Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 4
Formulation and Characterization of Minoxidil Oral Thin Film
Souli Dutta1, Jayanta Chattopadhyay2, Biswanath Ghosh2, Diptendu Goswami3 and Suchandra Goswami2*2Department of Pharmaceutical Chemistry, Bengal School of Technology, Sugandha, Hooghly, West Bengal, India
3Department of Pharmaceutical Chemistry, BCDA College of Pharmacy and Technology, Barasat, West Bengal, India
Suchandra Goswami, Department of Pharmaceutical Chemistry, Bengal School of Technology, Sugandha, Hooghly, West Bengal, India, Email: suchandra77@yahoo.co.in
Received: 16-Jul-2024, Manuscript No. DPC-24-141981; Editor assigned: 19-Jul-2024, Pre QC No. DPC-24-141981 (PQ); Reviewed: 26-Jul-2024, QC No. DPC-24-141981; Revised: 01-Aug-2024, Manuscript No. DPC-24-141981 (R); Published: 29-Aug-2024, DOI: 10.4172/0975-413X.16.4.406-417
Abstract
Objective: The primary aim of the current investigation was to formulate and assess immediate-release oral thin films of minoxidil, with the goal of increasing the bioavailability of the medication and accelerating its onset of action, thereby enhancing patient adherence.
Methods: Using a film-forming polymer such as hydroxypropyl methylcellulose, the solvent evaporation method was used to create instantrelease oral thin films of minoxidil.
Results: A number of physicochemical characteristics, such as drug content, in vitro drug release, folding endurance, tensile strength, thickness and weight change, were assessed for the films. Since the formed films F1-F9 were immediately wetted by the dissolving medium, no variations in the in vitro dissolution of the drug were observed. For the F8 formulations, the drug release was almost 94%.
Conclusion: The current formulation uses mouth-dissolved films that have a quick start of action to deliver medication properly and have good therapeutic efficacy when taken orally.
Keywords
Thin film; Minoxidil; Alopecia; Drug Release; Disintegration; Dissolution; Patient compliance; Solvent evaporation; Formulation; Drug delivery
Introduction
Due to its many benefits over other drug administration routes, including ease of consumption, discomfort avoidance and versatility, the oral route is currently the most popular method for drug delivery. Additionally, solid oral drug delivery systems do not require sterile conditions and are therefore less expensive. However, due to certain drawbacks specific to a particular patient class namely, elderly, paediatric and dysphasic patients associated with a variety of medical conditions solid oral drug delivery systems still require advancements. These patients struggle to swallow or chew solid dosage forms. Due to fear of choking, many elderly and paediatric patients refuse to undertake solid preparations. Because of their tablet-like form, there is still a risk of choking even with quickly dissolving tablets. Minoxidil is currently available as a 2% w/w and 5% w/w topical solution or formulation. Recently, the minoxidil tablet (Sublingual) has been approved and marketed in the US and India. However, no studies on oral thin films of minoxidil have been reported in the literature [1].
A collection of flat films that are inserted into the oral cavity are known as oral film or in related literature, oral wafers. Dissolvable oral thin films, also known as Oral Strips (OSs), originated in the confection and oral care industries a few years ago as breath strips. Over time, they developed an innovative and extensively used method by consumers to obtain vitamins and personal hygiene goods. The use of OTFs is currently an effective method for delivering over-the-counter drugs to APIs [2].
Minoxidil was first made orally available to treat severe and resistant hypertension in the 1970’s. Because individuals with baldness also exhibit generalized hypertrichosis and hair growth, a topical minoxidil formulation was accidentally developed to treat Androgenetic Alopecia (AGA), first in males and then in females. The 2% and 5% minoxidil solutions were first made available on the market in 1986 and 1993, respectively. Although minoxidil has been used for hair growth promotion for more than thirty years, its precise mechanism of action is still unknown [3].
Materials and Methods
Materials
The following materials that were used were of the best possible grade available and were supplied by the manufacturer without further purification or investigation. Minoxidil (Dr Reddys laboratory, India), hydroxy propyl methyl cellulose (loba cheme) are water soluble polymers, aspartame (Yarrowchem product) is a sweeting agent, menthol (yarrowchem product) is a flavouring agent and eucalyptus oil (yarrowchem product) is a taste modifier. Croscarmellose sodium (vivasolfrom Germany) superdisintegrant; crospovidone (yarrowchem product) superdisintegrant; sodium starch glycolate (Loba cheme) superdisintegrant; glycerol (fischer scientific) plasticizer; HCl (Merck) dissolution medium.
