Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
GC/MS Comparison Study of Pelargonium graveolens Essential Oils Extracted by Hydrodistillation and Microwave Assisted Hydrodistillation from North Regions in Kingdom of Saudi Arabia
Mahmood Salman1,2, El-Sayed S Abdel-Hameed1,2,3 and Azzah A Alshehri2,4*
1Natural Products Analysis Laboratory, Faculty of Science, Taif University, Saudi Arabia
2Chemistry Department, Faculty of Science, Taif University, Kingdom of Saudi Arabia
3Medicinal Chemistry Laboratory, Theodor Bilharz Research Institute, Giza, Egypt
4Chemistry Department, Bisha University, Kingdom of Saudi Arabia
- *Corresponding Author:
- Azzah A Alshehri
Chemistry Department
Faculty of Science
Taif University
Kingdom of Saudi Arabia
Abstract
Essential oils are complex mixtures of organic compounds that give characteristic odour and flavour to the plants. There are several techniques for essential oils extraction, including Hydrodistillation (HD). Microwave-Assisted Hydrodistillation (MAHD) is an advanced hydrodistillation technique, in which a microwave oven is used in the extraction process. MAHD and HD methods have been compared and evaluated for their effectiveness in the isolation of essential oils from fresh Pelargonium graveolens leaves that collected from north locations of KSA (Al-Riyadh, Mecca, and Al-Qatif). The composition of extracted essential oils was analyzed by Gas chromatography-Mass spectrometer (GC/MS). The identification will by the carried out by computer matching with NIST library as well as by comparison the retention time with standards. Results indicated that the use of microwave irradiation did not adversely influence the composition of the essential oils. A total of 67 compounds were identified in both HD and MAHD essential oils. The main constituents of HD oils were β-Citronellol (21.87-35.46%), Citronellyl formate (8.28-15.44%), and Geraniol (7.29-17.55%). While the main constituents of MAHD oils were β-Citronellol (24.66-32.95%), Citronellyl formate (10.51-13.71%), and Geraniol (6.84-9.68%). Results also indicated that the geographic location and the environment influenced in chemical composition of P. graveolens essential oil. So, we recommend further study on other plants grown in KSA.
Keywords
Pelargonium graveolens, Gas chromatography–Mass spectrometer, Essential oils, Hydrodistillation, Microwave assisted hydrodistilation
Introduction
Essential oils in plants are complex mixtures of volatile substances available in low concentrations [1]. These substances can be extracted by different methods. Hydrodistillation has been the most common approach to extract the essential oils from the medicine herbs and plants. Alternative methods, employing microwaves, have been developed in order to shorten extraction time, improve the extraction yield, and reduce the operational costs. Microwave-assisted procedures for isolating essential oils have become attractive for use in laboratories and industry. Novel Microwave-Assisted Extraction (MAE) methods such as Microwave-Assisted Hydrodistillation (MAHD) have proven to be fast and efficient methods for extracting essential oils from medicinal plants [2].
The identification of essential oil composition allows for a better and specially directed application [3]. Gas chromatography is widely used technique for the analysis of essential oils and aroma research [4]. Gas chromatography (GC) and mass spectroscopy (MS) combined together gives a perfect and effective separation process. The gaseous state of the compounds can use in both methods (GC and MS), so they can mix directly and easily together [5].
Pelargonium graveolens is a species indigenous to Southern Africa (widespread especially in the Cape Town region) belonging to the Geraniaceae family, which was introduced in Europe since the beginning of the eighteenth century [6]. The essential oil of the P. graveolens is employed in creams, cosmetics, perfumery, good antioxidant activity, soaps, aromatherapy products exhibit and has possible immune regulating impact on natural killer cells [7]. Also, it has been applied for several years in traditional medicine, such as antihepatotoxic, antiasthmatic, antiallergic, stomachic, antioxidant, haemostatic, antidiarrhoeic, diabetic, tonic, and diuretic [8].
Many previous researches pointed that essential oil composing of rose-scented geranium is affected through several of factors, including age of leaves, cultivars and method of distillation, location of growing, storage of oil, type of distillation unit used, seasonal changes and plant part distilled [9-13].
The objective of this study was the analysis of essential oils composition of P. graveolens that collected from north sites of Kingdom of Saudi Arabia (KSA) and extracted by two methods: hydrodistillation and microwave assisted hydrodistillation. To the best of our knowledge, and based on a literature surveys, this is the first report on the analysis of P. graveolens essential oils from KSA using GC-MS.
Materials and Methods
Chemicals
All standards and reagents were analytical and GC grade from Sigma-Aldrich Chemicals, USA.
Plant Material
The plant materials of P. graveolens were collected in March 2015 from different regions in Kingdom of Saudi Arabia such as Al-Riyadh, Macca- Benimalek, Mecca- Maysan, and Al-Qatif, Table 1 shows the altitude and sites, where this plant is frequently used in traditional medicine by the people. The collection plant was taxonomically kindly identified at the department of Biology, faculty of Sciences, Taif university.
| altitude | Longitude and latitude | ||
|---|---|---|---|
| Al-Riyadh | 597.6 m | N 24.65700º | E 46.75781º |
| Benimalek | 2153.9 m | N 20.59551º | E 41.07788º |
| Maysan | 2324.3 m | N 20.71758º | E 40.81146º |
| Al-Qatif | 5.6 m | N 26.49112º | E 50.01436º |
Table 1: The altitude and sites of P. graveolens collected from KSA
Essential oil extraction
Classical hydrodistillation apparatus and procedure
The samples preparation and essential oil extraction from the collected fresh plant samples, the leaves were released, typically 300 g of the fresh leaves were cut into smaller pieces and hydro distilled for 3 h using clevenger type apparatus producing a less colored essential oils at a yield of 0.5-0.9%. Oils were dried over of anhydrous sodium sulfate and filtered. The obtained oils were collected in a sealed vial and stored at -5ºC.
