Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 3
Green Rout of Synthesis of 3-(Ferfuraline-4-(5-P-tilyl-1,3,4 thiadiazole-2-ylimino)pentane-2-one imines and their Metal Complexes: A Study of Analytical and Microbial Activity
M.B. Zade1* and S.N. Ibatte22Department of Chemistry, Dayanand Science College, Latur, India
M.B. Zade, Department of Chemistry, Azad Mahavidyalaya, Ausa, India, Email: mbzade71@gmail.com
Received: 15-Feb-2024, Manuscript No. DPC-24-133193; Editor assigned: 20-Feb-2024, Pre QC No. DPC-24-133193 (PQ); Reviewed: 05-Mar-2024, QC No. DPC-24-133193; Revised: 17-May-2024, Manuscript No. DPC-24-133193 (R); Published: 14-Jun-2024, DOI: 10.4172/0975-413X.16.3.307-313
Abstract
A new schiff base ligand, H2A1 and its Zn(II) complex were prepared and characterized by various analytical spectroscopic techniques.1:1 composition of metal ion and ligand was taken for complex formation. New schiff base ligand was characterized by NMR. Infrared spectra reveal that metal ion is coordinated in tridentate manner. The synthesized complex was also studied using UV-visible, TGA and XRD. TG analysis used for thermodynamic and kinetic study. The antimicrobial activity of the ligand andits complexes was examined against a variety of pathogenic bacteria and fungi including Escherichia coli, Staphylococcus aureus and Candida albicans. The data obtained revealed that the free ligand enhanced the antimicrobial activity compared to metal ion in the complexes. The high efficiencies are towards S. aureus, E. coli and C. albican.
Keywords
Zn(II) complex; NMR; IR; TGA; Antimicrobial activity
Introduction
In the chemistry of transition elements coordination compounds have more importance. In the present era chemist synthesize newer and newer organic chelating agents coordinated transition metal ions using different methodologies. The electropositive elements are capable of binding with donor atoms. The researchers synthesize and study variety of schiff base metal complexes because of their wide range of applications in the field of science, biological, catalytic, chemical and physical properties [1].
Large numbers of biological processes are effective by metals; some of these processes are quite specific in the metal ions. The certain metal ions having specific oxidation state can complete necessity of catalytic activity in biological processes. It is now widely appreciated that metal complexes control variety of biological processes [2-4]. One of the principle themes of bioinorganic chemistry, the synthesis of metal complexes has the capability to mimic the functional properties of natural metallo proteins [5-7]. Macrocyclic ligand metal complexes perform in enzymes, proteins and some vitamins. Because of wide range of applications of the schiff bases or porphyrin metal complexes of multidentate ligands gained more attraction of researchers, multidentate ligand metal complexes have delocalized π-orbital. In the synthetic route of complexes recent convenient reversible catalyst can be employed.
The catalyst in free state is not much effective, so catalyst supported on solid silica will be more efficient. The catalyst can be reused five to six times after the completion of the reaction. During the synthesis of imines, various solvents have been reported, such as acetonitrile, dichloro methane, dichloro ethane, methanol, ethanol, DMF, chloroform and DMSO. The reaction can be performed in solvent free atmosphere too. Smooth reaction runes in solvents such as chloroform, methanol, THF, ethanol and DCM due to their intermediate polarity. The reaction gives good yield in methanol, ethanol, chloroform, DCM was obtained. In this work, ethanol is used due to the easy availability and nontoxic nature of the solvent. Some reactions proceed at room temperature simply by stirring for eight to twenty four hours.
1,3,4-thiadiazolederivatives possess interesting biological activity probably conferred to them by the strong aromaticity of this ring system, which leads to great in vivo stability and generally, a lack of toxicity for higher vertebrates, including humans. When diverse functional groups that interact with biological receptors are attached to this ring, compounds possessing outstanding properties are obtained. In present work, efforts have been taken to synthesizes 3-(4-substituted benzylidene)-4 -(substituted-1,3,4- thiadiazole-2-ylimino) pentane-2-one in one step instead of two steps along with solid supported Morphaline as a catalyst. Thiadiazole was derived with the standard procedure and used to imines synthesis.
