Research Article - Der Pharma Chemica ( 2018) Volume 0, Issue 0
Evaluation of Inhibitory Effect of Nerium oleander Leaf Extract on Mild Steel Corrosion in Aqueous Medium
Arockia Selvi J*, Pushpa Malini T, Arthanareeswari M, Kamaraj P, Mohan Kumar R, Sneh R Patel and Subasree N
Department of Chemistry, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur-603203, India
- *Corresponding Author:
- Arokia Selvi J
Department of Chemistry
Faculty of Engineering and Technology
SRM Institute of Science and Technology
Kattankulathur-603203, India
Abstract
Corrosion inhibitors are substances that are coated on the metal surface so as to avoid direct contact of the metal with the surrounding. Many natural products have inhibitory properties due to the presence of heterocyclic constituents such as alkaloids, flavonoids. Presence of tannins, cellulose and polycyclic compounds aids in formation of a protective film over the metal surface. In this article the inhibitory actions of Nerium oleander leaf extract as corrosion inhibitor for mild steel in 3.5% NaCl was investigated by weight loss method and electrochemical studies. Weight loss study reveals that N. oleander is a potential inhibitor in 3.5% NaCl. Satisfactory Inhibition Efficiencies (IE) were observed. It was also seen that the IE increases with increase in temperature, indicating chemisorptions of the inhibitor on metal surface. Linear polarisation studies coincided with the weight loss study. Potentiodynamic polarisation study reveals the inhibitor as the mixed type of inhibitor. The formation of the protective film on the metal surface was confirmed by Fourier Transform Infra-Red (FTIR) spectroscopy. Surface morphology was studied by Scanning Electron Microscopy (SEM).
Keywords
Nerium oleander leaf extract, Corrosion inhibitor, Mild steel, Aqueous medium, Electrochemical study, FTIR, SEM
Introduction
Corrosion is recognised as a major problem both in developed as well as developing countries. According to a survey carried out it was established that the losses due to corrosion were equivalent to 3.4% of the global GDP (2013). The chemical inhibitors used are reported to be more or less toxic to the human as well as other environmental resources and so there is huge demand for the greener alternatives. For instance, the use of hexavalent chromium in inhibitors for aerospace instruments has been found to have remarkably high carcinogenic content by the Environmental Protection Agency (EPA) [1]. Organic inhibitors are compounds that inhibit corrosion by means of adsorption. The use of various plant extracts in different mediums has been reviewed by Rani et al., and it governs the importance and need for the usage of plant extracts as potential inhibitors [2]. The presence of heterocyclic groups in plants is the reason for the inhibitory effects. Inhibition efficiency of Myrmecodia pendans on mild steel in 3.5% NaCl was studied and concluded that the optimum concentration for the maximum inhibition efficiency is 400 mg/l and confirmed the formation of a protective film on the metal surface [3]. In a comparative study between the inhibition efficiencies of zinc acetate, zinc acetylacetonate and zinc gluconate on mild steel in 3.5% NaCl, zinc gluconate was observed to be a more potential comparatively as it formed an insoluble corrosion product which aided in corrosion protection [4]. Study on the efficiency of natural honey as a natural indicator in 3.5% NaCl showed that the performance of the indicator dropped with time due to the growth of fungi [5]. Similar observation was observed in high degree saline medium as well [6]. The inhibitive properties of caffeine towards the corrosion of copper in neutral 0.5 mol-1 NaCl solution investigated by potentiodynamic polarization and EIS measurements, established that the caffeine was a very good cathodic inhibitor, with inhibition efficiency up to ≈ 92% [7]. Extracts of Andrographis paniculata, Strychnous nuxvomica, and Moringa oleifera showed their inhibition efficiencies on mild steel in HCl medium with A. paniculata having the maximum efficiency of 98% [8]. The use of various natural products in the corrosion inhibition of various metals in different corrosive media has been reviewed by Raja et al. [9]. The effect of aqueous extracts of Damsissa, Lupine and Halfa-bar on the corrosion of aluminium alloy in an aqueous solution of 0.5 M NaCl was reported to behave as cathodic inhibitors and that the mechanism was through adsorption of the plant extract on the metal plate [10]. Some inhibitors inhibit corrosion with increase in temperature while some work in the reverse manner. In a comparative study between imidazoline and amide it was studied that the inhibition efficiency decreases with increase in temperature. This can be attributed to the chemical instability of the inhibitor at high temperatures. Amide offers a better IE to imidazoline due to long term thermal stability [11]. Physisorption is the general mechanism regarded towards inhibitors whose efficiency decreases with increase of temperature [12]. It was observed to be an endothermic and spontaneous process in the inhibition by Curcuma longa extract [13] while the process was exothermic for Baphia nitida [14]. There are studies that also focus on the increase in efficiency with increase in temperature. It is a characteristic feature of chemisorption and there is abundant literature available to support this study [15,16]. The leaf extract of Ananas sativum also shows the effect on temperature in acidic medium [17].
