Research Article - Der Pharma Chemica ( 2019) Volume 11, Issue 3
Isolation and Characterization of Microcrystalline Cellulose from Papaya Stem
Nwajiobi CC*, Otaigbe JOE and Oriji ONwajiobi CC, Department of Pure and Industrial Chemistry, University of Port Harcourt, Nigeria, Tel: +2348034341173, Email: Cnwajiobi@yahoo.com
Abstract
Microcrystalline cellulose (MCC) was isolated from papaya stem, characterized and compared with a commercial brand MCC. The dried pulverized papaya stem was subjected to alkaline treatment using aqueous NaOH and NaOCl solutions. Alpha cellulose obtained was modified by partial hydrolysis using 2.5 M HCl at boiling temperature to produce microcrystalline cellulose. The Physicochemical properties such as bulk, tapped and true densities, degree of polymerization, particle sizes, moisture content, angle of repose etc were examined and compared with a commercial brand sample (C-MCC). Results have shown that the percentage yields of alpha cellulose and CU-MCC obtained from the starting dried stem material were 23.78 and 20.70, respectively. Fourier Transform Infrared (FT-IR) spectra of CU-MCC and C-MCC confirmed the presence of OH and (C=O) carbonyl functional group which are the main characteristics of cellulose. Scanning Electron Microscopy (SEM) showed individual rod-like and flat-shaped plank-like fiber structures with few bundled crystal packed forms for the CU-MCC and C-MCC respectively. The C-MCC has a high thermal stability at 408°C when compared to 367°C of the hydrolysed CU-MCC. This may be resulting from the morphological characteristics of the fiber structures. CU-MCC had poor powder flow properties and an appreciable thermal resistance.
Keywords
Cellulose, Characterization, Microcrystalline cellulose, SEM, FT-IR
Introduction
The amount of agricultural wastes generated in the African region annually demonstrates the inadequacies of various African countries to follow global trend of recycling [1]. Adeyi reported that large quantities of wastes produced in Nigeria are underutilized yearly [2]. The burning of agro-wastes and the inappropriate disposal of wastes (such as open dumping) are some of the regular practices of waste disposal and management in Nigeria. Previous studies have shown that burning of agro-wastes reduce crop yield [3], causes climate change and health complications mainly respiratory diseases such as bronchitis, eye irritation, asthma etc. [4]. The indiscriminate dumping of such waste also creates an unsightly and unaesthetic environment.
Papaya scientifically recognized as Carica papaya L, in the family Caricaceae [5], is a fast growing herbaceous, semi-succulent plant with a self-supporting stem which branches only when injured [6,7]. It is a big tree-like plant, having spirally arranged leaves confined at the uppermost part of the trunk and mostly a single stem of about 5-15 meters tall. Papaya leaves are 50-70 cm large in diameter and palmately lobed with 7 lobes. The large fruits maturing into 15 to 45 cm long and 10 to 30 cm diameter appear on the axils of the leaves [8]. Previous study has shown that the lignin, cellulose and hemicellulose content of papaya bast fiber (plantation and green house) are approximately 20%, 53% and 29%, respectively [9]. Papaya stem can be an interesting source of fiber for the production of cellulose if processed further instead of its disposal as waste, since the plants are normally replaced after 3-5 yrs when the fruit yield is said to be at maximum in the prime third year. At that point, the fiber material is said to be available in large scale and further processing into microcrystalline cellulose can be a great source of income to the producer.
Microcrystalline cellulose (MCC) is one of the natural polymers that have different functionalities. It is obtained from a specialized grade of alpha cellulose in its purified form by the process of acid hydrolysis or action of enzymes. Many of its vast applications include; pharmaceutical, cosmetics, food, cosmetics etc. Furthermore, the usefulness has also been extended in the making of paint, paper and nonwoven textiles, oil field services, medicine and composites due its properties such as high strength, aspect ratio and flexibility [10]. MCC is one of the few materials that has the capacity to perform dual functions of producing adequately hard and rapidly disintegrating tablets mainly as a result of the swelling of its particles and consequent weakening of the bond forces holding them together [11]. Wood and cotton linters have remained the main commercial sources of MCC. However, alternative low cost sources such as oil palm biomass [12], water gourd [13], Alfa grass [14], soy-bean hulls [15], Orange peel [16], Water Hyacinth [17], Oil palm frond [18], pod husk and stalk of fluted pumpkin [19] etc. have been reported.