Materials and Methods
The standard quality chemicals were used in the preparation of Schiff bases and their metal complexes. The purity of chemicals was checked by melting point, boiling point, thin layer chromatography etc. Thiosemicarbazide sigma aldrich (AR), acetoacetone sigma aldrich, furfural aldehyde sigma aldrich (AR) and zinc nitrate sigma aldrich (AR) chemicals were used in the preparation of 2-amino-(5-substituted aryl) 1, 3, 4-thiadazole, schiff bases and their metal complexes. All the solvents of HPLC grade were used for the preparation of Schiff bases and their metal complexes except for ethanol and methanol.
Physical measurements
The colors of the all ligands are yellow and buff in color. Obtained ligands were characterized by various analytical techniques. The melting point was recorded on Cotech digital melting point apparatus. Elemental C, H, N and S analysis was carried out on a Fison EA1108 analyzer. NMR spectra recorded on a Bruker 400 MHz FT-NMR instrument using solvent DMSO-d6. The UV-vis spectra of the ligand and its metal complex were recorded in DMSO using jasco V-530 UV-vis Spectrophotometer (path-length: 1 cm). The IR spectra were recorded using perkin-elmer FT-IR spectrometer (RX-1) in the range 400 cm-1-4000 cm-1 using KBr pallets.
Preparation of 2-ammino-5(substituted phenyl)1,3,4- thiadiazole
The commercially purchased all reagents and solvents of analytical grade were used without any purification.2-ammino-5(substituted phenyl)1,3,4- thiadiazole was prepared in laboratory by alkanoylation of thiosemicarbazide followed by dehydration. The reaction progress was screened by Thin Layer Chromatography (TLC). Molecular structure of derived moiety is as shown in Figure 1.
Synthesis of schiff base ligand
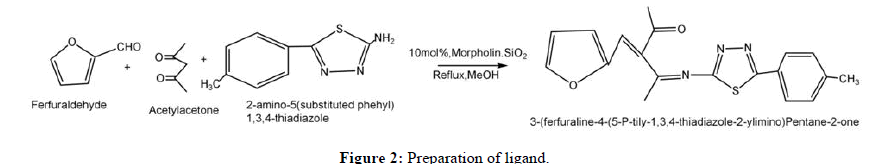
A round bottom flask charged with substituted aromatic aldehyde, acetoacetone (10 mmol) each and along with 10 mole % solid supported Morpholine in methanol (20 ml) are refluxed at 70°C by for 3 hours. The progress of reaction was monitored by using pet ether and ethyl acetate system (7:3 v/v). After that 2-amino-5-(substituted aryl) thiadiazole (10 mmol) added and continued refluxing for 1 hour. The progress of reactionwas monitored by benzene, acetone system (7:3 v/v). The reaction mixture was poured on flaks of ice. The obtained solid yellow precipitate of expected ligand was filtered, dried and recrystallized in methanol (Figure 2).
Synthesis of metal complex
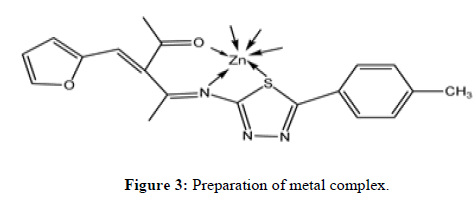
The metal ion solution was refluxed with HA1 (1:1) at 85°C for 1 hour in methanol and morpholine as a catalyst. The complex was dissolved in pet ether after filtration silica supported catalyst was separated and pure complex recollected using rota evaporator. The obtained complex used for various analytical tool screening. On the data the expected structure of the complex will be as shown in Figure 3.
Results and Discussion
The synthesized ligand and its Zn (II) complex are represented in Figures 1 and 2 respectively.
Characterization of ligand and its Zn (II) complex
The synthesized ligand and its corresponding metal complex have been screened by various analytical and spectroscopic techniques such as IR, 1H NMR, UV-vis, Molar conductance, etc. The synthesized ligand and its corresponding metal complex are moisture insensitive, air stable and soluble in DMSO. The analytical data are in good agreement with calculated values and are consistent with formation of mononuclear Zn (II) complex having metal to ligand ratio 1:1.
