Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 11
Influence of annealing on the optical properties of Mult-wall carbon nanotubes
Lora Alashi, Reema Aljehani, Amani Alsubyani, Rowan Alzahrani, Manar Hablin, Dhay Albeladi, Salha Alqarni and MM Abutalib*MM Abutalib, Department of physics, University of Jeddah, College of science, Jeddah, Saudi Arabia, Email: mmabotaleb@uj.edu.sa
Received: 29-Oct-2022, Manuscript No. dpc-22-78631; Editor assigned: 31-Oct-2022, Pre QC No. dpc-22-78631; Reviewed: 14-Nov-2022, QC No. dpc-22-78631; Revised: 16-Nov-2022, Manuscript No. dpc-22-78631; Published: 23-Nov-2022, DOI: 10.4172/0975-413X.14.11.1-4
Abstract
A uniform layer of MWCNTs was fabricated on the surface of a glass substrate with dimensions of (2.5 cm x 2.5 cm) by using a spin coater instrument. The Optical properties of MWCNTs at various temperatures were studied using UV-Vis spectrophotometer. The optical energy gap was calculated by using the equation tauc.
Keywords
MWCNTs; Uv Vis; Optical energy gap
INTRODUCTION
Carbon is considered one of the most important chemical elements due to its several applications and products, carbon has an atomic number equal to 6 with six electrons which are distributed in the atomic orbital 1S2, 2S2, and 2P2, carbon atomic orbitals have several hybridize such as sp, sp 2 or sp 3. Each of the carbon hybrids produces different type of material with different properties, for example, the hybrid Sp3 carbon atomic orbitals produce diamond, while Sp2 produces very constant nanometer size of bonded materials such as graphene, fullerenes, and carbon nanotubes. [1,2] Graphite was discovered in 1779, whereas the diamond was discovered after that by ten years of. Kroto, Smalley, and Curl discovered fullerene (C60) in 1985. Fullerene is a carbon allotrope with sp2 hybrid forms that have the same structure as graphite but with pentagonal rings rather than hexagonal rings. It comes in a variety of shapes, including spheres, ellipsoids, and cylindrical tubes, which are referred to as carbon nanotubes (CNTs). There are numerous classification methods for nanotubes, one of the most important of which is the classification of symmetries that depend on the unit cell. Taking into account several parameters used to represent the tubes, such as Chiral vector Ch = na1 + na2 o (n, m) and chiral angle = cos θ = (2n + m)/ (2*(n2 + m2 + n*m) Nanotubes are classified into three types: CNT ARMCHAIR, CNT ZIGZAG and CNT CHIRAL. The diversity of structure thickness is one of the most prominent classifications of CNTs, which has contributed to attracting a large number of researchers to conduct research on it among many nanomaterials. This classification is based on the number of layers that make up the tube, which is a key factor in distinguishing between the different types, and it is divided into two categories: single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT). Another type is Double Walled CNT, which can be included in the MWCNT. MWCNT are more accurately described as graphite tubes, which are a group of concentric graphene layers separated by 0.34 nm distances, ranging from 10 to 50 nm in diameter and over 10mu in length and similar in properties to graphite used in bulk, MWCNTs have two types based on layers configuration, first is the Russian Doll model which can identify as a bunch of CNTs arranged inside each other, while the second is Parchment model when a single graphene sheet is wrapped around itself many times. Whereas, the SWCNTs are visualized as a single layer of graphene with a diameter of 1 to 1.4 nm similar in structure and properties to fullerenes. CNTs have many properties that make them a very useful material, and all these improvements in properties are due to their very small size. In addition, its structure and bonding properties that gives it superior mechanical, electrical, and thermal properties. CNTs are considered very tough materials due to their mechanical properties such as tensile strength, which is hundreds of times stronger than steel, and flexibility CNTs have high flexibility when exposed to high forces. As for electrical properties, metallic carbon nanotubes are such materials that they can carry 1,000 times more current than silver and copper. Moreover, CNTs have a very high temperature stability of 28,000ºC which makes them better thermal conductors than other conventional conductors, and they can transmit 15 times more watts per minute than copper wires. CNTs absorb and emit light in the near-infrared spectrum. Finally, CNTs have many applications such as super capacitors, hydrogen storage, memory devices and sensors [3-10].
MWCNTs were fabricated by different methods such as laser ablation, a method that uses a pulsed and continuous laser to vaporize a graphite target in an oven which is filled with helium or argon gas to keep pressure. The ablation is governed by hydrodynamic motion caused by thermal expansion due to laser energy absorption. The pressure is relatively high because thermal stress is “confined” within a hot surface layer. And the most promising method is Chemical vapor deposition (CVD), where it allows the production of larger quantities of CNTs under more easily controllable conditions and at a lower cost. The overall kinetics of the growth efficiency process is greatly influenced by reactivity, competition of gas phase, surface, and free radicals as a result of hydrocarbon decomposition [11]. The fundamental objective of our paper is to analyze the optical properties (absorbance and reflection) of MWCNT samples that have been subjected to various temperatures (standard, 100, 250, 450) for use in the field of the memory devices and sensors.
