Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
Isolation and Identification of Metal Lead (Pb) Ressistent Microalgae on Branckish Water at Muaro Panjalinan Tabing, Padang, West Sumatera
Monaliza, Abdi Dharma and Zulkarnain Chaidir
Abstract
This research was done to obtain microalgae isolate which comes from West Sumatera that is Metal Lead (Pb) ressistent and knowing the efficiency and absorption capacity of Pb Metal from Microalgae Isolate. The microalgae sample was taken from branckish water in Muara Panjalinan, Padang, West Sumatera. Isolation was done by using 25 mg/L Pb metal. The isolate identification was done microscopically and mollecularly. On molleculare identification, 18S rRNA was amplified by using PCR with primary: forward (5’-CCTGGTTGATCCTGCCAG-3’) and reverse (5’-TTGATCCTTCTGCAGGTTCA-3’). The Pb absorption efficiency and capacity was analyzed by using Atomic Absorption Spectrophotometer (AAS). The growth of microalgae base on absorption value from spectrophotometer equipment. Based on the result of this research, can be concluded that in Branckish Water at Muara Panjalinan, Padang, West Sumatera, Ochromonas vasocystis microalgae that is ressistent towards heavy metal (Pb 25 mg/L) exists. Based on morphology and mollecular data, the isolate that was isolated can be identified as Ochromonas vasocystis and during the treatment of Pb metal towards microalgae isolated culture, cause the decrease of cell density which inhibit the growth of cell. Beside, absorption capacity and efficiency of metal tends to increase along with the increase of Pb initial concentration. Maximum absorption capacity and efficiency happens at the incubation time of 360 min.
Keywords
Microalgae, Heavy metal, Lead (Pb)
Introduction
The development of Industry and the increase of population lead to the increase of waste. If the waste is not managed properly, this can cause contamination that endangered living creature. One of the wastes that are resulted from Industry is Pb metal. If Pb metal is accumulated inside human body, this can cause intoxication. Because of that, a way to decrease that pollution is needed to be done.
Generally, industrial watste contains heavy metal with different concentration. Low concentration in ion form will be beneficial for living creature. But in high concentration, the metal ion will be dangerous. In high concentration, pollutant in form of metal ion will be difficult to be degraded [1].
Research to deal with pollution that is caused by heavy metal has been done many times and was based on ecofriendly attitude. Some examples that have often been researched and used is bacteria, fungi, and part of plants like skin and fruit also low staged plant such as microalgae [2]. The treatment of metal waste by using microalgae is much needed to avoid the bad impact towards living creature around the metal polluted environment.
Microalgae have a lotof benefit in many sectors, especially in energy, food, and environmental sector. One of the benefits of microalgae that can be used is its ability as biosorbent or metal absorbent. Some species of microalgae that is known as metal absorbent is Spyrogyra [3] and [4] Oudogonium urceolatum [5], Dunaliella [6], Chlorella marina [7], Spirulina and Chlorella [8].
When microalgae absorb the heavy metal, change happens on the whole metabolism system. That is why Microalgae is also often used as biocensor to detect the toxic effect of heavy metal [9]. The toxicity of heavy metal can cause: a) The inhibition of biologically important functional groups; b) The transfer or changing of essential metal ions from functional biomolleculars and units of cell and c) Cellular indcution from reactive oxygen species (ROS). High level of ROS will cause oxidation of protein, lipid, and nucleic acid. This high concentration level of ROS can trigger modification and deactivation of enzymes along with the breakdown of cell and cell organel membrane [10]. In this case, Kation will interact with residu of organic substance with negative charge to form complex substance as in the Figure 1.