Microwave-assisted hydrodistillation apparatus and procedure
Microwave-assisted hydrodistillation was performed at atmospheric pressure with a Milestone NEOS &GR microwave apparatus using a fixed power of 650 W for 30 min. Temperature was monitored by an external infrared sensor. The fresh plant materials (300 g, each) were ground into small pieces, then placed in a flat bottom flask (5 l) with 700 ml water, and subjected to microwave-assisted hydrodistillation using a Clevenger-type apparatus with cooling bath (-5ºC) system (30 min) (yield (v/w):between 0.6-1.0%. The obtained oils were collected in a sealed vial and dried over anhydrous sodium sulfate and stored at -5ºC.
Essential oil analysis
Sample preparation for gc/ms analysis
A quantity of 10 μl from the essential oil was mixed with 1 ml of GC grade n-hexane. The new mixture was agitated for 1 min, and 1 μl was injected into the GC–MS by using the auto sampler injector.
Gas chromatography-mass spectrometry system
The aim of the selection and definition of chromatographic condition is to achieve a proper separation of the components of the oil, both for the qualitative analysis, and for the proper quantification.
The analysis of the samples was performed using gas chromatograph (GC, Model CP-3800, Varian, Walnut Creek, CA, USA) coupled with a mass spectrometer (MS, Model Saturn 2200, Varian) and auto sampler (Model Combi Pal, Varian) system. The separation was done using a VF- 5ms fused silica capillary column (5% phenyl-dimetheylpolysiloxane, 30 m x 0.25 mm i. d., film thickness 0.25 μm, Varian). For MS detector, electron impact (EI) ionization system with ionization energy of 70 eV was used. Helium gas was used as a carrier gas at a constant low rate of 1 ml/min. Injector and mass transfer line temperature were set at 250 and 300°C, respectively. The Optimization condition for oven temperature was programmed for 1 min at 60°C, 60˗120°C at 1.5°C/min then hold for 1 min and 120˗260°C at 10°C/min then hold for 1 min at 260°C, solvent delay time 3 min. The injection of the samples was carried out with the auto-sampler for 1 μl with a split ratio 1/20. The conditions of analysis and specification of the instrument were optimized for a better separation and resolution. Identification of components was based on matching with mixed standard and Wiley and NIST electronic library.
Results and Discussion
Results
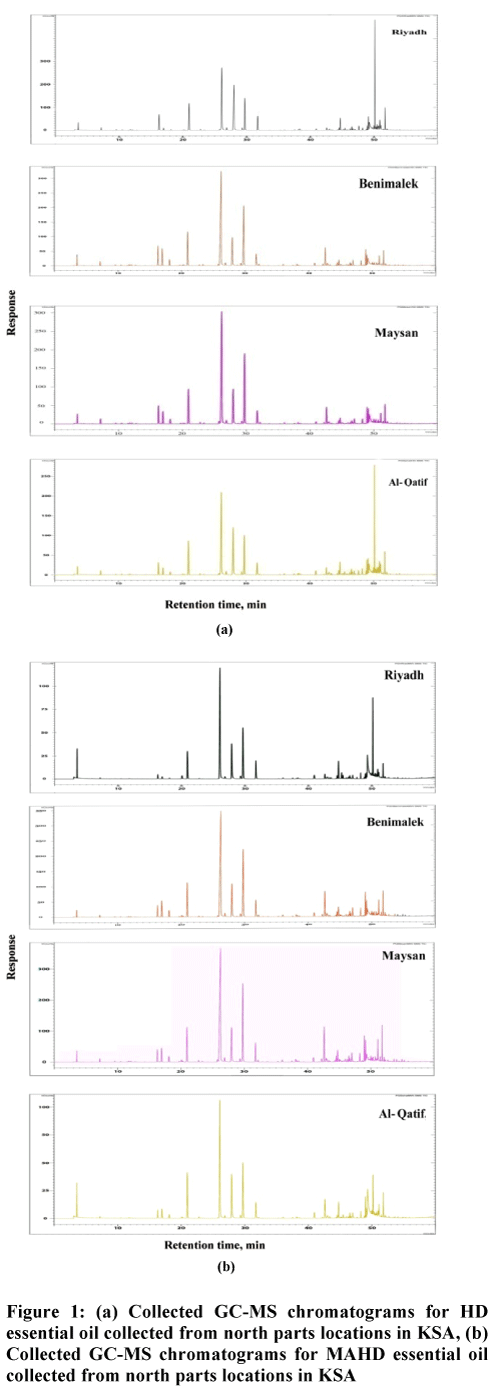
The leaves of P. graveolens were collected from north regions in Kingdom of Saudi Arabia. The essential oils of all samples were extracted by two methods; classical hydro-distillation (HD) and microwave-assisted hydro-distillation (MAHD). Analysis of the essential oils using GC/MS technique (Figure 1) revealed the presence of 67 components in both HD and MAHD essential oils.