Method of preparation of Oral Thin Films (OTFs):
• Weighing of all ingredients
• Preparation of polymer solutions
• Mixing aspartame, menthol and polymer solutions
• Addition of disintegrating agents
• Mixing of the above ingredients properly by triturating
• Finally, the drug solution was added, followed by glycerol
• Casting of film
• Cut the film to the desired size (1.6 × 2.2 cm2)
Preparation of the blank film
The preparation of OTF involved the dispersion of HPMC LV in a sufficient quantity of water. HPMC LV and distilled water were used at a ratio of 1:5. The HPMC LV was weighed carefully with a weighing balance and was kept on the side. The measured quantity of distilled water was poured into a can and heated at 70°C using a water bath. A mechanical stirrer was then fitted near the can. After that, the measured amount of HPMC LV was slowly added with continuous stirring for approximately 1 hr-2 hr. The tube was then kept in a refrigerator overnight. A clear HPMC slurry was obtained within 72 hours. Then, the components of the film were weighed as per the formula mentioned in Table 1 [4-7].
| Components | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Minoxidil | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg |
| HPMC LV | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg |
| Crosscarmellose sodium | 5 mg | 5 mg | 5 mg | 2.5 mg | 2.5 mg | 2.5 mg | 7.5 mg | 7.5 mg | 7.5 mg |
| Crosspovidone | 2.5 mg | 5 mg | 7.5 mg | 2.5 mg | 5 mg | 7.5 mg | 2.5 mg | 5 mg. | 7.5 mg |
| Aspartame | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg |
| Menthol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Eucaluptus oil | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Glycerol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
Table 1: Formulation table.
Measured amounts of aspartame and menthol were triturated. To this solution, drops of eucalyptus oil were added and the mixture was triturated. The HPMC slurry was added carefully and the mixture was triturated. Disintegrating agents were added and the mixture was triturated properly until it was mixed uniformly. Then, the required amount of water was added and mixed into the solution, followed by the addition of glycerol. It was then triturated properly. Finally, the film solution was cast on a glass plate and allowed to air dry. When the film was completely dry, it was cut to the desired size (1.6 × 2.2 cm2) [8,9].
Preparation of polymer solution
The preparation of OTF involved the dispersion of HPMC LV in a sufficient quantity of water. HPMC LV and distilled water were used at a ratio of 1:5. The HPMC LV was weighed carefully with a weighing balance and was kept on the side. The measured quantity of distilled water was poured into a can and heated at 70°C using a water bath. A mechanical stirrer was then used, after which the measured amount of HPMC LV was slowly added with continuous stirring for approximately 1 hr-2 hr. The tube was then kept in a refrigerator overnight. A clear HPMC slurry was obtained within 72 hours [10-12].
Evaluation of Oral Thin Films (OTFs)
Appearance of the film: Homogeneity, surface smoothness, color, transparency, odour and overall acceptance are the parameters observed for the assessment of physical appurtenance [13].
Weight variation: The films were prepared and cut from different areas into 10 patches of 1.6 × 2.32 cm2 each. Every patch was weighed and the mean and standard deviation were computed. It is necessary that every film patch has minimum weight variation, which can lead to differences in the dose of the drug in the films and in potent drug films, it is very harmful [14].
Surface pH: The surface pH of the film can cause irritation to the oral mucosa when it is placed into the mouth if its pH is acidic or alkaline, so it is important to determine the surface pH of the film. The film's surface pH should be neutral or 7 or close to 7. The pH of the films was assessed by moistening them with 0.5 ml of distilled water for 1 hr. The electrodes of the pH meter were brought in contact with the film surface and the readings were recorded when equilibrium was attained [15].
Folding endurance: Folding endurance is another metric used to estimate the mechanical properties of a film. The folding endurance of the nine films was studied by folding the film repetitively, folding one side of the film at the same place until it cracked. The folding endurance expresses the number of times the film is folded at the same place to develop visible cracks or to break the specimen [16].
Swelling properties: The results of the film swelling studies were verified using a simulated saliva solution. The initial weight of the film was determined and the film was placed in pre weighed stainless steel wire mesh. This mesh containing film was then dipped into the simulated saliva solution. An increase in the weight of the film is noted as constant predetermined time intervals until no more increase in weight is reached. The degree of swelling is determined by these parameters [17].
