Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Evaluation of the Anti-Hyperglycemic Property of the Philippine Duhat (Syzygium cumini), Crude Methanolic Leaf Extract on Glucose Challenged Hyperglycemic Mice
Allan L Hilario*
Department of Biochemistry and Molecular Biology, College of Medicine, University of the Philippines-Manila, 547 Pedro Gil St., Ermita, Manila 1000 Philippines
- *Corresponding Author:
- Allan L Hilario
Department of Biochemistry and Molecular Biology
College of Medicine
University of the Philippines-Manila
547 Pedro Gil St., Ermita, Manila 1000 Philippines
Abstract
Due to metabolic stress secondary to hyperglycemia and safety issues of primary pharmacologic agents used for the treatment of diabetes, safer plant-based natural products are being evaluated as alternative supplements in ensuring normal basal glucose level both in normal and diabetic patients. Recent studies have focused on developing such herbal drugs from plants like Syzygium cumini. This plant is readily used as an herbal drug in the Philippines due to its known medicinal properties; there are no sufficient studies currently available in the literature that validates the efficacy of the local duhat variety against hyperglycemia especially that of the leaf extract. In this study, crude methanolic extract of S. cumini leaves was evaluated for its anti-hyperglycemic property. Upon administration of treatment in glucose challenged hyperglycemic mice at 0, 0.5, 1.0, 1.5, and 2.0 h, results showed that the mean blood glucose levels of hyperglycemic mice treated with glibenclamide had a constant significant decrease as compared with the normal control (p=0.00045; p<0.05). Similar findings were noted with the mean blood glucose levels of hyperglycemic mice treated with S. cumini crude methanolic extract (p=0.00575; p<0.05). Hyperglycemia was controlled early on the observation period after glibenclamide administration and later after S. cumini crude methanolic leaf extract administration. It can be established that the crude methanolic extract of S. cumini leaves exhibits anti-hyperglycemic property on glucose challenged hyperglycemic mice. This plant can be a potential source of herbal drugs to supplement the pharmacologic treatment of diabetes.
Keywords
Hyperglycemia, Syzygium cumini, Glibenclamide, Glucose-challenged mice, Blood glucose levels
Introduction
Hyperglycemia is a serious condition that is usually involved in the dysfunction of insulin secretion. All though not a disease per se, avoidance of episodes of hyperglycemia like in pre and post prandial states in normal humans is optimal. In patients with diabetes being treated with pharmacologic drugs, hyperglycemia remains to be a challenge and should be prevented. Hyperglycemia in between intake of anti-diabetic drugs still poses metabolic stresses [1]. In persistent hyperglycemia, the cellular metabolic system is under the constant pressure due to glucose overloading. This causes glucotoxicity and activates several metabolic or signaling pathways that attempt to not only dispose excessive glucose, but also generate more reactive oxygen species that lead to oxidative stress and β cell failure. This condition is one the main pathogenic mechanisms of diabetes and its significant macrovascular and microvascular complications, making diabetes not only a metabolic disease but an inflammatory disease too [2-4].
The use of plants for healthcare has been found since the advent of human history. Traditionally, plants are used in treating various diseases such as diabetes, diarrhea, malaria, burns, and stomach disorders [5,6]. There is a wide option of oral hypoglycemic agents available in the market. However, since these drugs, although proven effective in the treatment of diabetes, are known to have adverse effects. Treatment control of diabetes with these drugs, even in the right dose and frequency intake, may not effectively address the intermittent state of hyperglycemia. The need to provide adjunct medications with safer pharmacologic profile is imperative. Hence, medicinal plants may be proven valuable source of effective natural products to be used as supplements in controlling blood glucose level [7]. There has been a recent exponential growth in the studies and development of herbal medicines in the Philippines largely due to their non-toxic nature, lesser side effects, and economic affordability [8,9].
Syzygium cumini, locally known as Philippine duhat [10], is one of the widely used traditional ethnomedicinal plants for treating various diseases throughout the world [11]. It is characterized as a tropical fruit tree [6], native to the Philippines and is found mostly in India, Indonesia, and throughout other Southeast Asian countries [5,10], and is considered of great economic importance since all parts of the tree can be used for various medicinal purposes [12]. However, although this plant is readily used as herbal drug in the Philippines due to its known medicinal properties, there are no sufficient studies currently available in the literature that validates the efficacy of the local duhat variety against hyperglycemia [8].