In Riyadh samples, sixty-six compounds were identified from the P. graveolens essential oil extracted by hydrodistillation. While, fifty-five compounds were identified in the P. graveolens essential oil extracted by microwave assisted hydrodistillation. The most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by hydrodistillation were found to be β-Citronellol (21.87%), Geraniol (17.55%), γ-Eudesmol (14.52%), Citronellyl formate (8.84%), Isomenthone (6.93%), Geranyl formate (3.88%), β-Linalool (3.65%), Germacrene A (2.53%), Geranyl tiglate (2.27%), Phenyl ethyl tiglate (2.07%), α-Eudesmol (1.67%).
Moreover, The most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by MAHD were found to be β-Citronellol (28.83%), Citronellyl formate (12.17%), γ-eudesmol (11.44%), Geraniol (9.04%), Isomenthone (6.17%), Geranyl formate (4.42%), Germacrene A (3.23%), Octane (2.32%), Geranyl tiglate (1.54%), δ-Eudesmol (1.28%), α-Eudesmol (1.27%), Germacrene D (1.09%).
From Mecca two samples were collected from two different regions, Benimalek and Maysan. In Benimalek samples, sixty-seven compounds were identified from the P. graveolens essential oils extracted by both methods hydrodistillation and microwave assisted hydrodistillation. The most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by hydrodistilation were found to be β-Citronellol (34.32%), Citronellyl formate (14.44%), Geraniol (7.29%), Isomenthone (7.03%), β -Linalool (3.77%), Aristolen (3.54%), cis- Rose oxide (3.12%), Geranyl formate (2.99%), Caryophyllene oxid (2.11%), Phenyl ethyl tiglate (1.42%), Geranyl tiglate (1.3%), Citronellyl tiglate (1.22%), trans- Rose oxide(1.18%), Germacrene A (1.03%).
Moreover, the most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by MAHD were found to be β-Citronellol (32.26%), Citronellyl formate (13.71%), Geraniol (7.27%), Isomenthone (6.03%), Aristolen (4.2%), Geranyl formate (3.43%), Geranyl tiglate (2.93%), Caryophyllene oxid (2.61%), cis- Rose oxide (2.5%), Citronellyl tiglate (1.92%), β-Linalool (1.79%), Phenyl ethyl tiglate (1.71%), Germacrene A (1.38%), Citronellyl butyrate (1.32%), Geranyl isobutyrate (1.1%), trans-Rose oxide (1.06%).
In Maysan samples, sixty-seven compounds were identified from the P. graveolens essential oil extracted by Hydrodistillation. While, sixty-six compounds were identified from the P. graveolens essential oil extracted by microwave assisted hydrodistillation. The most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by Hydrodistillation (HD) were found to be β-Citronellol (35.46%), Citronellyl formate (15.44%), Geraniol (8.09%), Isomenthone (6.68%), β-Linalool (3.24%), Aristolen (3.18%), Geranyl formate (2.92%), cis- Rose oxide (2.11%), Caryophyllene oxid (1.98%), Phenyl ethyl tiglate (1.82%), Geranyl tiglate (1.67%), Citronellyl tiglate (1.02%).
Moreover, the most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by MAHD were found to be β-Citronellol (32.95%), Citronellyl formate (13.66%), Geraniol (6.84%), Isomenthone (5.25%), Aristolen (4.93%), Geranyl formate (3.43%), Geranyl tiglate (2.95%), Caryophylleneoxid (2.36%), Citronellyl tiglate (1.95%), Phenyl ethyl tiglate (1.95%), cis- Rose oxide (1.83%), β-Linalool (1.76%), Germacrene A (1.5%).
In Al- Qatif samples, sixty-six compounds were identified from the P. graveolens essential oil extracted by hydrodistillation. While, sixty-two compounds were identified from the P. graveolens essential oil extracted by microwave assisted hydrodistillation. The most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by hydrodistillation were found to be β-Citronellol (22.71%), γ-Eudesmol (14.71%), Geraniol (11.82%), Citronellyl formate (8.28%), Isomenthone (6.88%), Geranyl formate (2.71%), Geranyl tiglate (2.43%), Germacrene A (2.29%), β-Linalool (2.19%), Phenyl ethyl tiglate (1.98%), Caryophyllene oxid (1.95%), α-Eudesmol (1.81%), Aristolen (1.45%), cis- Rose oxide (1.21%), Cubenol (1.09%).
Moreover, the most abundant constituents (≥ 1%) in the P. graveolens essential oil extracted by MAHD were found to be β-Citronellol (24.66%), Citronellyl formate (10.51%), Geraniol (9.68%), Isomenthone (8.59%), γ-Eudesmol (8.35%), Aristolen (3.3%), Geranyl formate (3.25%), δ-Eudesmol (2.78%), Germacrene A (2.48%), Caryophyllene oxid (2.42%), Geranyl tiglate (1.87%), Octane (1.87%), cis- Rose oxide (1.44%), Citronellyl tiglate (1.44%), β -Linalool (1.22%), β-Caryophyllene (1.2%), Phenyl ethyl tiglate (1.19%), Cubenol (1.18%). For all samples the chromatograms resulting from the analysis process by GC–MS as shown below.
