Research Article - Der Pharma Chemica ( 2018) Volume 0, Issue 0
Inhibition of Aluminium Corrosion in 1.0 M HCl Using Picralima nitida Leaves Extract
Ezeugo JNO1*, Onukwuli OD2 and Omotioma M3
1Department of Chemical Engineering, Chukwuemeka Odumegwu Ojukwu University, Nigeria
2Department of Chemical Engineering, Nnamdi Azikwe University Awka, Anambra State, Nigeria
3Department of Chemical Engineering, Enugu State University of Science & Tech, Enugu State, Nigeria
- *Corresponding Author:
- Ezeugo JNO
Department of Chemical Engineering
Chukwuemeka Odumegwu Ojukwu University, Nigeria
Abstract
The inhibitive action of the extract of Picralima nitida leaves toward 1.0 M HCl corrosion of aluminum was examined. Gravimetric (weight loss), potentiodynamic polarization and electrochemical impedance spectroscopy techniques were used in the corrosion inhibition process. In all the method used, the P. nitida leaves extract acted as good corrosion inhibitor of aluminum in 1.0 M HCl solution. Maximum inhibition efficiency of 84.09% was obtained with inhibition concentration of 1.2 g/l. It showed that the extract of P. nitida leaves has excellent inhibitive ability for the corrosion inhibition process. The values of kads are relatively small indicating that the interaction between the adsorbed extract of picralimanitida molecules and aluminum surface is a physical process. The P. nitida extract protected the aluminium against pitting corrosion. The level of protection increases with increasing extract concentration. The P. nitida leave extract inhibited both cathodic and anodic reactions and acted as mixed-type inhibitor.
Keywords
Aluminium, Corrosion inhibition, Picralima nitida extract, Potentiodynamic polarization, EIS
Introduction
Aluminum exhibit good corrosion resistance due to the formation of a thin and protective, naturally formed oxide film on its surface [1]. However, when aluminum is exposed to aggressive environment (such as acid pickling solutions, chemical etching, industrial cleaning or scale dissolving) the oxide film dissolves resulting to significant mass loss of aluminum [2]. In order to prevent and reduce aluminum dissolution, different types of corrosion inhibitors are used, mostly organic compounds, which in their structure contain atoms of nitrogen, sulphur, oxygen, phosphor [3-5]. Although most of the organic compounds have excellent inhibitive performance, their high cost and toxicity are the main setbacks in the use of these compounds as corrosion inhibitors [6,7]. In recent times, most corrosion inhibitors studies have been focused on the development of “environmental friendly” compounds in response to current trend to legislation changes concerning environmental protection. This term includes formulations that are not toxic to humans, have low environmental impact, optimal biodegradability and maintenance of high efficiency and cost-effectiveness [8]. Thus since the 1990s, some studies have confirmed the efficiency of natural compounds as corrosion inhibitors in line with the presence of complex organic compounds with variety of different adsorption centers (Such as heteroatom, double bonds and aromatic rings) [9-18].
Picralima nitida is a tree that can reach a height of 35 meters but is usually less. It is a commonly used herbal remedy in West Africa. All parts of the plant are bitter throughout its distribution areas; the seeds, bark, leaf and roots are used for pharmaceutical purposes [18,19]. Considerable researches have been carried out into the medicinal properties, much of it supporting the traditional uses. The stem, bark, fruit, leaf and seeds contain a number of phytochemicals. Picralima nitida leaves contain many organic compounds, such as phenolics, terpenoids and tannins as their major phytocompounds and also saponins, flavonoids and alkaloids in moderate amount to scavenge free radicals and induce detoxification. Before now, there is no reported work on inhibitive effects of P. nitida leaves extract on acidic corrosion of aluminum. Hence, the main objective of this work is to investigate the P. nitida leaves extract as potential inhibitor of the aluminum corrosion in 1.0 M HCl.
Materials and Methods
Gravimetric and electrochemical tests were carried out on Aluminum alloy (AA3003) specimen with the composition: 1.22% Mn, 0.55% Fe, 0.363% Si, 0.017% Cu, 0.064% Pb, 0.026% Ti, 0.009% V, 0.19% others 97.672% Al. Each sheet with 0.04 cm thickness was mechanically pressed cut into coupons of dimensions 2.6 cm × 2.6 cm. The coupons were degreased in absolute ethanol, dried in acetone, weighed and stored in moisture free desiccators prior to use.