Many studies have also been reported on the utilization of papaya stem fiber as a biosorbent to remove heavy metals [20-23], adsorbent to remove methylene blue (cationic dye) [24], reinforcement in green composite [25] and its wound healing property in albino rats [26]. No information exists on processing papaya stem fiber to microcrystalline cellulose and the evaluation of its physicochemical properties.
Materials and Methods
Cellulose precursor
The Carica papaya stem (height- 10 m tall) was sourced from a felled tree of paw-paw that had stopped producing fruits in Azigbo, Anambra State, Nigeria. The stem was debarked, washed, cut into irregular chips and dried properly. The dried chips of the papaya stem were milled and allowed to cool to room temperature. The pulverized substrate was sieved through 2.mm Laboratory test sieve to obtain uniform size.
Preparation of microcrystalline cellulose
A slight modification of the procedure by Ohwoavworhua [27] was employed. 900 g of the sieved stem powder was macerated in 13 l of 2% w/v aqueous NaOH solution at 80°C for 3 h in a stainless steel immersed in a JP Selecta water bath [Precisdig (6001197)]. The resulting de-lignified mass was treated with 4.320 l of 3.5% w/v aqNaOCl solution at 80°C for 30 min. The bleached mass was subjected to treatment with 2.680 l of 17.5% w/v aqNaOH solution at 80°C for 1 h. The resultant α-cellulose undergoes the final stage of bleaching with 1.920 l of (1:1) aq solution of 3.5% w/v NaOCl for 1 h. The resultant mass obtained was repeatedly rinsed with distilled water until it is neutral to litmus paper. Excess moisture was squeezed off using a muslin cloth and the small mass obtained was then oven-dried in a JP Selecta Digiheat Oven at 65°C for 12 h. The alpha cellulose obtained was then hydrolyzed using HCl at a boiling temperature according to the procedure reported by Nwajiobi [19].
Physicochemical analysis
The identification, organoleptic characteristics, solubility tests, starch and dextrin were carried out according to British Pharmacopoeia (BP) specifications [28].
Moisture content
2 g of the MCC was placed in a petri dish and then oven-dried at 105°C for 3 h to a constant mass. The computation of the moisture content (%) was based on the initial air-dried weight.
Bulk density (Bd)
5 g of the MCC was placed in a 50 ml clean, dry measuring cylinder. The occupied volume by the sample without tapping was noted as the bulk volume while the bulk density was computed by dividing the mass of the sample by its bulk volume as expressed:
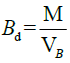
Where, M is the mass of the MCC, VB is the bulk volume of the MCC.
Tapped density (Td)
The tapping of the measuring cylinder containing the MCC on a wooden platform was carried out and after 500 taps; the occupied volume was noted as the tapped volume. The tapped density was then determined using the expression:
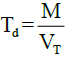
Where, M is the mass of the MCC, VT is the tapped volume of the MCC.
True density (Ttd)
The liquid displacement method was adopted in determining the true density. This was carried out using the immersion fluid xylene and immersing the sample completely in a 26 ml pycnometer bottle capacity. The volume of the liquid displaced was measured and the density was computed using the expression;
Ttd = [w/ {(a + w) –b} ×SG],
Where, w is the weight of MCC powder, SG is specific gravity of xylene, a represents sum of weights of bottle and solvent and b represents the sum of weights of bottle, solvent and the MCC powder.
Hausner’s Index (Har)
This was calculated from the tapped density and bulk density values of the MCC powder.
Har = Td/B
Powder porosity
This was determined from the true and bulk densities values when fitted into the equation below;
Pp = 1- Bd/Ttd × 100
Where Bd is the bulk density, Ttd is the true density and Pp is the porosity
Compressibility index CI
This was computed from the bulk and tapped densities values when fitted into the equation:
Compressibility index (C%) = [(Td – Bd)/ Td] × 100
Hydration capacity (Hc)
1 g of the MCC sample was introduced into each of the four 15 ml plastic centrifuge tubes and 10 ml distilled water was added, shaken and then mixed for 2 min. After 10 min of standing, the mixture was immediately centrifuged at 1000 rpm for 10 min on a Sorvall bench centrifuge. The weighed sediment obtained after decanting the supernatant was used to compute the hydration capacity as the ratio of sediment weight to the dry sample weight [29].