Experimental work
Material
MWCNTs were purchased from HANWHA CHEMICAL Company.
Preparation of MWCNTs
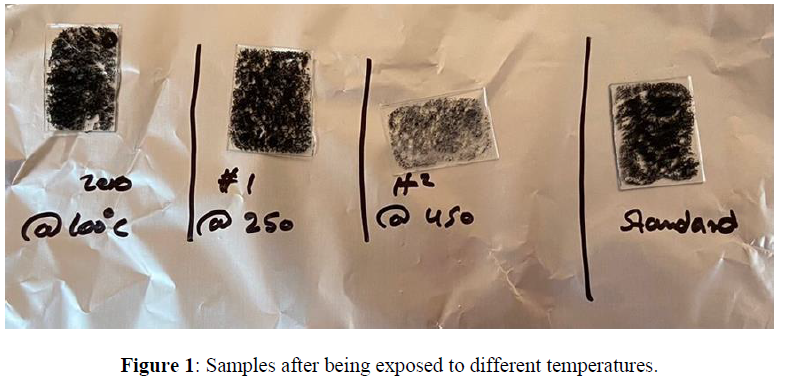
A uniform layer of MWCNTs was fabricated on the surface of a glass substrate with dimensions of (2.5 cm x 2.5 cm) by using a spin coater instrument. First, different suspensions of the MWCNTs were prepared in deionized water (DIW) using an ultrasonic actor for 10 min. Then, 100 microliters of the prepared MWCNTs suspension were spread over the glass substrate at 1000 RPM for 1 min. The obtained modified substrates were allowed to dry in a humid environment. The thickness of the MWCNTs layer was controlled based on the concentration of the used suspension. After that, we subjected the samples to different temperatures using a hot plate for a 30min to each sample with (100C , 250C , 450C) to be able to compare the difference in their optical properties (absorption and reflection) using the spectrophotometer instrument (Figure 1).
Characterization
We used a jasco V-770 spectrophotometer device to investigate the optical properties of MWCNTs. This device have a unique optical design with a single monochromatic and dual detector for the wavelength range from 190 nm to 2700 nm, it allows the researcher to obtain spectra by illuminating a sample and measuring the intensity of light returned relative to its wavelength. Measurements can be made with light transmitted through the sample, reflected from it, or even made to emit light by processes such as photoluminescence using this non-destructive technique.
RESULT AND DISCUSSION
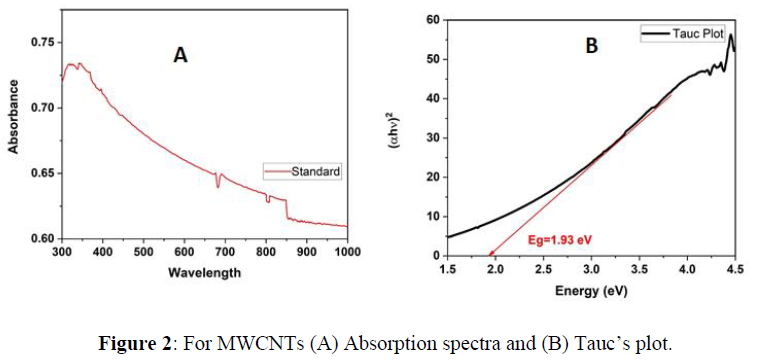
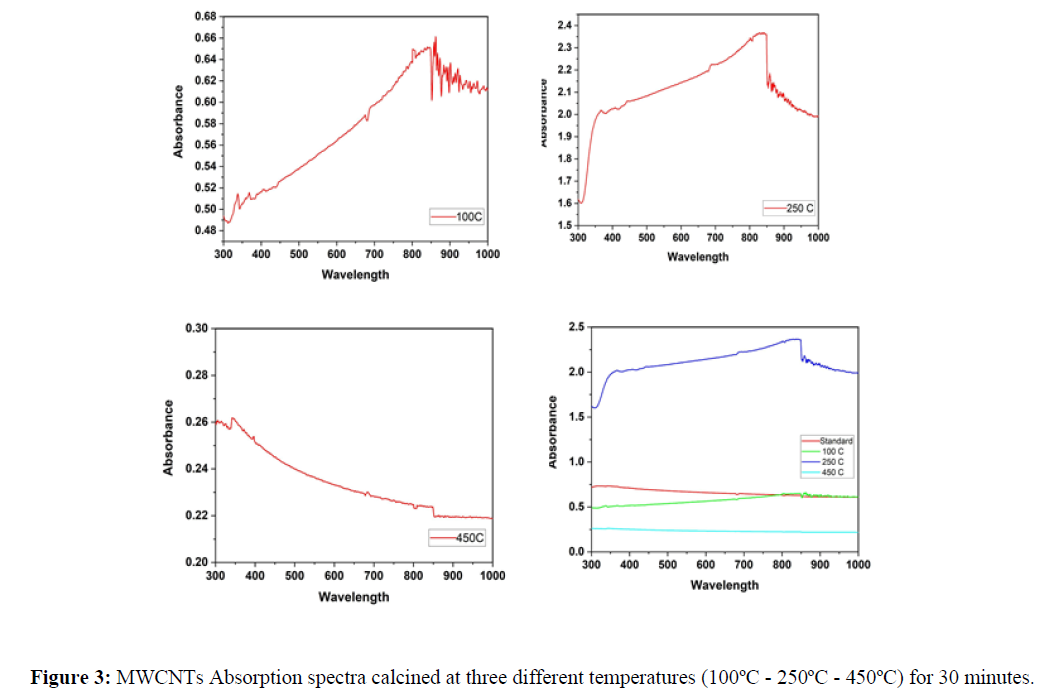
The Optical properties of MWCNTs at various temperatures were studied using UV-Vis spectrophotometer. The results were plotted using origin pro 2020b software as shown in figure1. The standard sample which has not been exposed to heat shows a maximum absorbance below 400nm and direct energy band gap of 1.93 eV found using Tauc’s plot (using Equation 1) as shown in figure 2 (A) and (B) respectively. Previous studies have shown that particle size increase with increasing temperatures. As it is known, there is a clear relationship between the band gap and the size of the particle, which means the existence of a relationship between the absorption and the size of the particles. According to quantum mechanics, the larger the particle size, the smaller the band gap, and vice versa. Based on previous studies, heat is directly related to absorption at lower temperatures. In this study, carbon nanotube samples were calcined at three different temperatures (a. 100C - b. 250C - c. 450C) for 30 minutes separately. The absorption of sample (a) decreased at visible wavelengths compared to the absorption of the