There have been few studies that have evaluated the extracts of various parts of S. cumini, especially the seeds and fruits, for its anti-hyperglycemic activity. It is observed that the blood and urine glucose levels of diabetic rats are significantly decreased upon treatment with ethanolic extract of duhat seeds [13]. Moreover, there is a recent study by Morales et al., which shows that the leaf extract of S. cumini is a nontoxic substance that can exhibit hypoglycemic activity or maintain the normal level of blood glucose in rats [14].
However, there are no studies regarding the anti-hyperglycemic effect of the methanolic leaf extracts of S. cumini, specifically its local variety in the Philippines. Most of the available studies on S. cumini are done on the seeds and fruits and on the variety locally found in India [7]. Hence, this study was conducted to evaluate the anti-hyperglycemic property of the crude methanolic leaf extract of Philippine duhat (S. cumini). Specifically, it evaluated the anti-hyperglycemic property of the crude methanolic leaf extract through Oral Glucose Tolerance Test (OGTT) in mice.
Materials and Methods
The animal experiment performed in this study was guided by the principles of animal use, care and welfare based on the Animal Welfare Act of the Philippines (RA 8485) and Administrative Order No. 45 of the Bureau of the Animal Industry and registered in their animal study registry. The study was approved by the Institutional Animal Care and Use Committee (IACUC), University of the Philippines-Manila with IACUC Protocol No. 2017-026. The study was registered to the Research Grants Administartion Office of the National Institutes of Health, University of the Philippines-Manila with registration no. 2017-1036.
Acclimatization of animals
Fifteen (15) male ICR mice of the same age (12-14 weeks) and more or less of the same weight (20-24 grams) were obtained from the National Institutes of Health, University of the Philippines-Manila. These animals were caged separately and kept at standard relative humidity (70-72%), temperature (20-22°C), and a 12-h light and dark cycle for one week before the start of the experiment. The animals were given access to standard animal pellet diet and water ad libitum.
Preparation of the crude methanolic extract of S. cumini
The leaves of the Philippine duhat (S. cumini) were acquired from the Bureau of Plant Industry (BPI, Manila, Philippines), and were authenticated by the Department of Biology, College of Arts and Sciences, De La Salle University, Taft, Manila. The fresh samples were then finely chopped, and dried over shade for 7 days, before soaking in methanol. After overnight soaking, the extract was subsequently collected by filtration. The remaining duhat leaves were re-soaked two more times with methanol. The generated extracts from the three batches were then pooled and dried using a rotary evaporator. The starting pressure was set at 300 mbar, and rotation speed of 3-4 times per second, heated at 37- 40°C, and then chilled at 4°C. The generated extract was kept in 4°C until further use. Each use of the generated extarct was suspended in distilled to make a solution of 500 mg/mL as the stock solution for use in the experiment.
Determination of anti-hyperglycemic property
Oral Glucose Tolerance Test (OGTT)
Fifteen test mice were fasted overnight, and then randomly divided into three groups, each receiving different treatments. The first group (Positive Control) received glibenclamide (10 mg/kg), and served as the positive control. The second group (Treatment Group) received 250 mg/kg of the crude methanolic leaf extract of S. cumini as the treatment group and the negative control group (Negative Control) only received distilled water as treatment. All treatments were given by oral gavage with a maximum volume of 0.5 ml.
After fasting, all mice were given a glucose challenge of three gms/kg given through oral gavage. The stock solution of the glucose used was 50% dextrose in 50 ml water (Euromed, Mandaluyong, Philippines). The blood glucose levels were measured after 30 min from the glucose challenge (0 hour). All mice were considered as hyperglycemic when blood glucose level after the glucose challenge is more than 11.1 mmol/l (200 mgs/dl). Once hyperglycemic, Positive Group received glibenclamide 10 mgs/kg via oral gavage (Euglocon™, Abbott Health Care Pvt Ltd, Mandaluyong, Philippines). Treatment Group received crude methanolic left extract of Philippine duhat (S. cumini) 250 mg/kg via oral gavage. Negative Group received 0.5 ml of distilled water via oral gavage.
After 0.5, 1, 1.5, and 2 hours, blood glucose levels were determined using a glucometer (GlucoX™, TaDoc Technology Corporation, New Taipei City, Taiwan). Blood glucose level was determined by making a vein prick using one of the tail veins. Once a good blood drop is produced after a vein prick, it was loaded onto the test strip. The blood glucose level was read after one minute. Test result from poor blood loading and read more than one minute were discarded.
