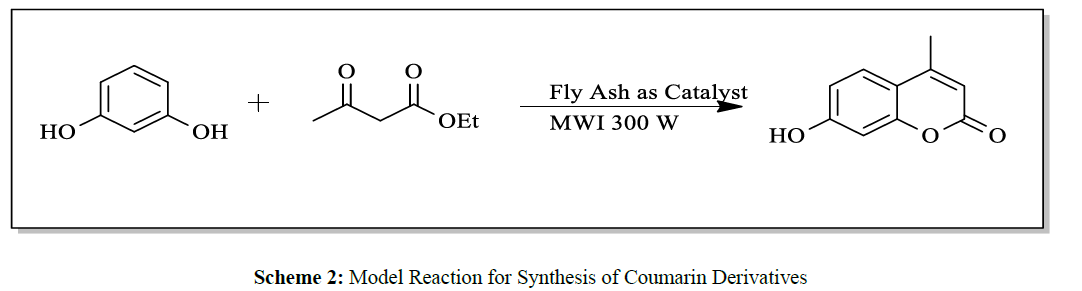
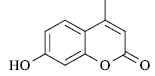
A mixture of resorcinol (10mmol), ethyl acetoacetate (10mmol) and fly ash (20 mol %) were subjected to microwave irradiation at 300W for appropriate time (Table 1). After completion of reaction, as monitor by TLC, the reaction mixture was cooled to room temperature, water was added and stirred for another two minutes, and precipitation was filtered off and recrystallized from methanol to afford pure 7-hydroxy-4- methylcoumarins as yellowish prism.
7-hydroxy-4-methylcoumarins
yield 98 %, mp 184-186 °C. 1H NMR (CDCl3) δ: 2.2 (s, 3H, Me), 6.1 (s, 1H), 6.83 (d, 1H, J 2.4 Hz), 6.97 (dd, 1H, J 8.7 and 2.4 Hz), 7.5 (d, 1H, J 8.7 Hz). IR (KBr, ν/cm-1): 2985, 1740, 1625. ES/MS, m/z: 175 (M-H).
Results and Discussions
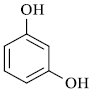
In summary, it can be stated that, the present protocol for synthesis of Coumarin by Pechmann condensation is highly efficient as it avoid use of organic solvents at any stage of reaction, under microwave irradiation technique at very low power (300W) and presence of fly ash as a byproduct from thermal power station as a catalyst (Scheme 2).
A mixture of substituted phenols and ethyl acetoacetate was subjected to microwave irradiation of very low power (300W) in presence of fly ash under solvent free condition (Scheme 1). The Progress of reaction was checked by chromatography (TLC). Optimization of reaction condition was achieved by using varying amounts of fly ash catalyst and best results of yields could be obtained by using 20 mol % of catalyst (Table 2).
Conclusion
Herein, we report the Pechmann condensation reaction of phenols and β-ketoesters using fly ash as a simple, efficient, easily available as a catalyst under microwave irradiation method and solvent free condition (Scheme 1). We carried out a series of substituted phenols with ethyl acetoacetate to obtain corresponding Coumarin derivatives in very good to best yield (Table 1).
Acknowledgements
Authors are thankful to the Principal, Badrinarayan Barwale College, Jalna for constant encouragement and providing necessary facilities for this work.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Kennedy RO and Thornes RD. Coumarins: Biology, Applications and Mode of action. 1997.
- Sahoo J, Mekap SK, Kumar PS et al., Uni for Sci. 2015, 9(2): p. 187-195.
- Basanagouda M, Jambagi VB, Nivedita N et al., Euro J of Med Chem. 2014, 74: p. 225-233.
- Naik MA, Mishra BG and Dubey A. Physicochem & Eng Aspects. 2008, 317: p. 234-238.
- Li Huang, Xiong Yuan, Donglei Yu et al., Virology. 2005, 332(2): p. 623-628.
- Von Pechmann H and Duisberg C. Chem Tech Ber. 1884, 17: p. 927-928.
- Adams R and Bockstahler TE. J Am Chem Soc. 1952, 74(21): p. 5346-5348.
- Johnson JR. Org React. 1942, 1: p. 210-210.
- Shirner RL. Org React. 1942, 1: p. 1-2
- Yavari I, Hekmat-shoar R and Zonouzi A. Tet Lett. 1998, 39(16): p. 2391-2392.
- Kotharkar SA, Bahekar SS and Shinde DB. Mendeleev Comm. 2006, 16(4): p. 241-242
- Rezaei R, Dorosty L, Rajabzadeh M et al., Chinese Chem Lett. 2011, 22: p. 1313- 1316.
- Das S, Majee A and Hajra A. Green Chem Lett & Review. 2011, 4(4): p. 349-353.
- Kokare ND, Sanghshetti JN and Shinde DB. Chinese Chem Lett. 2007, 18: p. 1309-1312.
- Kapade MM, Gawhale ND and Awjare NV. Ind J Chem. 2015, 54B: p. 671-675.
- Wang NN, Hu Q, Hao LL et al., Inter J Enviro Sci and Tech. 2019, 16: p. 89-100.
- Goel V. Orient J Chem. 2012, 28(4): p. 1725-1728.
- Dhokte AO, Khillare SL, Lande MK et al., J Korean Chem Soc. 2011, 55(3): p. 430-435.
Indexed at, Google Scholar, Crossref