Considering one factor at a time, the weight loss method (Gravimetric method) was carried out at different temperatures and with various concentrations of the P. nitida leaves extract. According to this method, weighed aluminum alloy coupons were separately immersed in 250 ml open beakers containing 200 ml of 1.0 M HCl. More so, Al coupons were separately immersed in 250 ml open beakers containing 200 ml of 1.0 M HCl with various concentrations of the P. nitida leaves extract. The variation of weight loss was monitored periodically at various temperatures in the absence and presence of various concentrations of the extract.
At the appropriate time, the aluminum coupons were removed, immersed in acetone, scrubbed with a bristle brush under running water, dried and reweighed. The weight loss was calculated as the difference between the initial weight and the weight after the removal of the corrosion product. Weight loss measurements were undertaken using a FAJA digital weighing balance of the range 0.0001 to 2000 g. The weight measurements were triplicate.
For potentiodynamic polarization and electrochemical impedance spectroscopy, test metal samples of aluminum for electrochemical experiment were machined into cylindrical specimens and fixed in Polytetrafluoroethylene (PTFE) rods by epoxy resin in such a way that only one surface of area 1 cm2 was left uncovered. The electrodes used were polished with emery papers (from 400 to 1200), rinsed with distilled water, degreased and dried. The electrochemical experiment were conducted in a three electrode corrosion cell using a VERSASTAT 400 complete dc voltammetry and corrosion system with V3 studio software for electrochemical impedance spectroscopy and potentiodynamic/Galvanostat corrosion system with E-chem software for potentiodynamic polarization experiments. A platinum sheet was used as counter electrode and a Saturated Calomel Electrode (SCE) was used as reference electrode. The calomel electrode was connected via a luggins capillary. Impedance measurement were performed in aerated and unstirred solutions at the end of 1800s at 30 ± 1°C. The measurements were made at corrosion potentials (Ecorr) over a frequency range of 100 kHZ-0.1 HZ with a signal amplitude perturbation of 10 mV [18].
Potentiodynamic polarization studies were carried out in the potential range ± 250 mV versus corrosion potential at a scan rate of –250 to 400 mVs-1. Each test was run in triplicate to verify the reproducibility of the data. All experiments were carried out in freshly prepared solution at constant temperature 30 ± 1°C using a thermostat.
Results and Discussion
Weight loss measurements
Table 1 presents the results of the inhibition efficiency as function of time and inhibitor (extract) concentration. The inhibition efficiency was time and temperature dependent. It increases with increase in Picralimanitida extract concentration. Maximum inhibition efficiency of 84.04% was obtained with inhibition concentration of 1.2 g/l. It showed that the extract of P. nitida leaves has excellent inhibitive ability for the corrosion inhibition process.
| Concentration (gl-1) | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 6 | 8 | 12 | 13 | 14 | 15 | |
| Inhibition efficiency, IE (%) | |||||||||
| 0.2 | 26.76 | 35.8 | 43.67 | 47.55 | 53.43 | 56.12 | 56.13 | 56.12 | 56.13 |
| 0.45 | 30.11 | 48.04 | 50.00 | 55.81 | 60.02 | 63.13 | 63.13 | 63.13 | 63.13 |
| 0.7 | 34.03 | 50.05 | 54.14 | 60.51 | 65.00 | 70.8 | 70.84 | 70.84 | 70.84 |
| 0.95 | 37.82 | 54.7 | 60.00 | 67.11 | 77.17 | 80.59 | 80.59 | 80.59 | 80.59 |
| 1.2 | 40.04 | 59.00 | 65.12 | 71.16 | 80.09 | 84.09 | 84.08 | 84.08 | 84.08 |
Table 1: Results of IE (%) of Al in HCl versus time at various concentrations of Picralima nitida leaves extract
Adsorption studies
In order to obtain the adsorption isotherm, the degree of surface (θ) for various concentrations of the inhibitor has been calculated according to the following Equation [19].