Data Processing and Statistical Analysis
The blood glucose level was presented in mean. The independent student t-test was used for statistical comparison. Statistical significance was considered if the calculated p-value was less than 0.5.
Results and Discussion
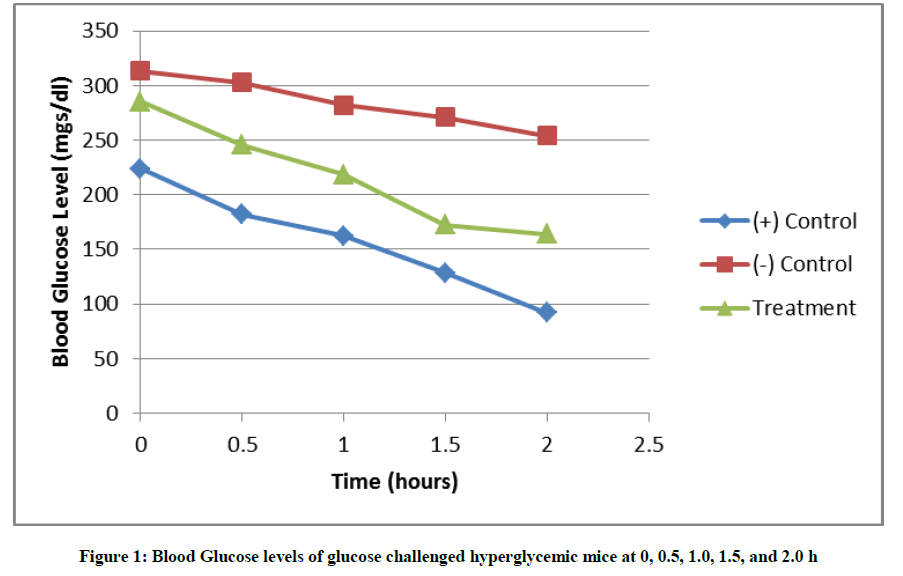
In this study, the crude methanolic extract of S. cumini leaves was evaluated for its anti-hyperglycemic property. Figure 1 shows that there was a continuous decrease in the blood glucose levels of the glucose challenged hyperglycemic mice after being treated with glibenclamide and the crude methanolic leaf extract of S. cumini at 0.5, 1.0, 1.5 and 2.0 h. It also shows that the positive control group had an immediate decrease to non-hyperglycemic state at 0.5 h and returned to normal blood glucose level at 1.5 and 2 h after the administration of glibenclamide, while the same was observed only at 1.5 and 2.0 hours upon the administration of the crude methanolic leaf extract. Table 1 shows the mean blood glucose levels of the different groups and time points in this study.
| Time points (Hour) | Groups | ||
|---|---|---|---|
| Negative Control | Treatment Group | Positive Control | |
| 0 | 17.4 | 15.9 | 12.4 |
| 0.5 | 16.8 | 13.6 | 10.1 |
| 1 | 15.6 | 12.2 | 9 |
| 1.5 | 15.1 | 9.6 | 7.2 |
| 2 | 14.1 | 9.1 | 5.1 |
Table 1: Blood Glucose levels of glucose challenged hyperglycemic mice at 0, 0.5, 1.0, 1.5, and 2.0 h
Table 1 shows that hyperglycemia persisted in the negative group. This animal model of hyperglycemia is a good, reproducible and less toxic to the animal subject for the evaluation of anti-hyperglycemia of any products in pre-clinical studies. The positive group returned to normal values at two hours after glucose challenge. In the treatment group, hyperglycemia persisted late in the observation period at one hour after glucose challenge and became non-hyperglycemic at 1.5 and 2.0 h. Hyperglycemia is the elevation of blood glucose level above 11.1 mmol/l (200 mg/dl). In mice, the normal fasting blood sugar is in the range of 3.4 to 7.2 mmol/l while for non-fasted mice, the range is 6.3 to 9.6 mmol/l [15]. In humans, the fasting blood sugar is in the range of 3.9 to 5.5 mmol/l. The target blood glucose for diabetic humans is in the range of 5.0 to 7.2 mmol/l [16].
The goal in the treatment of both type 1 and 2 diabetes mellitus with insulin and oral hypoglycemic drugs respectively, is to mimic the normal basal blood glucose level in body. Studies report that uncontrolled hyperglycemia such as in pre and post prandial state and in between intake of medications or injection with insulin still pose problems and complications associated with diabetes. It may contribute to various microvascular complications like retinopathy and nephropathy. Despite several treatment options, safer alternatives like medicinal plants may supplement diabetic control [17,18].