Table 2 lists the composition together with the percentage and retention time of P. graveolens essential oils from different localities in KSA that were extracted by HD and MAHD. The most abundant components found in HD essential oils were β-Citronellol (21.87-35.46%), Citronellyl formate (8.28-15.44%), Geraniol (7.29-17.55%), Isomenthone (6.68-7.03%). β-Linallol (2.19-3.77%), Geranyl formate (2.71-3.88%), γ- Eudesmol (0.47-14.71%), Phenyl ethyl tiglate (1.42-2.07%), cis-Rose oxide (0.48-3.12%), Aristolen (0.58-3.54%), Germacrene A (0.91-2.53%), Geranyl tiglate (1.30-2.43%), and Citronellyl tiglate (0.46-1.22%). Furthermore, the main components of MAHD essential oils were β- Citronellol (24.66-32.95%), Citronellyl formate (10.51-13.71%), Geraniol (6.84-9.68%), Isomenthone (5.25-8.59%). Geranyl formate (3.25- 4.42%), β-Linallol (0.83-1.79%), γ-Eudesmol (0.55-11.44%), Aristolen (0.97-4.93%), Citronellyl tiglate (0.74-1.95%), Germacrene A (1.38- 3.23%), Geranyl tiglate (1.54-2.95%), cis-Rose oxide (0.45-2.50%), and Phenyl ethyl tiglate (0.94-1.95%).
| % of oil from sample of | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oil constituent | tR | Al-Riyadh | Mecca-Benimalek | Mecca- Maysan | AL-Qatif | ||||
| HD | MAHD | HD | MAHD | HD | MAHD | HD | MAHD | ||
| Octane (C8) | 3.56 | 0.54 | 2.32 | 0.67 | 0.46 | 0.57 | 0.48 | 0.5 | 1.87 |
| α-Pinene | 7.19 | 0.36 | 0.13 | 0.5 | 0.2 | 0.55 | 0.25 | 0.44 | 0.17 |
| β -Pinene | 9.57 | 0.12 | - | 0.15 | 0.06 | 0.11 | 0.11 | 0.07 | - |
| α-Phellandrene | 10.48 | 0.12 | - | 0.13 | 0.07 | 0.12 | 0.07 | 0.11 | 0.06 |
| p-Cymene | 11.48 | 0.04 | - | 0.07 | 0.05 | 0.06 | 0.04 | 0.06 | |
| D-Limonene | 11.74 | 0.13 | - | 0.17 | 0.09 | 0.15 | 0.1 | 0.13 | 0.05 |
| β -Phellandrene | 11.83 | 0.13 | - | 0.11 | 0.07 | 0.11 | 0.06 | 0.08 | - |
| Eucalyptol (p-Cineol) | 11.94 | 0.09 | - | 0.06 | 0.04 | 0.04 | 0.06 | 0.05 | - |
| β -Linalool | 16.28 | 3.65 | 0.83 | 3.77 | 1.79 | 3.24 | 1.76 | 2.19 | 1.22 |
| cis- Rose oxide | 16.96 | 0.48 | 0.45 | 3.12 | 2.5 | 2.11 | 1.83 | 1.21 | 1.44 |
| trans-Rose oxide | 18.11 | 0.16 | 0.19 | 1.18 | 1.06 | 0.84 | 0.79 | 0.51 | 0.64 |
| Camphor | 19.75 | 0.04 | - | 0.16 | 0.13 | 0.15 | 0.14 | 0.04 | - |
| β -Citronellal | 20.11 | 0.13 | 0.81 | 0.13 | 0.31 | 0.14 | 0.31 | 0.18 | 0.26 |
| Menthone | 20.3 | 0.08 | - | 0.23 | 0.17 | 0.15 | 0.15 | 0.15 | 0.12 |
| Isomenthon | 20.98 | 6.93 | 6.17 | 7.03 | 6.03 | 6.68 | 5.25 | 6.88 | 8.59 |
| Menthol | 22.8 | 0.24 | 0.14 | 0.21 | 0.19 | 0.31 | 0.23 | 0.25 | 0.29 |
| α -Terpineol | 23.37 | 0.11 | - | 0.29 | 0.13 | 0.23 | 0.14 | 0.12 | 0.05 |
| Nerol | 25.79 | 0.21 | - | 0.36 | 0.29 | 0.64 | 0.49 | 0.37 | 0.14 |
| β - Citronellol | 26.25 | 21.87 | 28.83 | 34.32 | 32.26 | 35.46 | 32.95 | 22.71 | 24.66 |
| cis-Citral | 26.91 | 0.65 | 0.52 | 0.56 | 0.56 | 0.63 | 0.54 | 0.63 | 0.44 |
| Geraniol | 27.98 | 17.55 | 9.04 | 7.29 | 7.27 | 8.09 | 6.84 | 11.82 | 9.68 |
| trans-Citral | 29.34 | 0.68 | 0.67 | 0.58 | 0.63 | 0.67 | 0.56 | 0.73 | 0.68 |
| Citronellyl formate | 29.8 | 8.84 | 12.17 | 14.44 | 13.71 | 15.44 | 13.66 | 8.28 | 10.51 |
| Geranyl formate | 31.76 | 3.88 | 4.42 | 2.99 | 3.43 | 2.92 | 3.43 | 2.71 | 3.25 |
| Unkown | 32.18 | - | - | 0.34 | 0.31 | 0.32 | 0.28 | 0.11 | 0.19 |