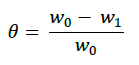 (1)
(1)
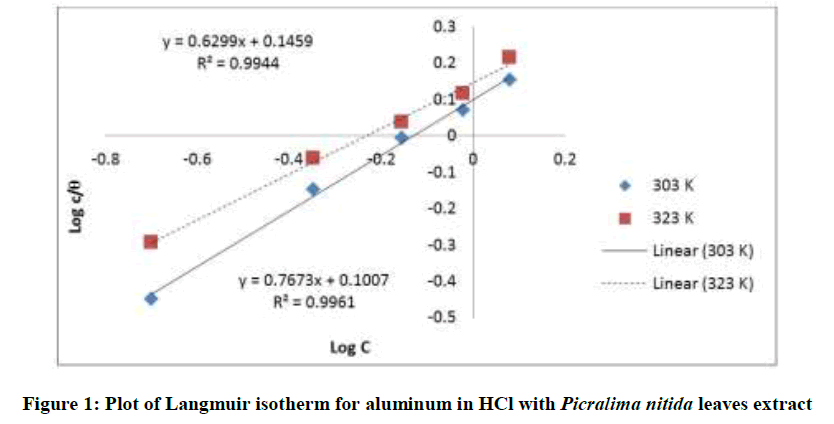
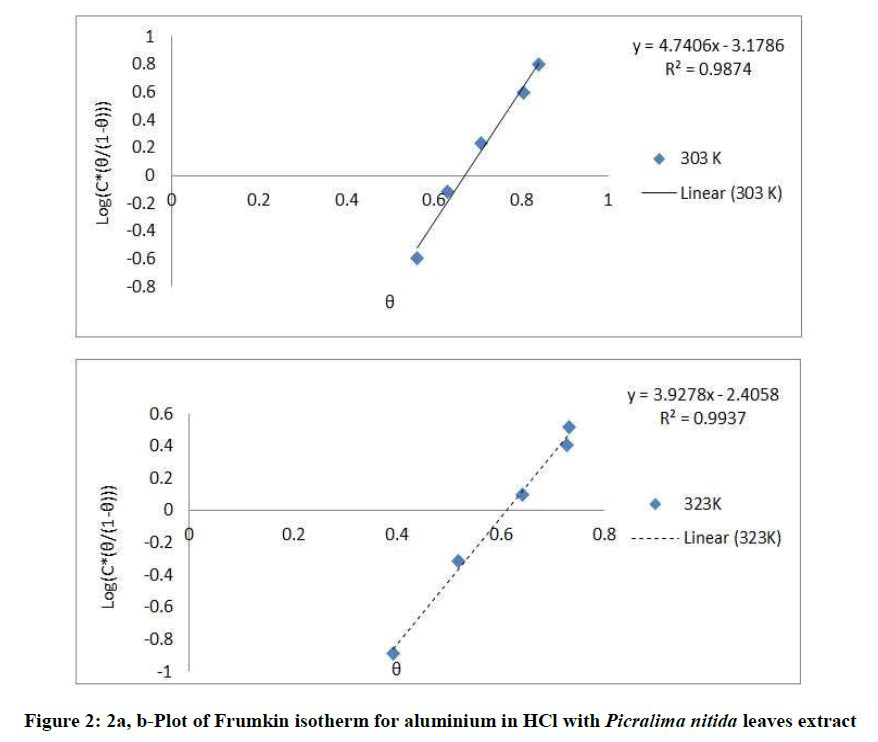
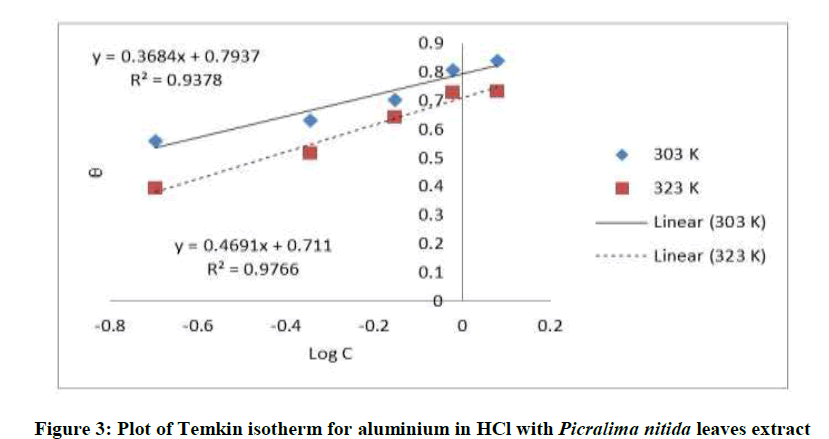
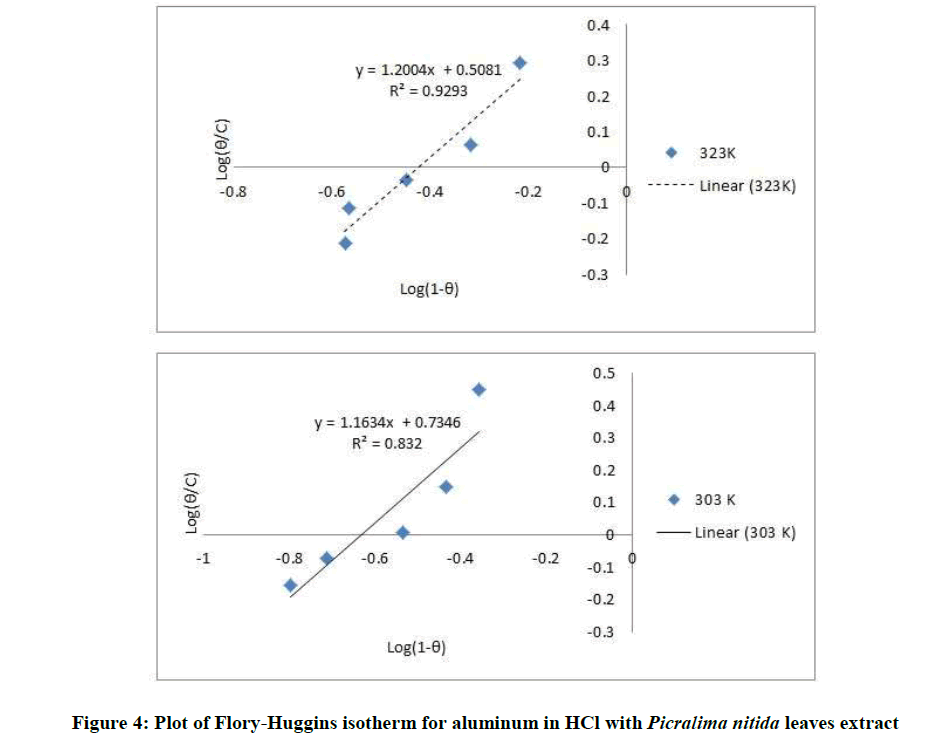
Where, w1ad w0 are the weight loss values in presence and absence of inhibitor, respectively. In these studies, Langmuir, Temkin, Frumkin and Flory-Huggins are tested for the P. nitida leaves extract at three temperature ranges of: 303K, 313K and 323K. Straight lines, Figures 1-4) indicating that the inhibition process obeyed all the tested adsorption isotherms, were given by the following Equations [20].
Langmuir: logθ/c = logc – logk (2)
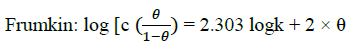 (3)
(3)
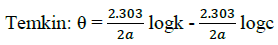 (4)
(4)
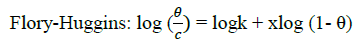 (5)
(5)
Where, θ is the degree of surface coverage, K is the equilibrium constant of the adsorption isotherm, C is the concentration of the inhibitor in the bulk solution. The linear regression coefficient is close to unity, hence, adsorption of inhibitor follows Langmuir, Frumkin, Temkin and Flory- Huggins adsorption isotherms. The four adsorption isotherms are based on the assumption that each site of Al surface holds one molecule of the adsorbed species. Therefore, one adsorbed water molecule is replaced by one molecule of the inhibitor adsorbed (Picralimanitida extract) on the aluminum surface. The apparent free energy of adsorption (ΔG0ads) was calculated from the relation:
ΔG0ads = -2.303 RT log 55.5 Kads (6)
The thermodynamic parameters are presented in Table 2. In respect to the fitted data to the Langmuir isotherm, the R2 value is close to unity, indicating excellent adherence to Langmuir adsorption isotherm [20-22]. The negative values of ΔG0ads indicate the spontaneous adsorption of the P. nitida extract. The value of the free energy of adsorption is not only negative but is less than the threshold value of – 40 kl.mol-1 required for chemical adsorption. This is in compliance to the previous studies [23,24]. From the Frumkin adsorption parameter, the lateral interaction term (α) gave positive values suggesting attractive behavior of the inhibitor on the aluminum surface. From Temkin adsorption parameter, the attractive value (a) is negative, showing that repulsion exists in the adsorption layer [21,25]. The value of the size parameter (x) is positive, the positivity of the value indicates that the adsorbed species of the extract is bulky [21,26]. The values of kads are relatively small indicating that the interaction between the adsorbed extract of P. nitida molecules and aluminum surface is a physical process. This is also supported by lower negative (ΔG0ads) values for P. nitida extract [27].