The use of plant inhibitors, their effectiveness, their advantages, disadvantages, need for their incorporation in the market and the problems dealt which are highlighted in a recent review by Ismail et al. [18]. This study mainly focuses on the inhibitory effects of Nerium oleander leaf extract on mild steel in aqueous medium 3.5% NaCl at two different temperatures of 303K and 328K. Weight loss and electrochemical polarisation studies were carried out at various concentrations and it was seen that the corrosion rates decreases with increase in inhibitor concentration and in turn lead to increase in inhibition efficiency. FTIR study was done to confirm the protective layer formed on the metal surface. Surface analysis was done by scanning electron microscopy (SEM).
Experimental Section
Material preparation
Mild steel coupons of 4 × 1 × 0.2 cm dimensions were taken for the study. They were polished with fine grade emery sheets and then degreased with trichloroethylene. They were dried with a drier and cooled in desiccator and subjected for weight loss study. The composition of the mild steel used was investigated by Chemical Optical Emission Spectroscopy. The percentage composition of the metal constituents was given in Table 1.
| Fe | C | Mn | Si | Cr | Mo | S | P | Al |
|---|---|---|---|---|---|---|---|---|
| 97.106% | 0.419% | 0.76% | 0.239% | 1.079% | 0.274% | 0.031% | 0.014% | 0.032% |
Table 1: Composition of mild steel plate
Extract preparation
100 grams of shade dried N. oleander leaves were soaked in 1000 ml of ethanol for 24 h. The ethanol solution was filtered with Whatman filter paper 42 and was evaporated at 60°C for the removal of ethanol. The dried powder of 1 g was dissolved in Dimethyl Sulphoxide (DMSO) and then made up to 100 ml with distilled water. It was filtered to remove the suspended particles and the filtrate was used to carry out the studies.
Solution preparation
3.5% NaCl was prepared by dissolving 35 g of analar grade sodium chloride in 1000 ml of distilled water.
Weight loss determination
Metal coupons in duplicate were immersed in 100 ml beaker with varying concentrations of the inhibitor in 3.5% NaCl. The tests were carried out for a span of 24 h and 72 h at 303K. After the immersion, the coupons were taken out and washed with running water, dried and cooled in a desiccator. The weight of the metal coupons were measured before and after immersion. Difference in weight was noted as weight loss and the Corrosion Rate (CR), Inhibition Efficiency (IE) were calculated. Similar procedure was done for the weight loss analysis at 328K in a water bath for 6 h.
Inhibition efficiency was calculated by the following formula:
 ---------- (1)
---------- (1)
Where, w=Weight loss in the absence of inhibitor, w1=Weight loss in the presence of inhibitor.
Corrosion rate was calculated by the following formula:
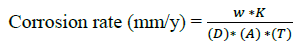 ----------- (2)
----------- (2)
Where, w=Weight loss in mgs, K=87.6 (constant), D=Alloy density=7.86 g/cm3, A=Exposed area (cm2), T=Exposure time (h)
Potentiodynamic polarisation studies
The electrochemical polarisation measurements were carried out using three-electrode setup comprised of counter electrode (Platinum electrode), a working electrode (a mild steel coupon), and a reference electrode (Saturated calomel electrode). Electrochemical measurements were carried out using biologic work station. The metal was immersed in the test solutions for 5 min at OCP to attain a stable state. The potentiodynamic current-potential curves were recorded by changing the electrode potential from-250 to +250 mV vs OCP with a scan rate of 1 mVs-1. The studies were performed for varying concentrations for a temperature of 328K. Inhibition efficiency was calculated by the following formula:
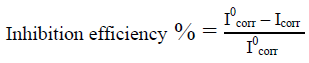 ---------- (3)
---------- (3)
Where, I0corr and Icorr are the corrosion current densities without and with inhibitor. The corresponding Tafel plots were plotted and studied.