| α -Cubebene | 35.32 | 0.08 | 0.09 | 0.06 | 0.1 | 0.06 | 0.09 | - | 0.08 |
| Citronellyl acetate | 36 | 0.14 | 0.26 | 0.34 | 0.36 | 0.3 | 0.33 | 0.17 | 0.27 |
| α -Copaene | 37.56 | 0.16 | 0.23 | 0.13 | 0.13 | 0.12 | 0.17 | 0.23 | 0.2 |
| β -Bourbonene | 38.13 | 0.2 | 0.19 | 0.33 | 0.38 | 0.3 | 0.44 | 0.31 | 0.3 |
| Neryl acetate | 38.36 | 0.39 | 0.28 | 0.18 | 0.2 | 0.16 | 0.2 | 0.32 | 0.21 |
| β –Cubebene | 38.61 | 0.11 | 0.2 | 0.09 | 0.2 | 0.07 | 0.22 | 0.16 | 0.22 |
| β -Caryophyllene | 40.95 | 0.42 | 0.92 | 0.63 | 0.78 | 0.5 | 0.76 | 0.9 | 1.2 |
| β-Guaiene | 42.23 | 0.05 | 0.05 | 0.32 | 0.38 | 0.27 | 0.47 | 0.12 | 0.23 |
| Aristolen | 42.61 | 0.58 | 0.97 | 3.54 | 4.2 | 3.18 | 4.93 | 1.45 | 3.3 |
| Citronellyl propanoate | 42.92 | 0.1 | 0.34 | 0.41 | 0.49 | 0.37 | 0.48 | 0.28 | 0.26 |
| Isoledene | 43.03 | 0.31 | 0.41 | 0.31 | 0.4 | 0.29 | 0.46 | 0.53 | 0.52 |
| α -Humulene | 43.34 | 0.12 | 0.28 | 0.18 | 0.23 | 0.15 | 0.24 | 0.27 | 0.3 |
| Aromadendrene | 43.58 | 0.09 | 0.23 | 0.06 | 0.07 | 0.05 | 0.1 | 0.12 | 0.09 |
| β -Cadinene | 44.35 | 0.15 | 0.12 | 0.18 | 0.21 | 0.17 | 0.25 | 0.22 | 0.17 |
| Geranyl propionate | 44.54 | 0.49 | 0.62 | 0.53 | 0.61 | 0.52 | 0.67 | 0.64 | 0.48 |
| Germacrene A | 44.75 | 2.53 | 3.23 | 1.03 | 1.38 | 0.91 | 1.5 | 2.29 | 2.48 |
| β -Gurjunene | 45 | 0.02 | - | 0.2 | 0.25 | 0.19 | 0.32 | 0.05 | 0.14 |
| β-Eudesmene | 45.12 | 0.07 | 0.04 | 0.19 | 0.25 | 0.18 | 0.28 | 0.15 | 0.15 |
| Germacrene D | 45.26 | 0.15 | 1.09 | 0.12 | 0.18 | 0.1 | 0.2 | 0.16 | 0.17 |
| γ-Elemene | 45.47 | 0.42 | 0.86 | 0.07 | 0.12 | 0.07 | 0.13 | 0.51 | 0.6 |
| α -Muurolene | 45.67 | 0.07 | 0.07 | 0.13 | 0.07 | 0.11 | 0.2 | 0.15 | 0.14 |
| Unkown | 45.86 | 0.1 | 0.06 | 0.06 | 0.11 | 0.07 | 0.09 | 0.17 | 0.13 |
| Pentadecane | 45.97 | 0.08 | 0.24 | 0.23 | 0.32 | 0.21 | 0.37 | 0.17 | 0.33 |
| γ-Muurolene | 46.28 | 0.27 | 0.25 | 0.17 | 0.25 | 0.19 | 0.29 | 0.35 | 0.26 |
| γ-Cadinene | 46.53 | 0.6 | 0.52 | 0.47 | 0.64 | 0.44 | 0.62 | 0.82 | 0.64 |
| δ-Cadinene | 46.69 | 0.3 | 0.25 | 0.35 | 0.46 | 0.32 | 0.48 | 0.46 | 0.33 |
| Citronellyl butyrate | 46.98 | 0.13 | 0.56 | 0.91 | 1.32 | 0.64 | 0.92 | 0.46 | 0.63 |
| α -Gurjunene | 47.07 | 0.14 | 0.14 | 0.03 | 0.04 | 0.13 | 0.18 | 0.24 | 0.16 |
| Geranyl isobutyrate | 48.24 | 0.38 | 0.83 | 0.72 | 1.1 | 0.63 | 0.95 | 0.82 | 0.76 |
| Caryophyllene oxid | 48.97 | 0.7 | 0.72 | 2.11 | 2.61 | 1.98 | 2.36 | 1.95 | 2.42 |
| Phenyl ethyl tiglate | 49.16 | 2.07 | 0.94 | 1.42 | 1.71 | 1.82 | 1.95 | 1.98 | 1.19 |
| δ-Eudesmol | 49.68 | 0.36 | 1.28 | 0.29 | 0.28 | 0.33 | 0.26 | 0.6 | 2.78 |
| Cubenol | 50 | 0.79 | 0.96 | 0.67 | 0.78 | 0.69 | 0.77 | 1.09 | 1.18 |
| γ-Eudesmol | 50.16 | 14.52 | 11.44 | 0.47 | 0.58 | 0.47 | 0.55 | 14.71 | 8.35 |
| δ-Cadinol | 50.32 | 0.3 | 0.18 | 0.5 | 0.6 | 0.49 | 0.57 | 0.44 | 0.3 |
| β-Eudesmol | 50.52 | 0.66 | 0.4 | 0.21 | 0.08 | 0.11 | 0.23 | 0.55 | 0.19 |
| τ-Cadinol | 50.67 | 0.31 | 0.16 | 0.33 | 0.48 | 0.36 | 0.47 | 0.38 | 0.23 |
| α-Eudesmol | 50.95 | 1.67 | 1.27 | 0.29 | 0.33 | 0.27 | 0.3 | 1.81 | 0.63 |
| Citronellyl tiglate | 51.11 | 0.46 | 0.74 | 1.22 | 1.92 | 1.02 | 1.95 | 0.92 | 1.44 |
| 1-Tetradecanol | 51.5 | 0.08 | 0.11 | 0.23 | 0.41 | 0.18 | 0.52 | 0.14 | 0.18 |