W=weight of film at time interval t.
Wo=weight of film at time 0.
Uniformity of drug content: Using a mechanical shaker, the film was placed in a 100 ml flask and the volume was increased with 0.1 N HCl. The film was then shaken constantly for two hours. Subsequently, Whatman grade I filter paper was used to filter the solution at a concentration of approximately 50 μg/ml. After that, a test solution was made using one millilitre of the prior solution and a double-beam UV-visible spectrophotometer was used to measure the absorbance at a wavelength of 279.4 nm. The absorbance was measured three times [18,19].
Percent moisture loss: One measure of a film's hygroscopicity is its percent moisture loss. Usually, this parameter is determined by first determining the initial weight of the film and then placing it in a hot air oven. At 30-minute intervals, the films were removed and weighed until a constant weight was reached. Moisture loss is determined by applying the following formula [18].
Disintegration test: A film was placed in a petri dish to measure the disintegration time. After the film was covered with distilled water, the amount of time it took for it to dissolve was recorded [19].
In vitro dissolution studies: In accordance with USP recommendations (USP, 2013), in vitro dissolution studies were conducted using a USP apparatus type ॥ (spinning paddle) in 900 ml of 0.1 N HCl at 50 rpm and 37°C ± 0.5°C. Throughout a half-hour period, sampling was performed every 5 minutes. Each time, a 5 ml sample was taken and replaced with an equivalent volume of fresh media. Before the samples were analyzed using the verified spectrophotometric method, they were filtered through 0.45 μm filter paper [20].
Thickness: The film thickness of six samples was measured from five different locations using filler gauze, after which the average thickness and standard deviation were calculated [20].
Percent elongation: The percent elongation of the strip was determined by stretching the films and was calculated by the following formula:
When a film sample is subjected to stress, it stretches and is referred to as being under strain. In essence, strain is the film's distortion divided by its initial dimension. Generally, the flexibility of the film increases as the plasticizer concentration increases. The percentage elongation was calculated by measuring the increase in the length of the film after tensile strength measurement by using the following formula. When stress is applied to the film, the sample stretches and is alluded to as a strain [21,22].
Percentage elongation =[{(L-L0) / L0*100],
where L=final length and L0=initial length.
Results
IR spectroscopic study
Appearance: The film prepared with the HPMC LV showed good film-forming properties. Mouth- dissolved films were visually evaluated and all the F1 to F9 films exhibited good transparency, homogeneity and smoothness.
Weight variation: Three films from each batch were taken and the mean value was calculated. The mean weight of the films was then considered. The data are presented in the table below.
Surface pH: All of the formulations had pH values between 5.31 and 7.25, which are almost neutral. Formulations F8 and F5 may cause less irritation to the buccal mucosa since their pH is closer to that of saliva. As previously established, the buccal mucosa becomes irritated by surfaces with basic or acidic pH.
Folding endurance: A small strip of film was folded repeatedly at the same location until it broke to determine the folding endurance values of the films, which ranged from 121 to 174.
Swelling properties: Three films of the optimized formulation were taken and the degree of swelling was determined.
Degree of swelling=[final weight (w)-initial weight (wo)]/initial weight (wo)]
W=weight of film at time interval t.
Wo=weight of film at time 0.
Content uniformity: A drug content uniformity test was conducted on each of the 10 formulations. The outcomes ranged between 97.6 and 114%.
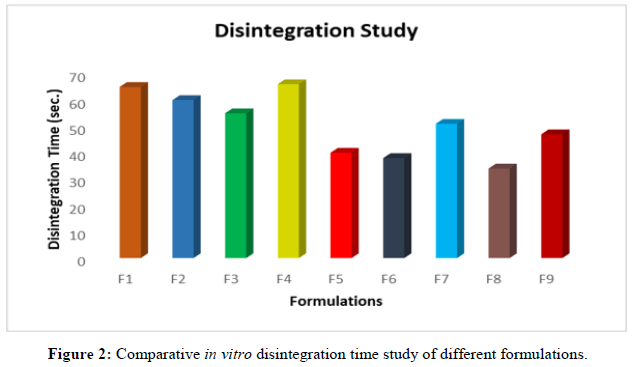
In vitro disintegration studies: In vitro disintegration studies were carried out for all nine formulation.