HC = [(sediment weight – tube weight)/ dry sample weight]
Swelling capacity (Swell ability)
This was determined alongside with the hydration capacity according to the study reported by Ohwoavworhua [27] and was calculated as follows:
Swelling capacity (Sc) = [(V2-V1)/V1]
V1 = Occupied tapped volume prior to hydration
V2 = Volume occupied after hydration
Angle of repose (a)
The method as reported by Achor [13] was used in determining the static angle of repose. The tangent of the angle of repose was computed using the equation.
Tan a = 2h/D
Where, h is the height of the heap of powder and D is the diameter of the base of the heap of powder
Particle size analysis
This was determined by microscopic method using a trinocular microscope (SXY-m50) and s-viewer application was used in taking accurate readings. The average diameters of the magnified particles were determined for the sample size of 100 particles.
Degree of polymerization and Molecular weight determination
The procedure as established in a study reported by Nwajiobi [19] was adopted in determining the degree of polymerization and Molecular weight.
Scanning Electron Microscopy (SEM)
SEM was carried out on the MCC powder to study the surface morphology. The MCC powders were sputter-coated with gold for 3-4 min and then observed at 10 KV accelerated voltage in a SEM, JSM 5400 (JEOL ltd., Japan)
Fourier Transform (FTIR) Spectroscopy
The MCC powders were characterized using a Shimadzu FTIR-8400S by mixing about 5μg with a little amount of KBr and compressed into its disc. It was then scanned between 4000-500 cm-1 and the spectra was recorded and studied.
Thermo-gravimetric analysis (TGA)
Perkin-Elmer Thermal analysis controller was used to determine the thermal stability of the MCC powder at a heating rate of 10°C/min in nitrogen atmosphere.
Conclusion
The characterization of CU-MCC obtained from paw-paw stem shows that the sample has poor powder and flow properties. From the thermal analysis, the sample has fair thermal resistance when compared to other established reports. The SEM revealed long strands of rod-like shaped fiber particles and FT-IR identified the main functional groups associated with cellulose. CU-MCC may be used for other high-temperature related applications since it has an appreciable thermal stability.
References
- L.G. Bakre, O.J. Ajala, Nig. J. Pharmaceut. Sci., 2012,11(2), 21-30.
- O. Adeyi, J. Appl. Sci. Environ. Management.,2010,14 (1), 55-58.
- M. Auffhammer, V. Ramanathan, J. Vincent, Proceedings of the National Academy of Sci.,2006, 103(52), 19668-19672.
- http://www.cerdi.org/uploads/sfCmsContent/html/323/kumar.pdf
- P. Jaiswal, P. Kumar, V.K. Singh, D.K. Singh, J. Pharm. Res., 2010, 3(5), 998-1003.
- G. Dick, Papaya: A tantalizing taste of the Tropics, Maricopa County Master Gardener Volunteer information, University of Arizona Cooperative Extension, 2003.
- V.J. Mello, M.T. Gomes, F.O.Lemos, J.L. Delfino, S.P. Andrade, M.T. Lopes, C.E. Salas, Phytomed., 2008, 15, 237-244.
- S. Rahman, M. Ismail, N. Muhammad, F. Ali, A.K. Chisthi, M. Imran, Afr. J. Pharmacol., 2011, 5(8), 1170-1174.
- A. Kempe, A. Göhre, T. Lautenschläger, A. Rudolf, M. Eder, C. Neinhuis, Annual Res. Rev. Biol., 2015, 6(4), 245-252.
- S.S.Z. Sheriff, Biocrystals Journal, 2016, 1(1), 26-38.
- L. Pachuau, D.C. Vanlalfakawma, S.K. Tripathi, J. Appl. Pharmaceut. Sci., 2014, 4 (11), 087-094.
- M.K.M. Haafiz, S.J. Eichhorn, A.M. Hassan, M. Jawaid, Carbohydrate Polymer, 2013, 93(2), 628-634.