| Geranyl tiglate | 51.78 | 2.27 | 1.54 | 1.3 | 2.93 | 1.67 | 2.95 | 2.43 | 1.87 |
| Farnesol | 52.08 | 0.12 | 0.22 | 0.14 | 0.26 | 0.16 | 0.29 | 0.19 | 0.17 |
Table 2: The composition together with the percentage and retention time of Pelargonium graveolens essential oils that were extracted by MAHD and HD
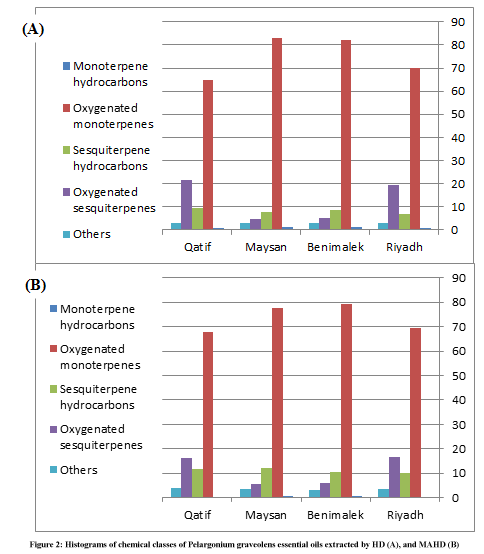
Table 3 and Figure 2 shown chemical classes of P. graveolens essential oils extracted by HD and MAHD. The essential oils which extracted by HD, contained monoterpene hydrocarbons (0.87-1.13%), oxygenated monoterpenes (64.87-83.05%), sesquiterpene hydrocarbons (6.84-9.48%), oxygenated sesquiterpenes (4.87-21.70%), and other components (2.87-3.18%). While the essential oils which extracted by MAHD, contained monoterpene hydrocarbons (0.13-0.64%), oxygenated monoterpenes (67.90-79.44%), sesquiterpene hydrocarbons (10.14-12.32%), oxygenated sesquiterpenes (5.79-16.64%), and other components (3.31-3.88%).
| Chemical classes | % of oil from sample of | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Riyadh | Mecca- Benimalek | Mecca-Maysan | Qatif | Total | ||||||
| HD | MAHD | HD | MAHD | HD | MAHD | HD | MAHD | HD | MAHD | |
| Monoterpene Hydrocarbons | 0.9 | 0.13 | 1.13 | 0.53 | 1.1 | 0.64 | 0.87 | 0.28 | 1 | 0.4 |
| Oxygenated Monoterpenes | 69.95 | 69.42 | 82.31 | 79.44 | 83.05 | 77.57 | 64.87 | 67.9 | 75.05 | 73.58 |
| Sesquiterpene Hydrocarbons | 6.84 | 10.14 | 8.61 | 10.71 | 7.79 | 12.32 | 9.48 | 11.68 | 8.18 | 11.21 |
| Oxygenated Sesquiterpenes | 19.44 | 16.64 | 5.01 | 6.01 | 4.87 | 5.79 | 21.7 | 16.26 | 12.76 | 11.18 |
| Others | 2.87 | 3.67 | 2.94 | 3.31 | 3.18 | 3.69 | 3.08 | 3.88 | 3.02 | 3.64 |
Table 3: Chemical classes of Pelargonium graveolens essential oils extracted by HD and MAHD
Discussion
Essential oils from leaves of P. graveolens were obtained by classical hydrodistillation, and microwave-assisted hydrodistillation). The oils were investigated by GC/MS. Both HD and MAHD oils of P. graveolens were strongly characterized by the presence of oxgenated monoterpenes, comprising (64.87-83.05%) of HD oils and (67.90-79.44%) of MAHD oils with β-Citronellol, Citronellyl formate, Geraniol, and Isomenthone dominating this fraction. Monoterpene hydrocarbons were poorly represented. Sesquiterpenes appeared as hydrocarbons (6.84-9.48%), (10.14- 12.32%) in HD and MAHD oils, respectively. Oxygenated sesquiterpenes representing (4.87-21.70%), (5.79-16.64%), respectively in HD and MWHD oils. Figure 2 shown histograms of chemical classes of P. graveolens essential oils extracted by HD and MAHD. The previous studies (obtained by hydrodistillation) agreement with this result was published by (Boukhris et al. 2012; Boukhatem et al. 2013(a); Boukhatem et al. 2013(b); Boukhris et al. 2013; Bouzenna and Krichen 2013; Afifi et al. 2014; Džamić et al. 2014; Hatami et al. 2014) [8,10,14-19].
The yields obtained by both methods: HD and MAHD are (0.5-0.9%), (0.6-1.0%), respectively. They are similar to each other while the differences appeared in the extraction time. The extraction time of MAHD is clearly shorter than that of classical HD, the full extraction took 30 min, whilst 3 h is required by HD.