In this study, the leaves of S. cumini) of the Philippine variety has anti-hyperglycemic property comparable with glibenclamide, a representative drug of the sulfonylurea family of hypoglycemic drugs. But unlike S. cumini, sulfonylureas are associated with hypoglycemia which could be fatal, one of the many side effects of these drugs. This side effect of sulfonylureas can lower blood glucose level even when the blood glucose level of diabetic individual is already controlled. This can also happen to normal individuals who may accidentally take this drug. Hypoglycemia may lead to shock and could be fatal [16,19].
Conclusion and Recommendations
In this study, the crude methanolic extract of S. cumini) leaves showed blood glucose lowering property similar to glibenclamide in glucose challenge hyperglycemic mice. S. cumini) can be a potential source of plant-based pharmacologic agent, which can be used in controlling unwanted hyperglycemic states in diabetic individuals. In order to provide more information regarding the pharmaceutical properties of the local variety of S. cumini leaves, it is recommended that other medicinal properties, such as anti-coagulant, gastro-protective, and reno-protective properties, be further investigated using the crude methanolic leaf extracts of Philippine Duhat.
Acknowledgement
The author would like to thank Ms. Judy Ann Cocadiz for her invaluable support and contribution in the experimentation and preparation of the manuscript.
References
- D. Yao, Y. Zhao, X. Gao, Y. Zhou, Life Sci., 2016, 144, 148-155.
- J. Wu, L.J. Yan, Diabetes Metab. Syndr. Obes., 2015, 8, 181-188.
- W. Hunt, S. Zughaier, D. Guentert, M. Shenep, M. Koval, N. McCarthy, J. Hansen, Am. J. Physiol. Lung Cell Mol. Physiol., 2013, 306, L43-L49.
- The Diabetes Control and Complications Trial Research Group, N. Engl. J. Med., 1999, 329, 977-986.
- R. Haque, M. Sumiya, N. Sakib, O. Sarkar, T.T. Siddique, S. Hossain, A. Islam, A. Parvez, A. Talukder, S. Dey, Adv. Microbio., 2017, 7, 195-204.
- G. Jagetia, J. Explor. Res. Pharma., 2017, 2, 2, 54-66.
- S. Sarkar, A. Sengupta, A. Mukhrjee, A. Guru, A. Patil, A. Kandhare, S. Bodhankar, Pharmacol., 2015, 6, 7, 273-281.
- M. Ayyanar, P. Subash-Babu, S. Ignacimuthu, Complement. Thera. Med., 2013, 21, 232-243.
- S. Srivastava, D. Chandra, J. Sci. Food Agri., 2013, 93, 9, 2084-2093.
- C. Ragasa, O. Torres, C.C. Shen, M.K.E. Lachica, A.B. Sulit, D.B.D.L. Chua, A.D.M. Ancheta, C. Ismail, F.T. Bernaldez, D. Raga, Chem. Nat. Comp., 2014, 50, 5, 942-944.
- M. Ayyanar, P. Subash-Babu, Asian Pac. J. Trop. Biomed., 2012, 240-246.
- B. Chaudharu, K. Mukhopadhyay, Int. J. Pharm. Bio. Sci., 2012, 2, 1, 46-53.
- V.T. Chagas, L.M. Franca, S. Malik, A.M. Front, Pharmacol., 2015, 6, 259.
- S. Morales, J.M. Camad, R. Merida, P.L. Sanchez, R.J. Vidal, M.C. Wamimal, Root Gatherers, 2014, 7, 57-73.
- T.M. Wallace, D.R. Matthews, Int. J. Med., 2000, 93, 6, 369–374.
- D.L. Kasper, A.S. Fauci, S.L. Hauser, D.L. Longo, J.L. Jameson, J. Loscalzo. Harrison's Principles of Internal Medicine, McGraw-Hill Education, New York, USA, 2015, 19th (Edn.), 417-421.
- UK Prospective Diabetes Group Study, Lancet., 1998, 352, 837-853.
- R. Robertson, J. Harmon, P. Tran, Y. Tanaka, H. Takahashi, Diabetes., 2003, 52, 3, 581-587.
- M. Stewart. Glibenclamide for diabetes, 2016.