- M. Achor, Y.J. Oyeniyi, A. Yahaya, J. Appl. Pharmaceut. Sci., 2014, 4(1), 57-60.
- D. Trache, A. Donnot, K. Khimeche, R. Benelmir, N. Brosse, Carbohydrate Polymer., 2014, 104, 223-230.
- A. Merci, A. Urbano, M.V.E. Grossmann, C.A. Tischer, Mali, Int. J. Resour., 2015, 73, 38-43.
- C.E. Akhabue, N.T.Osubor, Ife J. Sci., 2017, 19(2), 227-235.
- M.A.H. Latif, Y.F. Mahmood, J. Pure &Appli. Sci., 2018, 31(1), 180-188.
- M.H. Husin, N.A. Husin, I. Bello, N. Othman, M.A. Bakar, M.K.M. Haafiz, Int. J. Electrochem. Sci., 2018,111, 717-721.
- C.C. Nwajiobi, J.O.E. Otaigbe, O. Oriji, Chem. Sci. Int. J., 2018, 25(4), 1-11.
- A. Saeed, M.W. Akhter, M. Iqbal, Separa. Purifi. Technol., 2005, 45, 25-31.
- M. Iqbal, A. Saeed, Enzyme Microbial Technol., 2006, 39, 996-1001.
- W.E.Igwegbe, B.C. Okoro, J.C. Osuagwu, Int. J. Environ. Chem. Ecol. Geol. Geophys. Engg., 2015, 9(12), 1400-1404.
- A. Amatussalam, M.N. Abubacker, B. Rajendran, Indian J. Experim. Biol., 2011, 49, 925-931.
- R.R. Krishni, K.Y. Foo, B.H. Hameed, Desalination and water treatment, 2014, 52, 6088-6095.
- A. Kempe, A. Göhre, T. Lautenschläger, A. Rudolf, M. Eder, C. Neinhuis, Annual Res. Rev. Biol., 2015, 6(4), 245-252.
- A. Marzha, A. Liwayway, Int. J. Biosci. Biochem. Bioinform., 2016, 6(2), 68-73.
- F.O. Ohwoavworhua, T.A. Adelakun, Indian J. Pharmaceut. Sci., 2010, 72(3), 295-301.
- Annonymous, British Pharmacopoeia, Her Majesty Stationery Office, University Press Cambridge, Volume 11, A366-A327, 2009.
- S.S. Kornblum, S.B. Stoopak, J. Pharmaceut. Sci., 1973, 62(1), 43-49.
- J.M. Sonnergard, Eur. J. Pharmaceut. Biopharmaceut., 2006,63, 270-277.
- G. Thoorens, F. Krier, B. Leclercq, B. Carlin, B. Evrard, B. Int. J. Pharmaceut.,2014,473 (1-2), 64-72.
- A.F. Stamm, Wood and Cellulose Science, New York: The Ronald Press Company, 1964,pp. 132-165.
- J.S. Wu, H.O. Ho, M.T. Sheu, Eur. J. Pharmaceut. Sci., 2011, 12, 417-425.
- A. Nokhodchi, Pharmaceut. Technol., 2005, 29(1), 46-66.
- E. Emery, J. Oliver, T. Pusssley, J. Sharma, J. Zhou, Powder Technol., 2008.
- B.B. Mohammed, A.B. Isah, M.A. Ibrahim, Nigerian J. Pharmaceut. Sci., 2009, 8(1), 80-88.
- N. Johar, I. Ahmad, A. Dufresne, Indus. Crops Prod., 2012, 37, 93-99.
- A.P. Mathew, K. Oksman, M. Sian, J. Appl. Polymer Sci., 2006, 101, 300-310.
- S.M.L. Rosa, N. Rehman, M.I.G. De Miranda, S.M.B. Nachtigall, C.L.D. Bica, Carbohydrate Polymers., 2012,87, 1131-1138.
- M. Nuruddin, A. Chowdhury, S.A. Haque, M. Rahman, S.F. Farhad, M.S. Jahan, Cellulose Chemical and Technol., 2011, 45, 347-354.
- M.S. Jahan, A. Saeed, Z. He, Y. Ni, Cellulose., 2011, 18, 451-459.