Physical observation and molar conductance
The color of synthesized ligand was yellow and its Zn (II) complex was reddish black. The color of the complex is redisblack. On analytical data and color of complex, it is confirmed that the sulphur taking part in coordination physical parameters of ligand and metal complex are shown in Table 1. The synthesized Zn (II) complex was dissolved in DMSO solvent and molar conductance of 10-3 solution was measured at room temperature. The value of molar conductance indicates that the metal complex is non-electrolyte (Table 1) [8].
| Melting Points, Elemental Analytical data (%) and m/z values of ligand and its Zn (II) complex. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Ʌm Ω-1 mol-1cm-1 |
Color | M.P. (°C) | Elements | m/z | ||||
| C | H | N | O | S | |||||
| C19H17N3O2S (A1) | -- | Yellow | 107 | 63.7 | 5.05 | 12.38 | 9.43 | 9.45 | 351 |
| (Zn (A1)) (C19H17N3O2SZn) | 15.14 | Reddish black | >250 | 55.5 | 4.89 | 9.71 | 7.39 | 7.41 | 432 |
Table 1: Physical parameters of ligand and metal complex.
Electronic spectra
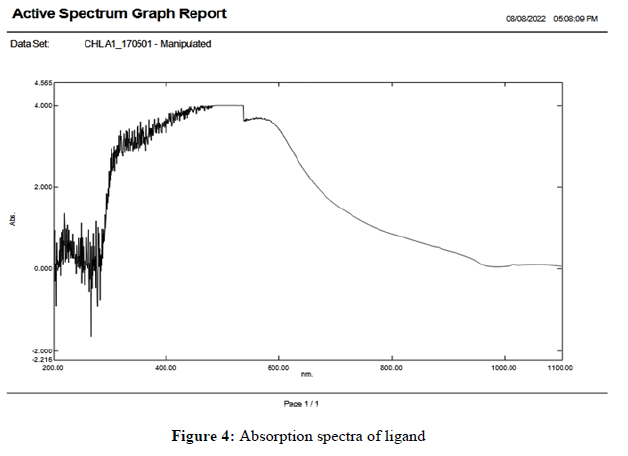
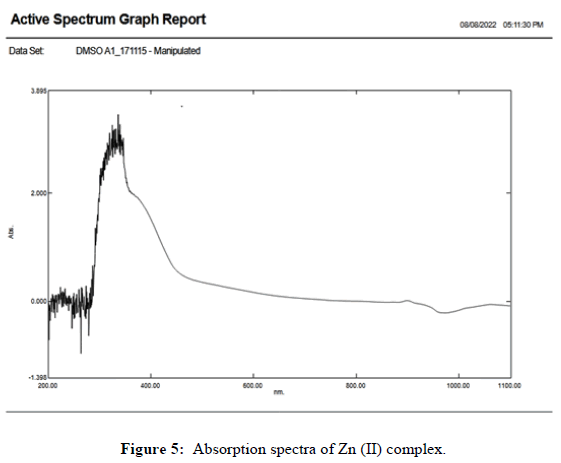
The UV-Vis absorption spectra of synthesized ligand in chloroform and its Zn(II) metal complex were recorded in DMSO (10 M-5 M) at room temperature and are shown in Figures 4 and 5. The electronics spectrum of the ligand shows the absorption maxima n→π* transition at 10682 cm-1 and π→π* transition at 9960 cm-1 the latter being more intense bands in the respective of complex, the n→π*band is blue shifted to 11135 cm-1. In the low and high energy π→π* band also blue shifted 10626 cm-1 respectively. The band observed in UV visible region with absorption maximum at 28751 and 27027 cm-1 in all complexes may be conjugated to strong ligand to metal charge transfer transition. 4T1g (F)→4T2g (F) and 4T1g (F)→4T1g (F) with high spin octahedral geometry. On the basis of all these complexes shows octahedral geometry in which ligand act as trident it and stability is found in complexes.