| Adsorption Isotherm | Temperature (K) | R2 | Kads | ∆G0ads | Isotherm |
|---|---|---|---|---|---|
| Langmuir isotherm | 303 | 0.994 | 0.7161 | – 9.278 | - |
| 323 | 0.996 | 0.7943 | – 10.169 | - | |
| a | |||||
| Frumkin isotherm | 303 | 0.987 | 0.017 | – 2.114 | 2.37 |
| 323 | 0.993 | 0.093 | – 4.329, | 1.9635 | |
| A | |||||
| Temkin isotherm | 303 | 0.937 | 0070 | – 2.382 | – 3.1291 |
| 322 | 0.976 | 0.0305 | – 1.414 | – 2.4552 | |
| x | |||||
| Flory-Huggius isotherm | 303 | 0.832 | 5.4200 | – 14.378 | 1.163 |
| 323 | 0.929 | 3.2211 | – 13.929 | 1.200 |
Table 2: Adsorption parameters for the corrosion inhibition of aluminum in HCl by Picralima nitida leaves extract
Results of the potentiodynamic polarization study
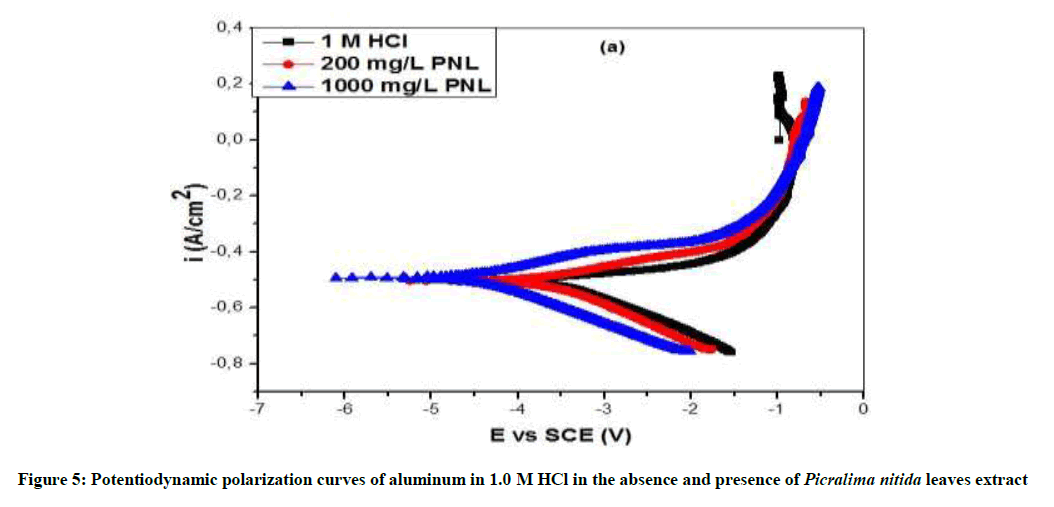
The potentiodynamic polarization curves of Al in the absence and presence of P. nitida leaves extract are shown in Figure 5 for the samples in 1.0 M HCl solution. The values of the polarization parameters are provided in Table 3, where Ecorr and Icorr are the corrosion potential and current density respectively. They were obtained from the extrapolation of the anodic and cathodic Tafel slopes with respect to the Ecorr values. The results revealed that in the presence of P. nitida leaves extract and at higher concentration of 1000 mg/L-1 aluminum displayed lower Icorr and more positive Ecorr values in studied environment. In Table 4, the result shows that the introduction of P. nitida leaves extract reduces both the cathodic and the anodic corrosion current densities, implying that the corrosion rate of Al sample in the presence of the inhibitor was reduced when compared to the uninhibited Al. Also, the Ecorr of the inhibited Al is more positive (anodic) than the uninhibited Al. This shows that in the absence of P. nitida leaves extract, Al has a higher susceptibility to corrosion in acidic environment than the uninhibited Al sample. It is evident that the extract shifts both the anodic and cathodic curves to lower values of current densities. The polarization curves for the Al samples in 1.0 M HCl environment exhibited passivation behavior and do not differ in nature of transition from active to passive states. The similarity of the polarization curves of both the uninhibited and inhibited Al samples indicates that the mechanism of the corrosion of Al in the absence of the inhibitor did not changed even when P. nitida leaves extract was introduced into the aggressive solution.