standard sample. At the same time, the peaks shifted towards the larger wavelengths, and this redshift can be attributed to the agglomeration of particles. On the other hand, (b) sample showed an increase in absorption at wavelengths ranging from 400-850 nm, which is consistent with the results in previous studies that indicate a direct relationship between heat and absorption. However, sample c showed an anomaly result, as a large amount of material on the surface has been evaporated, which significantly reduced the absorption.
The absorbance peak at 450ºC shifted to lower wavelength as the temperature of MWCNT increased, high-temperature causing weight loss started to occur due to the oxidation of the remaining amorphous carbon and of MWCNTs themselves as shown in Figure 3. The Energy band gap of MWCNT was calculated from Tauc’s plot of absorption spectra with following formula:
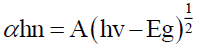
where α is absorption coefficient, h is Plank’s constant, v is frequency of incidence light, A is a constant and Eg is optical band gap of material.
CONCLUSION
The effect of temperatures on the adsorption was studied in a limited interval for three samples and compared with the original sample of MWCNTs that was prepared by the spin-coater device. The absorption was studied using UV-Avis method, which results express a variation in absorption of every sample. Sample (A) has low absorption in wavelengths range while sample (B) absorption was increased in the range of 400 - 850, while sample (C) get a stringed results due to high decreasing in its absorption because the material was evaporated from the surface. We conclude that the absorption increases with temperature increases and there is some strange status in which its absorption doesn't increase due to evaporation of the sample from the surface.
References
- Eatemadi A, Daraee H, Karimkhanloo H, et al., Nanoscale research letters. 2014, 9(1): p. 1-13.
- Aqel A, Abou El-Nour KM, Ammar RA, et al., Arabian Journal of Chemistry. 2012, 5(1): p. 1-23.
- Karna SK, Holmestad R. Properties and applications of carbon nanotubes.
- Khanna S, Islam N. In Organic and Medicinal Chemistry International J. 2019, 7(1): p. 1-6.
- Jensen KA, Bøgelund J, Jacobsen NR, et al., Academic research. 2015, 12: p. 57.
- Jang SH, Kawashima S, Yin H. Materials. 2016, 9(4): p. 220.
- Lehman, J. H., Terrones, M., Mansfield, E., Hurst, K. E., & Meunier, V. (2011). Evaluating the characteristics of multiwall carbon nanotubes. Carbon, 49(8), 2581-2602.
- Ganesh EN. IJITEE. 2013, 2(4): p. 311-320.
- Saleh HM, Koller M. IntechOpen. 2019, p. 3-9.
- Ren Z, Lan Y, Wang Y. Springer Science & Business Media. 2012.
- Pandey P, Dahiya M. Carbon. 2016, 1(4): p. 15-21.
Indexed at, Google Scholar, Crossref