NMR spectra
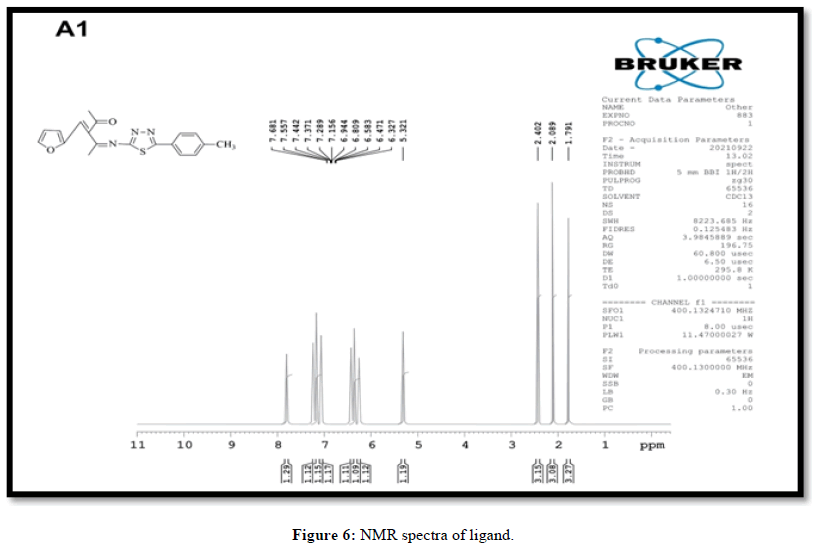
The 1H NMR spectra of the synthesized ligand, 3-(ferfuraline-4-(5-P-tilyl-1,3,4 thiadiazole-2-ylimino)pentane-2-oneImine is shown in Figure 6. It showed a singlet at 9.17 ppm due to azomethine proton (-CH=N-). The signal at 10.29 ppm is because of hydroxyl proton (-OH).
Infrared spectra
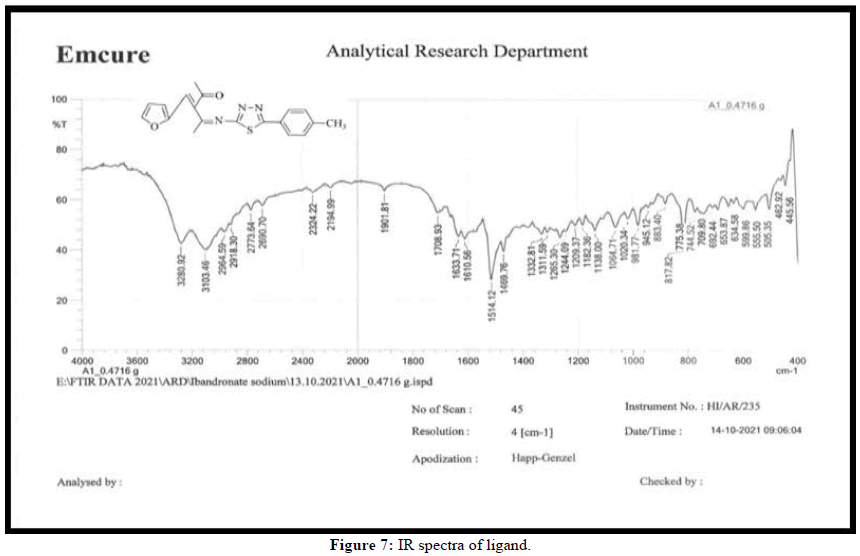
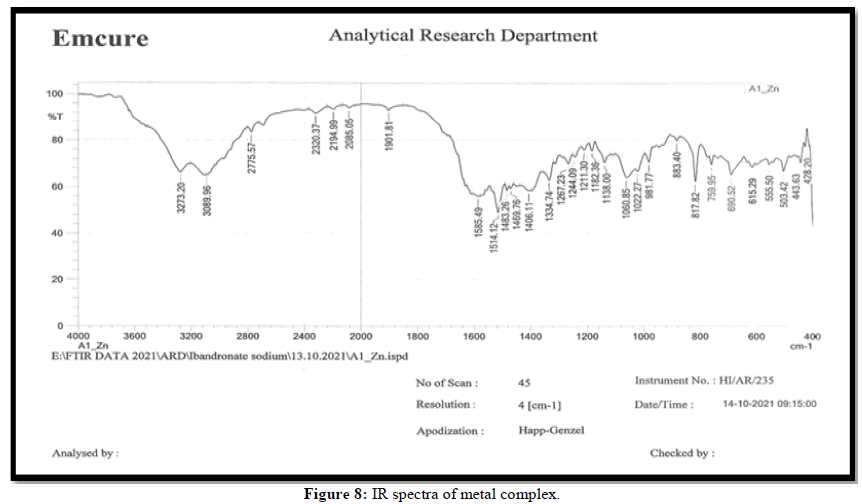
The metal ion and ligand coordination has been studied using IR data of ligand and its Zn(II)complex. The IR spectra of synthesized ligand and its Zn(II)complex are shown in Figure 7 respectively. The shifts in the stretching frequencies of Zn(II)complex compared with free ligand. The ligand shows a characteristic strong band at 1708 cm-1 due to ʋ(-C=O) this band shift to 1585 cm-1 in the complex, indicating participation of oxygen in complexation. A band at 1633 cm-1 due to ʋ(-C=N) of ligand this band shift to 1514 cm-1 in the complex, indicating participation of nitrogen in complexation. A band at 945 cm-1 due to ʋ(-C-S) of ligand this band shift to 883 cm-1 in the complex, indicating participation of sulphur in complexation. Therefore from the above data and elemental analysis together indicates that the synthesized imines is tridentate coordinate to Zn(II) with N, O and S. The complex possibly has tetrahedral geometry due to d10 configuration of Zn (II) ion (Figures 7 and 8) [9].