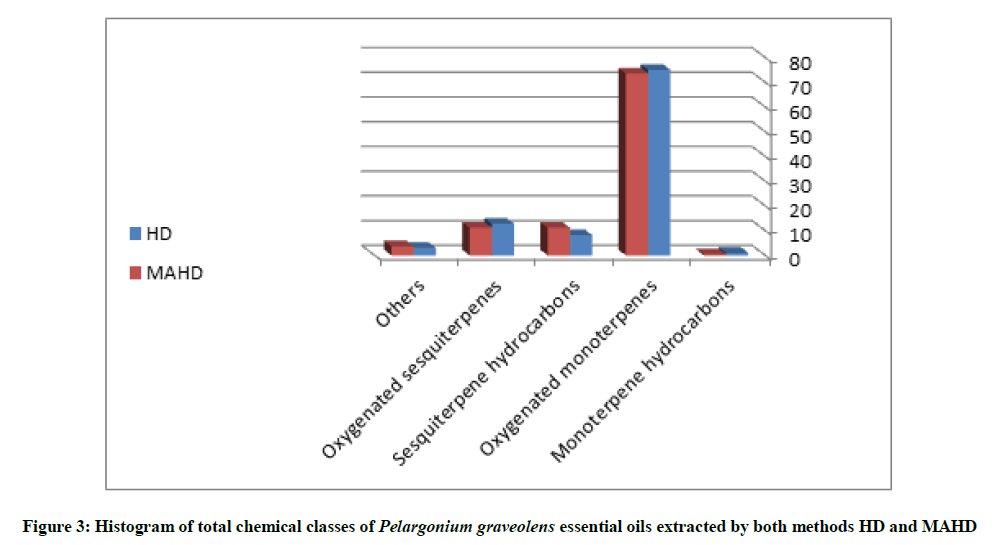
In general, there is no big different in chemical composition of extracted essential oils by both methods HD, and MAHD. Figure 3 is a histogram shows the total chemical classes of P. graveolens essential oils extracted by both methods HD and MAHD.
Prior to the present study the composition of P. graveolens oil obtained by hydrodistillation was studied by several researches and findings indicate that β-Citronellol is a prominent part of volatile oil as in the present study [1,20-29]. Other studies regarding the analysis of P. graveolens essential oils and they were similar to our result. But, it is interesting to mention that the main component was not Citronellol but Geraniol [11,30]. In addition, the study that carried out by (Juárez et al. 2016) [31] which extracted the essential oil by hydrodistillation coupled with microwave and the main component was not β-Citronellol but Geraniol. Among the different studies, including the present study, the greatest variation was observed with the two main compounds of the monoterpenes, namely Geraniol, and Linalool. This due to that the plant materials were collected from different geographical regions. Influence of climatic and environmental conditions may contribute to the observed qualitative and quantitative variations in composition [1,15,18,23,26,28].
There was an obvious disparity in the percentage of essential oil components in all samples which collected from north sites of KSA and extracted by HD which is the reference method in essential oil extraction. The essential oils extracted from Riyadh and Qatif samples, on altitude of 597.6 and 5.6 m, respectively, revealed that the oxygenated monoterpenes were 69.95 and 64.87% with β-Citronellol 21.87 and 22.71%, in Riyadh and Qatif oils, respectively, that represented the lowest percentage in samples. While, oxygenated sesquiterpenes were 19.44 and 21.70%, with γ-Eudesmol 14.52 and 14.71% in Riyadh and Qatif oils, respectively, that represented the highest percentage in samples.
The essential oils of P. graveolens extracted from Bnimalek, and Maysan, on altitude of 2153.9, and 2324.3 m, respectively, revealed that oxygenated monoterpenes percentage were 82.31, and 83.05 % with β-Citronellol 34.32, and 35.46 %, respectively, that represented the highest percentage in samples, while, the oxygenated sesquiterpenes were 5.01 and 4.87% respectively for same samples. Additionally, the sesquiterpene hydrocarbons were 8.61, and 7.79%, respectively, that represented more percentage than oxygenated sesquiterpenes.
The Citronellol to Geraniol (C/G) ratio is an important factor which determines the quality of essential oil of geranium. Generally, geranium oil which a C/G ratio 0.5-2.0 possesses a good odor value and is accepted by the perfume industry [23]. Thus, the present study showed that the samples Riyadh and Qatif with a C/G ratio (HD-MAHD) were (1.25-3.19), and (1.92-2.55), respectively. Additionally, (4.71-4.44), and (4.38- 4.82), representing the C/G of Bnimalek, and Maysan samples, respectively.
Conclusion
The essential oil of P. graveolens is one of the most famous twenty essential oil in the world with wide implementation in flavor industry, cosmetic industries and perfumery. There are many techniques used for extraction the essential oil. Microwave assisted hydrodistillation is a combination of microwave and Clevenger type (or hydrodistillation). The apparatus is relatively simple. One of the advantages of the MAHD method is rapidity. An extraction time of 30 min with MAHD provides yields comparable to those obtained after 3 h by means of HD, which is the reference method in essential oil extraction. The compositions of the oils were analyzed by using GC/MS, that widely applied for the analysis of essential oils and aroma study. According to our results which proved that there is no obvious difference in the chemical composition between samples which were extracted by different methods. But, there is obvious difference between samples which were collected of different regions or environments. From these results it can be concluded that the chemical composition of the essential oil obtained from the leaves of P. graveolens collected from different sites of KSA and extracted by HD and MAHD, have different qualitative and quantitative properties.