| Concentration (gl-1) | Ecorr (mv) | Icorr (uAcm-2) | q | IE (%) |
|---|---|---|---|---|
| 1.0 M HCl | -510 | 187.2 | - | - |
| 200 | -506 | 72.15 | 0.613 | 61.3 |
| 1000 | -498 | 31.7 | 0.831 | 83.1 |
Table 3: Parameters obtained from polarization curves at different concentration of inhibitor for 1.0 M HCl
| Concentration (gl-1) | Rs (W cm2) | RL1 (W cm2) | Rct (W cm2) | Qdl (F cm-2) | L |
|---|---|---|---|---|---|
| 1.0 M HCl | 2.36 | 5.7 | 310 | 2.39 | 4.34 |
| 200 | 5.45 | 776 | 811 | 2.32 | 793 |
| 1000 | 6.89 | 1240 | 1709 | 20.02 | 1692 |
Table 4: Impedance parameters for aluminum in 1.0 M HCl in absence and presence of different concentrations of Picralima nitida leave extract
Impedance spectroscopic results
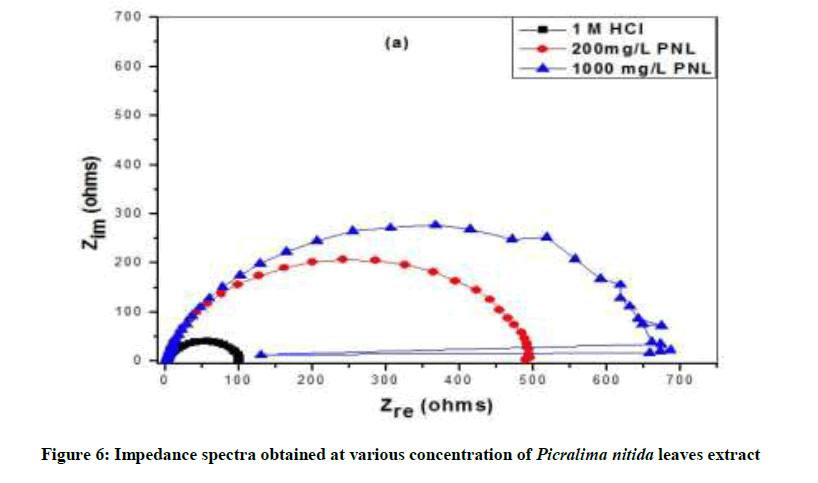
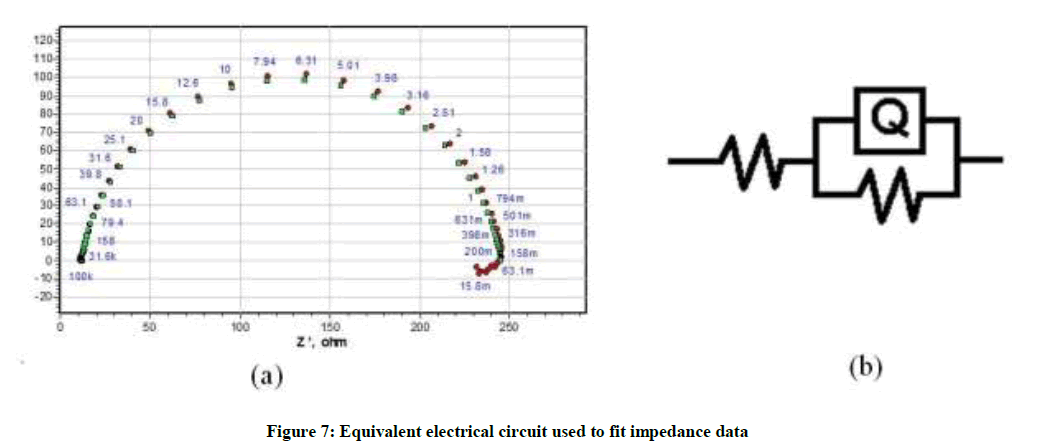
Results obtained by impedance spectroscopy are shown in Figure 6. The Nyquist plots showed a capacitive loop followed by an inductive loop for aluminum samples in 1.0 M HCl solution. The diameter of the semi circles are related to charge transfer resistance. The size of the capacitive loops was greater in the presence of P. nitida leaves extract, compared to that in inhibitor-free environment, an indication of a higher corrosion resistance for aluminum in the presence of the inhibitor. The occurrence of an inductive loop for the aggressive solutions may indicate certain non-faradic processes, such as adsorption and desorption of corrosion products, occurring at the sample/electrolyte interface. The equivalent circuit model shown in Figure 7 was used to model the impedance results obtained for aluminum in HCl solutions, after fitting with Zsimpwin software.