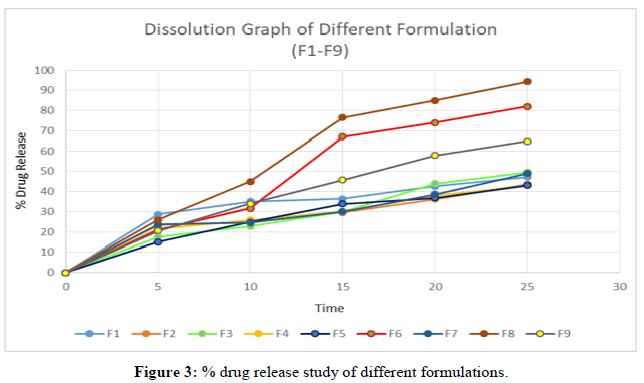
In vitro dissolution studies: A drug release study of the optimized formulation was performed. The table below represents the percentage of cumulative drug release of the optimized formulation.
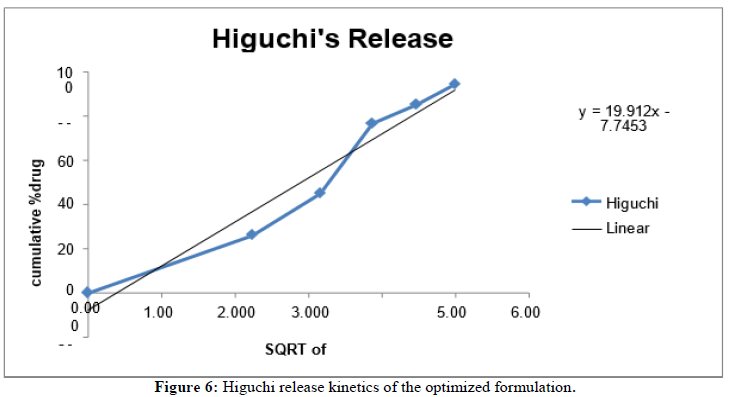
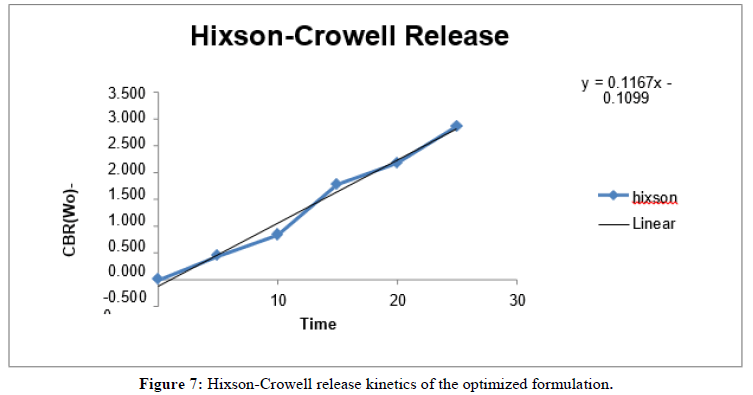
In vitro release kinetics: The release kinetics study of the optimized formulation was carried out on various kinetic models.
Trinocular microscopy images
Images of the minoxidil oral thin films were taken and evaluated. The surface of the film and the distribution of the polymer and drug within the films were examined. From the images below, it appears that the hydroxypropyl methyl cellulose-containing film (f8) is porous and has a short disintegration time (34 s) and a smooth surface (Figures 1-8).
Discussion
Minoxidil is under the brand name of Loniten, Mintop eva, Minodyl etc. Minoxidil comes under the classification of vasodilators. It helps to stimulate the hair growth in adult men and women who suffers from alopecia. In this study we have prepared an oral thin film of minoxidil and determined the critical parameters like physical properties, disintegration time, dissolution rate, moisture content, swelling properties etc.