In conclusion, there are some studies have shown the possibility of using essential oil of P. graveolens not only as flavor agents but, this oil also introduced in many medical synthetics, including anti-oxidant property, treatment of skin diseases, anticancer activity, anti-inflammatory, antibacterial properties and arthritis. As well as, this aromatic oil are an important in impacting tonsillitis, rheumatism, poor circulation, curing burns and eczema, in addition, this essential oil assists in the enhanced circulation and stress associated condition.
As the current information shows, it is also possible that volatile components, of P. graveolens, and might be useful in the development of new drugs to treat various diseases. Of course, a further study is necessary to further elaborate the biological activities of P. graveolens essential oils for various applications and some test should be conducted in future, in order to evaluate the safety of these oils before human uses.
References
- E. Mousavi, H. Dehghanzadeh, and A. Abdali, Bull. Env. Pharmacol. Life Sci., 2014, 3, 10, 182-184.
- H.S. Kusuma, M. Mahfud, South African Journal of Chemical Engineering., 2016, 21, 1, 49-53.
- M. Lahlou, Phytother. Res., 2004, 18, 6, 435-448.
- S. Maurya, A.K. Kushwaha, and G. Singh, Advances in Natural Science, 2013. 6, 4, 84-95.
- T.F. Jorge, J.A. Rodrigues, C. Caldana, R. Schmidt, J.T. Van Dongen, J. Thomas-Oates, C. António, Mass Spectrom. Reviews., 2015, 35, 5, 620-649.
- G. Carmen, G. Hancu, Adv Pharm Bull., 2014, 4, 2, 511-514.
- J. Saraswathi, K. Venkatesh, N. Baburao, M.H. Hilal, A.R. Rani, J. Med. Plant. Res., 2011, 5, 13, 2587-2598.
- M. Boukhris, M. Bouaziz, I. Feki, H. Jemai, A. El Feki, S. Sayadi, Lipids Health Disease., 2012, 11, 1, 81-90.
- Wany, S. Jha, V.K. Nigam, D.M. Pandey, Int. J. Adv. Res.,2013, 1, 6, 504-521.
- M. Hatami, R. Ghafarzadegan, M. Ghorbanpour, Journal of Medicinal Plants, 2014. 13, 49, 5-14.
- R. Verma, R. Verma, A. Yadav, A. Chauhan., Ind. J. Nat. Prod. Reso., 2010, 1, 367-70.
- B. Eiasu, J. Steyn, P. Soundy, New Zealand Journal of Crop and Horticultural Science., 2008, 36, 4, 285-294.
- B.K. Eiasu, P. Soundy, J.M. Steyn, Hort. Science., 2008, 43, 2, 500-504.
- M.N. Boukhatem, A. Kameli, M.A. Ferhat, F. Saidi, M. Mekarnia, Libyan J. Med., 2013, 8, 1, 22520-22526.
- M.N. Boukhatem, A. Kameli, F. Saidi, Food Control.,2013, 34, 1, 208-213.
- M. Boukhris, M.S. Simmonds, S. Sayadi, M. Bouaziz, Phytother. Res., 2013, 27, 8, 1206-1213.
- H. Bouzenna, L. Krichen, Nat. Prod. Res., 2013, 27, 9, 841-846.
- F. Afifi, V. Kasabri, R. Abu-Dahab, I. Abaza, European J. Med. Plants.,2014, 4, 2, 220-233.
- A.M. Džamić, M.D. Soković, M.S. Ristić, S.M. Grujić, K.S. Mileski, P.D. Marin, J. appl. pharm. sci., 2014, 4, 03, 001-005.
- V.S. Rana, J.P. Juyal, A.M. Blazquez, International Journal of Aromatherapy.,2002, 12, 4, 216-218.
- M. Singh, S. Singh, M. Yaseen, Journal of Spices and Aromatic Crops., 2008, 17, 3, 247-250.
- S.A. Fayed, Res. J. Agric. & Biol. Sci., 2009, 5, 5, 740-747.
- A. Chauhan, R.S. Verma, Med. Aromat. Plant. Sci. Biotechnol., 2010, 4, 1, 77-79.
- A. Naeini, M. Nazeri, H. Shokri, J. Mycol. Med., 2011, 21, 2, 87-91.
- A. Ghannadi, M.R. Bagherinejad, D. Abedi, M. Jalali, B. Absalan, N. Sadeghi, Iran J. Microbiol., 2012, 4, 4, 171-176.
- A.B. Hsouna, N. Hamdi, Lipids in Health and Disease., 2012, 11, 1, 167.
- A. Ben Slima, M.B. Ali, M. Barkallah, AI. Traore, T. Boudawara, N. Allouche, R. Gdoura, Lipids in Health and Disease., 2013, 12, 1, 30-38.
- F.S. Sharopov, H. Zhang, and W.N. Setzer, Am. J. Essent. Oil. Nat. Prod., 2014, 2, 1, 13-16.
- Z.N. Juárez, H. Bach, E. Sánchez-Arreola, L.R. Hernández, J. Appl. Microbiol., 2016, 120, 5, 1264-1270.
- S. Ćavar, M. Maksimović, Food Control., 2012, 23, 1, 263-267.
- I. Atailia, A. Djahoudi, Phytothérapie., 2015, 13, 3, 156-162.