The inductance, L and charge transfer resistance, (Rct) characterize the processes beneath the store of charges. The result shows that the Qpo value was lower in the inhibited environment compared to the inhibitor-free aluminum. Similarly; the value of inductance was greater in the inhibited Al sample than for the uninhibited. It shows that introduction of NPL extract can modify the electrochemistry of the aluminum sample by reducing the penetration of electrolyte into the substrate electrolyte interface, thus, decreasing the rate of the corrosion in the acid solution. This can also be evidenced by the higher value of Rct for Al in the presence of the inhibitor than the value obtained in the absence of P. nitida leaves. Furthermore, addition of the inhibitor also reduces the rate of penetration of the electrolyte. Hence, the rates of the electrochemical processes in the aluminum electrolyte interface. From the present result, the introduction of the P. nitida leaves extract in the 1.0 M HCl solution seems to reduce the susceptibility of the Al substrate to dissolution and to the electrochemical processes occurring at the substrate/electrolyte interface. This was made evident by the shift of Ecorr to more positive potentials, the reduction of current densities in the inhibited environment and the increased size of the diameter of the Nyquist plots. It can be deduce that the adsorption of P. nitida leaves extract serve as a barrier blocking the contact between the Al substrate and the electrolyte solutions.
Surface morphology
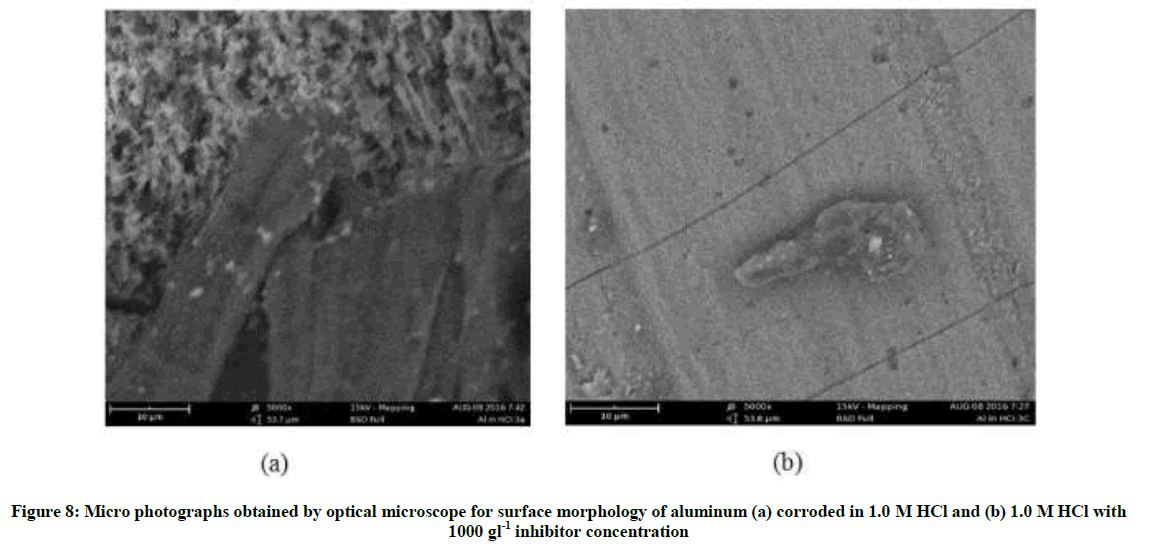
Optical microscope test revealed that when a smooth surface of aluminum alloy is immersed in 1.0 M HCl solution, in absence of P. nitida extract, corrosion pits were created on the surface of the aluminum due to aggressive attack of the HCl solution. A rough surface with flakes of dark color is observed (Figure 8a) which indicates deposition of corrosion products and/or chloride ions on the surface. But in the presence of inhibitor as shown in (Figure 8b), smooth layers were observed due to adsorption of organic species present in the extract at most active sites. On the basis of the information provided by the optical microscope, it can be concluded that plant extract protect aluminum surface in hydrochloric acid environment, forming good protective film on the surface of the metal. The outcome of this process revealed that the adsorption of the extract is spontaneous, stablesss and follows the mechanism of physical adsorption.