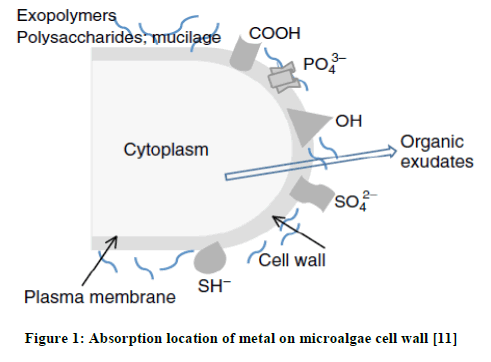
There are a lot of microalgae in Indonesia’s waterbecause most of the area of Indonesia consists of water, be it land water or ocean water. Based on that, writer is interested to do the research of microlagae from branckish water at Muaro panjalinan, Tabing, Padang, West Sumatera. The isolated microalgae willl be identified morphologically and continued by species determination process by using Polymerase Chaine Reaction Methods (PCR).
The goal of this research is to isolate and eliminate microalgae species that is Lead Metal ressistent (Pb) with high concentration from branckish water at Muaro Panjalinan, Padang City, identify the microalgae species that is isolated and screened microscopically and mollecularly, analyze the influence of metal towards the growth of screened microalgae species and to analyze the absorption efficiency and capacity of Metal Pb from the screened microalage isolate.
Materials and Methods
Equipments and materials
Equipments which were Net Planton, Analytic scake, Nikon binoculer microscopic E200, incubatir, autoclave, test tube, double beem spectrophotometer, Polymerase Chain Reaction (PCR) tools, Laminar air flow, Atomic Absorption Spectrophotometer (AAS), Electrophorethor (BIO – RAD), pipette, and other glass tools.
Material are Bolt Bassal’s medium (BBM), which are NaNO3, CaCl2.2H2O, KH2PO4, MgSO4.7H2O, NaCl, PCR, Kit, Primary microalgae mollecular identification, DNA Purification kit, Pb(NO3)2, HNO3, aquabides.
Sampling method
Microalgae sample were taken from branckish water at Muaro Panjalinan Tabing, Padang, West Sumatera by using plankton net with the hole sized of 30 micron and were done at good weather situation (not raining). In purpose so the microalgae that was obtained actually belong to that particular branckish water.
Breeding and observation of microalgae
The branckish water sample that contains microalgae was strained by usint plankton net, culturized into a tube that is filled with microalgae vegetation media. The growth media that was used is Bolt Basal Medium (BBM). Microalgae were grown for about 4–7 days on that medium for later be observed by using microscope E200 with magnification up to 100x. The species of microalgaed that is contained in the sample were observed and identified.
Screening and isolation of lead (Pb) ressistent microalgae
Microalgae that was grown and observed were screened by using Lead Metal in form of ion with the concentration of 25 mg/L. The goal of this screening is to obtain the microalgae species that can resist Lead Metal. This observation was done for 30 days until the resistent microalgae species were obtained. The resistent microalga after being screened by lead, later was isolated and transferred to a new without lead growth medium [12].
Observation and culture process of isolate microalgae species
Lead screened Isolate microalgae species was grown into a tube that contains the BBM growth medium. The microalgae cell growth is observed daily until 5-7 days. After the culture of light green colored microalgae, the isolate was sub-cultured into a new place that is bigger like a glass bottle that is filled with BBM medium growth with the volume of 100 up to 500 mL, with the comparison between microalgae and medium (1:9)
Identification of microalgae molleculare isolate
The microalgae isolate that was isolated was identified on sequence of 18S rRNA. DNA isolation will be done by using QIAamp DNA Blood Mini Kit (QIA-GEN K.K, Tokyo, Japan). 18S rRNA was amplified using PCR with primary: primary forward (5’-CCTGGTTGATCCTGCCAG-3’) and primary reverse (5’-TTGATCCTTCTGCAGGTTCA-3’). The PCR product will be electrophoresified by agarose 1%. The DNA ribbon was extracted by using NucleoSpin® GEL and PCR clean-up kit. Direct sequencing was directly done towards the DNA from electrophoresis result.