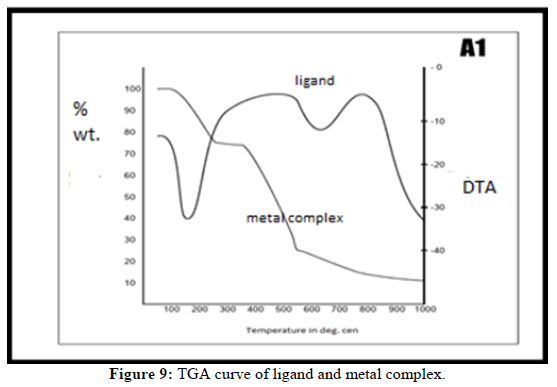
Thermogravimetric analysis
The synthesized Zn (II) metal complex of ligand ((C19H17N3O2SZn)) was analyzed through TGA with heating rate 20°C min-1. The TG analysis predicts the nature of volatile compounds produced while heating [10,11]. In the first phase temperature range 50°C to 200°C weight loss was found due to dehydration of the complex. In the further heating weight loss of complex found gradually due to combustion of moieties present out of sphere and inner sphere. It was confirmed that above temperature 800°C the final product occurred ZnO. It predicts the calcination temperature was 800°C. The TGA curves are shown in Figure 9 using this curves the thermodynamic parameters are calculated.
Calculation of kinetic and thermodynamic parameters from TGA data
The kinetic and thermodynamic parameters were calculated by considering the decomposition thermal reaction as first order reaction. Rate equation of first order reaction is shown in equation 1.
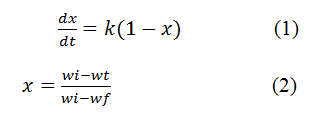
wi is the initial weight, wt of sample at particular time t and wf is final weight. Equation (1) can be written as:
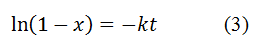
Using equation (3) graph was plotted; the value of slope of obtained straight line is equal to rate constant (k). The half-life period (t1/2) was determined by using equation (4):

The thermodynamic parameters were calculated by using basic thermodynamic equations. Both parameters are shown in Table 2. On the TGA data, it is confirmed that the decomposition of metal complex reaction is endothermic in nature. The positive values of ΔH and ΔG and negative value of ΔS reveals that the reaction is non-spontaneous.
| Metal Complexes | Temp (K) | K (min-1) | t1/2 (min) | Ea (Jmol-1) | ΔH (Jmol-1)(×103) | ΔS (Jmol-1K-1) | ΔG (Jmol-1)(×104) |
|---|---|---|---|---|---|---|---|
| (Zn (A1)) (C19H17N3O2SZn) |
623 | 0.3 | 2.31 | 2.4942 | 5.1821 | -281.04 | 18.02701 |
Table 2: Kinetic and Thermodynamic parameters of [Zn (A1)] / (C19H17N3O2SZn).
XRD
Powder XRD diffractogram of Zn(II) complex were recorded in the range 22°-80° at wavelength 1.540 A° . The diffractogram and associated data depict the 2θ value of each peak, relative intensity and inter planer spacing major relaxes were used to determine corresponding inter planer distance. The X-ray diffraction pattern of Zn(II) complex with respect to major peaks having relative intensity greater than 10% has been indexed by using computer program. Miller indices, unit cell parameters and unit cell volume were also obtained from above indexing method. The unit cell of Zn(II) complex yield value of lattice constants a=11.2235, b=7.5331 , c=5.0391 and unit cell volume 425.44 A°. Also the condition as a≠b≠c andα=γ=900 ≠β for a sample to be monoclinic were tested and found satisfactory. Hence Zn(II) complex have monoclinic crystal system..