Conclusion
From the gravimetric method, maximum inhibition efficiency of 84.04% was obtained with inhibition concentration of 1.2 g/l. It showed that the extract of P. nitida leaves has excellent inhibitive ability for the corrosion inhibition process. The adsorption process of P. nitida leaves extract on the surface of the aluminum is spontaneous, stable and follows Langmuir adsorption isotherm. The values of kads are relatively small indicating that the interaction between the adsorbed extract of picralimanitida molecules and aluminum surface is a physical process. The P. nitida extract protected the aluminium against pitting corrosion. The level of protection increases with increasing extract concentration. The P. nitida leave extract inhibited both cathodic and anodic reactions and acted as mixed-type inhibitor.
References
- Y.S. Ulutas, H. Dal, Appl. Surf. Sci., 2006, 253, 919.
- S. Safak, B. Duran, A. Yurt, G. Turkoglu, Corro. Sci., 2012, 54, 251.
- J.M. Lashgari, A.M. Malek, Electrochem. Acta., 2010, 55, 5253.
- E.A. Noor, Mater. Chem. Phys., 2009, 114, 533.
- E. Kalman,. In proceedings of 7th European symposium on corrosion inhibitors (7SIEC) Ferrara, Italy, 17-21 September, 1990.
- C.A. Grillo, M.V. Mirifico, M.L. Morales, M.A. Reigosa, M.F. Lorenzo de Mele, J. Hazard. Mater., 2009, 170, 1173.
- S.A. Umoren, U.M. Eduok, A.U. Israel, I.B. Obot, M.M. Solomon, Green Chem. Lett. Rev., 2012, 5, 303-313.
- U.F. Ekanem, S.A. Umoren, I.I. Udousoro, A.P. Udoh, J. Mater. Sci., 2010, 45, 5558-5566.
- L.R. Chauham, G. Gunasekaran, Corros. Sci., 2007, 49, 1143-1161.
- A.Y. El-Etre, Appl. Surf. Sci., 2006, 252, 8521-8525.
- A.Y. El-Etre, Mater. Chem. Phys., 2008, 108, 278-282.
- A.Y. El-Etre, J. Colloid Interf. Sci., 2007, 314, 578-583.
- S.A Umoren, I.B. Obot, N.O. Obi-Egbedi, J. Mater. Sci., 2009, 44, 274-279.
- I. Radiojcic, K. Berkovic, S. Kovac, Vorkapicfurac, J. Corros. Sci., 2008, 1498-1504.
- A.A. Rahim, E. Rocca, J. Steinmetz, M.J. Kassim, R. Adnan, M.S. Ibrahim, Corros. Sci., 2007, 49, 402-417.
- J.J. Fu, S.N. Li, L.H. Cao, L. Wang, L.H. Yan, I.D. Lu, J. Mater. Sci., 2010, 45, 979-986.
- E.E. Oguzie, E.E. Ebenso, Pig. Res. Tech., 2010, 35, 30-35.
- E. Stupnisek-Lisac, A. Gazivoda, M. Madzarac, Electrochem. Acta., 2002, 47, 4189.
- L.A. Nnanna, I.O. Owate, O.C. Nwadiuko, N.D. Ekeke, W.J. Oji, Inter. J. Mater. Chem., 2013, 3, 10-16.
- V.G. Vasudha, P.K. Shanmuga, Res. J. Chem. Sci., 2013, 3, 21-26.
- O.D. Onukwuli, M. Omotioma, J. Chem. Tech. Metall., 2016, 51(3), 302-314.
- O.M. Ndibe, M.C. Menkiti, M.N.C. Ijomah, O.D. Onukwuli, EJAF Che., 2011, 10, 2847-2860.
- E. El-Ouariachi, J. Paolini, M. Bouklah, A. Elidrissi, A. Bouyanzer, B. Hammouti, J.M. Desjobert, J. Costa, Acta Metal. Sin (Engl. Lett.)., 2010, 23, 13-20.
- M. Abdulwahab, A. Kasim, K.A. Bello, J.O. Gaminana, Adv. Mater. Res., 2010, 367, 319-325.
- J.T. Nwabanne, V.N. Okafor, J. Tren. Eng. Appl. Sci., 2011, 2(4), 619-625.
- D. Bracher, A.D. Mercer, British Corrosion J., 1968, 3, 120.
- A.S. Fouda, El-dosky, A.M. Hasan, Int. J. Electrochem. Sci., 2013, 8, 5866-5886.