Measuring of optic density of microalgae velocity growth
Pb metal solution that were measured with concentration of 0,5, 10, 15, 20, 25 and 30 mg/L was provided about 100 ml. 25 mL of microalgae culture were added to metal solution that had been provided. The solution was incubated and the growth velocity was measured by using optic denisity method. Growth velocity was measured by using spectrophotometer UV-VIS tools. To measure the growth velocity through optic density method, optimation of microalgae sample absorbant is needed to use as the basic to determine the wave length that will be used. The measurement was done every day until the growth of microalgae reached the stationer or death phase.
Microalgae isolate biosorption towards metal Pb ion
Pb metal solution that were measured with concentration of 0, 5, 10, 15, 20, 25 and 30 mg/L was provided about 100 ml. 25 mL of microalgae culture were added to metal solution that had been provided. Each solution was incubated for 30 min, 60 min, 120 min and 6 hours. After being incubated, the suspension was strained and the filtrate was taken to measure the absorption capacity using Atomic Absorption Spectrophotometer (AAS) tools. To count the absorption of metal and the metal absorption efficiency of Microalgae, we use the formula:
a. Metal absorption capacity
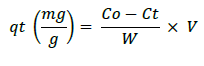
b. Metal absorption Efficiency
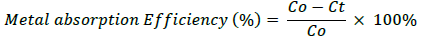
Explanation:
qt=Metal absorption capacity
W=Biosorbent mass ( g)
Co=Initial metal concentration (mg/L)
Ct=Residual metal concentration (mg/L)
V=Volume (L)
Conclusion
From the research that was conducted, can be concluded that 1). at the branckish water at muara panjalinan padang city west sumatera, exists microalgae that can survive the heavy metal treatment (Pb 25 mg/L), 2). Morphologically and mollecularly, the isolate that was isolated can be identified as Ochromonas vasocystis, 3). The Pb treatment towards microalgae isolate culture causes pb to inhibit the growth of microalgae and the low density of cell, which cause the inhibition of cell growth, 4). The capacity and efficiency absorption tends to increase along the increase of Pb initial concentration. The maximum capacity and efficiency absorption of Pb happened during the incubation time of 360 min.
References
[1] H.V. Perales-Vela, J.M. Pena-Castro, R.O. Canizares-Villanueva, Chemosphere., 2006, 64, 1-10.
[2] J.W. Forster, Taylor & Francis e-Library., 2003, 1, 221-225.
[3] B.R. Abbas Rezaee, F. Ganati, Majid, A. Solimanian, J. Biol. Sci., 2006, 6, 695-700.
[4] J.I.N.K.A.C. Oommen, J. Environ. Biol., 2002, 33, 27-31.
[5] M.F.N. Atif Yaqub, K. Mahmood Anjum, N. Mazhar Ali, W. Ahmad Khan, Z. Iqbal Khan, Biologia., 2009, S99-S109.
[6] S.R. Saber Imani, Z. Medrdad Hashemi, H. Borna, J. Med. Plant. Res., 2011, 5 (13), 2775-2780.
[7] K.S. Dinesh, Int. J. Fish. Aquacul., 2014, 6, 1-8.
[8] G.K.S. Khan, Int. J. Environ. Eng. Manag., 2013, 4, 573-580.
[9] D. Claude, G.H. Andriy, C. Jean-Marc, 2007, Lyon, France.
[10] S.S. Sharma, K.J. Dietz, Trends. Plant. Sci., 2009, 14, 43-50.
[11] D. Kaplan, 2013, 602-611.
[12] H.B. Swale, 1982, Institute of Terrestrial Ecology, Los Angeles.
[13] A.A. Zamani, R. Shokri, M.R. Yaftian, A.H. Parizanganeh, Int. J. Environ. Sci. Technol., 2012, 10, 93-102.
[14] G.W. Prescott, Brown Company Publisher, 2002.
[15] Serediak Algae Identification, Agriculture and Agrifood Canada Ottawa, Ontario, 2006.
[16] http://www.algalweb.net/