Here in this study 2 different types of disintegrants (crosscarmellose sodium and crosspovidone) are used in different amount (2.5 mg, 5 mg, 7.5 mg). Based on the amount of disintegrating agents we plotted the disintegration time and % of drug release. Tables 11 and 14 showed the disintegration time and % drug release. Here we observed that formulation 6 and formulation 8 showed less disintegration time (F6 38 sec and F8 34 sec) and high % of drug release which were F6 82% and F8 94% in 25 minutes (Tables 2-16).
| Sl No | IR Bands (cm-1) | Assignments |
|---|---|---|
| 1 | 2362 | CH (Stretching aromatic and aliphatic) |
| 2 | 1646 | C=N (Stretching aromatic) |
| 3 | 1555, 1468 | Aromatic C=C (Stretching N-H bending) |
| 4 | 1234 | N-O (Stretching aromatic C-N stretching) |
Table 2: FTIR spectrum band assignments.
| Formulations | W1 (mg) | W2 (mg) | W3 (mg) | Mean weight (mg) | Std. Dev. |
|---|---|---|---|---|---|
| F1 | 139 | 136 | 138 | 137.6 | 1.41 |
| F2 | 141 | 140 | 143 | 141.3 | 1.24 |
| F3 | 142 | 144 | 141 | 142.3 | 1.24 |
| F4 | 135 | 137 | 134 | 135.3 | 1.24 |
| F5 | 137 | 134 | 138 | 136.3 | 1.69 |
| F6 | 140 | 142 | 141 | 141 | 0.81 |
| F7 | 142 | 144 | 139 | 141.6 | 2.05 |
| F8 | 143 | 144 | 143 | 143.3 | 0.47 |
| F9 | 145 | 148 | 144 | 145.6 | 1.69 |
Table 3: Study of the weight variations of the films.
| Formulations | Surface pH |
|---|---|
| F1 | 6.31 |
| F2 | 6.55 |
| F3 | 6.51 |
| F4 | 6.34 |
| F5 | 6.6 |
| F6 | 6.53 |
| F7 | 6.43 |
| F8 | 6.89 |
| F9 | 6.32 |
Table 4: Surface pH of the film.
| Formulations | 1 | 2 | 3 | Mean | Std. Dev. |
|---|---|---|---|---|---|
| F1 | 128 | 128 | 127 | 127.66 | 0.57 |
| F2 | 151 | 149 | 148 | 149.33 | 1.52 |
| F3 | 121 | 128 | 126 | 125 | 3.6 |
| F4 | 141 | 144 | 153 | 146 | 6.24 |
| F5 | 135 | 122 | 132 | 129.66 | 6.8 |
| F6 | 118 | 127 | 119 | 121.33 | 4.93 |
| F7 | 135 | 124 | 125 | 128 | 6.08 |
| F8 | 181 | 174 | 168 | 174.33 | 6.5 |
| F9 | 148 | 131 | 129 | 136 | 10.4 |
Table 5: Folding endurance of the film.
| Optimized formulations | Initial weight (W0) gm | Final weight (Wt) gm | Degree of swelling (W) |
|---|---|---|---|
| Strip-1 | 140 | 446.7 | 2.19 |
| Strip-2 | 144 | 457.9 | 2.17 |
| Strip-3 | 138 | 418.7 | 2.03 |
Table 6: Degree of swelling of the optimized formulations.
| Degree of Swelling (W) | Average degree of swelling | Std. Dev |
|---|---|---|
| 2.19 | 2.13 | 0.07 |
| 2.17 | ||
| 2.03 |
Table 7: Average degree of swelling of the optimized formulation.
| Optimized formulations (F8) | Initial weight (W0) mg | Final weight (Wt) mg | % Moisture loss |
|---|---|---|---|
| Strip1 | 136 | 123 | 9.5 |
| Strip2 | 142 | 128 | 9.85 |
| Strip3 | 139 | 126 | 9.35 |
Table 8: Percentage moisture loss of optimized formulations.
| % Moisture loss | Average % moisture loss | Std. Dev |
|---|---|---|
| 9.5 | 9.56 | 0.2 |
| 9.85 | ||
| 9.35 |
Table 9: Average percentage of moisture loss of the optimized formulations.
| Formulations | Amount of drug present (mg) | % Drug content |
|---|---|---|
| F1 | 5.7 | 114 |
| F2 | 5.5 | 110 |
| F3 | 5.2 | 104 |
| F4 | 4.9 | 98 |
| F5 | 5.6 | 112 |
| F6 | 5.1 | 102 |
| F7 | 4.88 | 97.6 |
| F8 | 5.7 | 114 |
| F9 | 5.1 | 102 |
Table 10: Average percentage of moisture loss of the optimized formulations.