Biological activity
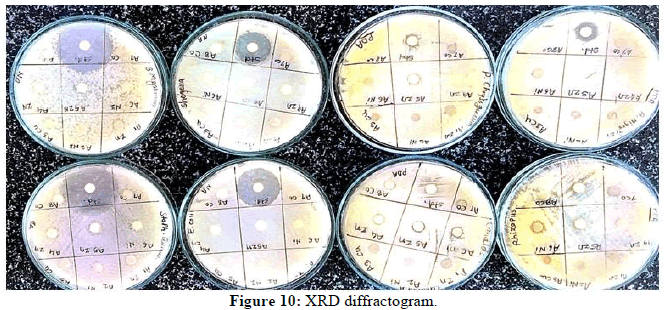
The in vitro antifungal and anti-microbial activities [12,13] of synthesized schiff bases have been studied by disc diffusion method. The antifungal and antibacterial activities were done at 100 μg/mL concentration in chloroform solvent using four fungal and bacterial strains. Aspergillusniger, Candida albicans, Chrysogenum, Rhizopus and Shigella, Staphylococcus aureus, Escherichia coli, Bacillus megaterium by Minimum Inhibitory Concentration method (MIC). These fungal and bacterial strains were incubated for 24 h at 28ºC. Standard fluconazole and streptomycin drugs were used for comparison under similar condition. Activity was determined by measuring the diameter of the zone of inhibition (mm). It was observed that, the ligands are more active against the fungal and bacterial strains Aspergillus niger and Staphylococcus aureus as compare to other fungal and bacterial strains. The metal complex of the ligand shows moderate antifungal and antibacterial activity against these four fungal and bacterial strains. The synthesized ligand is more feasible to fungal and bacterial strains than its Zn(II) complex due to greater polarity of ligand than its metal complex (Figure 10) [14,15].
Conclusion
The ligand 3-(ferfuraline-4-(5-P-tilyl-1,3,4 thiadiazole-2-ylimino)pentane-2-one and its Zn (II) complex have been synthesized and screened against analytically and biologically. The ligand moiety exhibits tri dentate coordination mode in Zn (II) metal complex. Solid reflectance spectra reveal that complex is monoclinic crystal system, diamagnetic and tetrahedral structure. The TGA and kinetic data indicate that the reaction is first order and spontaneous in nature. In vitro ligand exhibit more active biologically than its metal complex because of the multi vacancy of sulphur, while in metal complex sulphur coordinated with metal.
References
- Raman N, Thangaraja C. Transit Met Chem. 2005; 30: p. 317-322.
- Parashar RK, Sharma RC, Kumar A, et al. Inorganica Chim Acta. 1988; 151(3): p. 201-208.
- Sorenson JR, Soderberg LS, Chang LW. Proc Soc Exp Biol Med. 1995; 210(3): p. 191-204.
- Tripathi V, Dey A. Journal of the Indian Chemical Society. 1975; 52(3): p. 269-270.
- Biradar NS, Patil BR. Monatsh Chem. 1977; 108: p. 581-588.
- Gruber SJ, Harris CM, Sinn E. J Chem Phys. 1968; 49: p. 2183-2191.
- Gruber SJ, Harris CM, Sinn E. J Chem Phys. 1968; 49(5): p. 2183-2191.
- Aswar AS, Bhave NS. J Indian Chem Soc. 1974; 74: p. 75-78.
- Battin SN, Dave L D. Indian J Chem; 1974; 12: p. 984-990.
- Hassan AM, Heakal BH, Awad B, et al. Al-Azhar Bul Sci. 2017; 9: p. 157-176.
- Zhang Q, Zhang JB, Cao LH, et al. J Chin Chem Soc Taip 2010; 57: 992-997.
- Huma R, Mahmud T, Awan SJ, et al. Arab J Chem. 2022; 15: p. 103640.
- Yousif E, Majeed A, Al-Sammarrae K, et al. Arab J Chem. 2017; 10: p. 639-644.
- Rao PV, Narasaiah AV. Indian J Chem. 2003; 42: p. 1896-1899.
- Das A, Mishra DK, Sinha DB. J Coord Chem. 2017; 70: p. 3035-3047.
- Pravin N, Raman N. Eur J Med Chem. 2014; 85: p. 675-687.