| Formulations | Disintegration time (Sec.) |
|---|---|
| F1 | 65 |
| F2 | 60 |
| F3 | 55 |
| F4 | 66 |
| F5 | 40 |
| F6 | 38 |
| F7 | 51 |
| F8 | 34 |
| F9 | 47 |
Table 11: Disintegration time of the formulations.
| Formulations | W1 (mg) | W2 (mg) | W3 (mg) | Percent elongation (n=3) | Std. Dev. |
|---|---|---|---|---|---|
| F1 | 13.58 | 13.57 | 13.88 | 13.67 | 0.17 |
| F2 | 20.15 | 19.25 | 19.25 | 19.55 | 0.51 |
| F3 | 18.96 | 18.02 | 18.05 | 18.34 | 0.53 |
| F4 | 16.02 | 14.58 | 14.87 | 15.15 | 0.76 |
| F5 | 16.25 | 16.88 | 15.87 | 16.33 | 0.51 |
| F6 | 11.87 | 17.58 | 11.88 | 13.77 | 3.29 |
| F7 | 14.58 | 14.78 | 14.57 | 14.64 | 0.11 |
| F8 | 18.31 | 17.18 | 18.78 | 18.09 | 0.82 |
| F9 | 14.54 | 14.88 | 14.68 | 14.7 | 0.17 |
Table 12: Percentage elongation.
| Formulations | Film- 1 | Film- 2 | Film-3 | Thickness (mm) | Std. Dev. |
|---|---|---|---|---|---|
| F1 | 0.12 | 0.14 | 0.11 | 0.12 | 0.01 |
| F2 | 0.11 | 0.15 | 0.13 | 0.13 | 0.02 |
| F3 | 0.15 | 0.13 | 0.14 | 0.14 | 0.01 |
| F4 | 0.13 | 0.13 | 0.12 | 0.12 | 0.005 |
| F5 | 0.11 | 0.1 | 0.14 | 0.11 | 0.02 |
| F6 | 0.14 | 0.12 | 0.11 | 0.12 | 0.01 |
| F7 | 0.14 | 0.13 | 0.1 | 0.12 | 0.02 |
| F8 | 0.11 | 0.11 | 0.12 | 0.11 | 0.005 |
| F9 | 0.15 | 0.13 | 0.1 | 0.12 | 0.02 |
Table 13: Thickness of oral thin film.
| Time (min) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 28.7 | 23.5 | 17.6 | 21.67 | 15 | 21 | 23.66 | 26 | 20.51 |
| 10 | 35 | 24.8 | 22.7 | 25.89 | 24.8 | 31.6 | 24.54 | 44.9 | 34 |
| 15 | 36.31 | 29.5 | 30 | 30.21 | 33.83 | 67 | 30 | 76.54 | 45.76 |
| 20 | 42.6 | 36.3 | 44 | 38 | 36.76 | 74 | 38.65 | 85 | 57.89 |
| 25 | 47.24 | 43.6 | 49.5 | 42.98 | 43 | 82 | 49 | 94.3 | 64.89 |
Table 14: In-Vitro drug release of drug formulation.
| S. no | Kinetics | Slope | Intercept | R2 |
|---|---|---|---|---|
| 1 | Zero order | 3.8865 | 5.8752 | 0.9613 |
| 2 | First order | -0.0495 | 2.1048 | 0.9601 |
| 3 | Higuchi model | 19.91 | -7.7453 | 0.9485 |
| 4 | Hixson-crowell model | 0.116 | -0.1099 | 0.9859 |
Table 15: Release Kinetics study of optimized formulation.
| Components | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Minoxidil | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg |
| HPMC LV | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg | 120 mg |
| Crosscarmellose sodium | 5 mg | 5 mg | 5 mg | 2.5 mg | 2.5 mg | 2.5 mg | 7.5 mg | 7.5 mg | 7.5 mg |
| Crosspovidone | 2.5 mg | 5 mg | 7.5 mg | 2.5 mg | 5 mg | 7.5 mg | 2.5 mg | 5 mg | 7.5 mg |
| Aspartame | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg | 5 mg. | 5 mg |
| Menthol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Eucaluptus oil | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
| Glycerol | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml | 1 ml |
Table 16: Composition of the various formulations.
Conclusion
Fast dissolving film when placed in the oral cavity quickly dissolve and stick onto the site of application; then disintegrate to release the drug. In this study, a fast dissolving oral thin film of Minoxidil were formulated and characterized. Various formulation was prepared in which F8 formulation was found to have optimal quality profile (disintegration time 34 sec, dissolution rate 94.3% in 25 minute) Folding endurance also found adequate (mean 174). Fast dissolving film can be a potential novel drug dosage form for paediatric, geriatric and also for general population.
Acknowledgment
The authors acknowledge support from the Bengal school of Technology and the multiple assistance associated with this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author's Contributions
JC designed the experiment. SD performed the experiment, wrote the manuscript. SD and SG performed the analysis of results.
Conflicts of Interest
All authors have none to declare.
References
- Nishigaki M, Kawahara K, Nawa M, et al. Int J Pharm. 2012; 424(1-2): p. 12-17.
[Crossref] [Google Scholar] [PubMed]
- Simons P, Olofsen E, van Velzen M, et al. Front Pain Res. 2022; 3: p. 946486.
[Crossref] [Google Scholar] [PubMed]
- Shimoda H, Taniguchi K, Nishimura M, et al. Eur J Pharm. Biopharm. 2009; 73(3): p. 361-365.
[Crossref] [Google Scholar] [PubMed]
- Yir-Erong B, Bayor MT, Ayensu I, et al. Ther Deliv. 2019; 10(7): p. 443-464.
[Crossref] [Google Scholar] [PubMed]
- Özakar RS, Özakar E. Turk J Pharm Sci. 2021; 18(1): p. 111.
[Crossref] [Google Scholar] [PubMed]
- Prajapati VD, Chaudhari AM, Gandhi AK, et al. Int J Biol Macromol. 2018; 107: p. 2075-2085.
[Crossref] [Google Scholar] [PubMed]
- Wennerberg A, Frojd V, Olsson M, et al. Clin Implant Dent Relat Res. 2011; 13(3): p. 184-196.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Quan P, Fang L. Int J Pharm. 2015; 496(2): p. 314-322.
[Crossref] [Google Scholar] [PubMed]
- Traas AM, Fleck T, Ellings A, et al. Am J Vet Res. 2010; 71(6): p. 610-614.
[Crossref] [Google Scholar] [PubMed]
- Gupta S, Kumar TP, Gowda DV. Recent Pat Drug Deliv Formul. 2020; 14(2): p. 88-97.
[Crossref] [Google Scholar] [PubMed]
- Kumar GP, Phani AR, Prasad RG, et al. Int J Pharm. 2014; 471(1-2): p.146-152.
[Crossref] [Google Scholar] [PubMed]
- Karimiyan H, Hadjmohammadi MR, Kunjali KL, et al. Molecules. 2019; 24(20): p. 3664.
[Crossref] [Google Scholar] [PubMed]
- Mushtaque M, Muhammad IN, Hassan SM, et al. Pak J Pharm Sci. 2020; 33(1).
[Google Scholar] [PubMed]
- Hashimoto Y, Ueda M, Kohiga Y, et al. Dent Mater J. 2018;37(3): p. 408-413.
[Crossref] [Google Scholar] [PubMed]
- Shamma R, Elkasabgy N. Drug Del. 2016; 23(2): p. 489-499.
[Crossref] [Google Scholar] [PubMed]
- Alaei S, Omidi Y, Omidian H. Eur J Pharm Sci. 2021; 166: p. 105965.
[Crossref] [Google Scholar] [PubMed]
- Khadra I, Obeid MA, Dunn C, et al. Int J Pharm. 2019; 561: p. 43-46.
[Crossref] [Google Scholar] [PubMed]
- Yardley MM, Huynh N, Rodgers KE, et al. Alcohol. 2015; 49(6): p. 553-539.
[Crossref] [Google Scholar] [PubMed]
- Cao B, Wang Y, Li N, et al. Dent Mater J. 2013; 32(2): p. 311-316.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Lu A, Thakkar R, et al. Pharmaceutics. 2021; 13(10): p. 1613.
[Crossref] [Google Scholar] [PubMed]
- Adrover A, Varani G, Paolicelli P, et al. Pharmaceutics. 2018; 10(4): p. 222.
[Crossref] [Google Scholar] [PubMed]
- Wang T, Ansai T, Lee SW. J Chromatography B. 2017; 1041: p. 133-140.
[Crossref] [Google Scholar] [PubMed]