Fourier Transform Infra-Red (FTIR) analysis
The samples were examined with a Brucker Fourier transform Infrared spectrometer. The corresponding spectra were recorded in the absorbance mode, giving a linear scale for quantitative measurements. The dried plant extract was analysed by FTIR and the metal coupon dipped in best system was studied by Fourier Transform Infrared Reflection-Absorption Spectroscopy (FT-IRAS). From this single reflection spectrum was obtained to give the corresponding spectrum.
Scanning Electron Microscopy (SEM) analysis
The surface morphology measurements of carbon steel were examined using model FEI Quanta FEG 200-High resolution scanning electron microscope. It provides the information about the sample's surface topography.
Results and Discussions
Weight loss analysis
Weight loss data, corrosion rate and the inhibition efficiencies for different concentrations at a temperature of 303K and 328K were calculated and tabulated.
Effect of immersion period on inhibition efficiency
The effect of inhibitor for two different immersion periods (24 and 72 h) was studied with the concentrations ranging from 0.5 ml to 2.5 ml (with 0.5 ml increment) in 3.5% NaCl. The results were noted and tabulated in Table 2. A gradual increase in IE was observed with increase in inhibitor concentration. The IE increased from 2.38% for 0.5 ml N. oleander to about 16.66% for 2.5 ml N. oleander. There was significant decrease in CR as well. However there was very less inhibition observed with small amounts of inhibitor concentration.
| Duration of immersion | Conc. of Nerium oleander leaf extract (ml) |
CR (mm/y) |
IE (%) |
|---|---|---|---|
| 24 h | Blank | 0.09751 | 0 |
| 0.5 | 0.09519 | 2.38 | |
| 1 | 0.09287 | 4.76 | |
| 1.5 | 0.08823 | 9.53 | |
| 2 | 0.0859 | 11.9 | |
| 2.5 | 0.08126 | 16.66 | |
| 72 h | Blank | 1.4937 | 0 |
| 0.5 | 1.4627 | 2.07 | |
| 1 | 1.424 | 4.66 | |
| 1.5 | 1.3931 | 6.73 | |
| 2 | 1.3466 | 9.84 | |
| 2.5 | 1.3157 | 11.91 |
Table 2: Corrosion rate and inhibition efficiency of various concentrations of Nerium oleander leaf extract for mild steel in 3.5 % NaCl, Immersion Period: 24 and 72 h
The tests were then performed for a span of 72 h identical observations of increase in IE with increase in inhibitor concentration were observed. On comparing the IE between 24 h and 72 h it was observed that the IE decreased with increase in inhibition duration. For example, IE at 2.5 ml N. oleander in 3.5% NaCl for 24 h was calculated to be 16.66% while the IE at 2.5 ml N. oleander in 3.5% NaCl for 72 h was calculated to be 11.91%. The inhibition efficiency increases with increase in concentration. The inhibition efficiency decreased with increase in immersion period.
These results can be justified on the basis that as the concentration of the inhibitor increases the concentration of heterocyclic atoms also increases which results in the reduction of loss of electrons from the metal surface. Hence with increase in concentration there is increase in IE observed. Decrease in IE with time can be attributed to the fact that there is no strong bonding of the inhibitor film with the metal. With increase in immersion period the chloride ions present in the medium attacks the protective film making it weaker, therefore reducing the efficiency.
Effect of temperature on corrosion inhibition efficiency
In this study inhibitor concentration ranged from 2 ml to 12 ml (2 ml increment). The data is tabulated in Table 3. Here the effect of temperature was analysed. The IE when compared between 303K and 328K, IE was observed to be more for 328K. For example IE was 54.36% for 12 ml N. oleander at 303K and increased to about 88.88% for the same concentration at 328K.
| Temperature | Conc. of Nerium oleander leaf extract (ml) |
CR (mm/y) | IE (%) |
|---|---|---|---|
| 303K | Blank | 0.1068 | 0 |
| 2 | 0.0975 | 8.69 | |
| 4 | 0.0835 | 21.73 | |
| 6 | 0.0789 | 26.08 | |
| 8 | 0.065 | 39.13 | |
| 10 | 0.058 | 45.65 | |
| 12 | 0.0487 | 54.34 | |
| 328K | Blank | 0.3339 | 0 |
| 2 | 0.2597 | 22.22 | |
| 4 | 0.2226 | 33.33 | |
| 6 | 0.1484 | 55.55 | |
| 8 | 0.1298 | 61.11 | |
| 10 | 0.0927 | 72.22 | |
| 12 | 0.0371 | 88.88 |
Table: 3 Corrosion rate and inhibition efficiency of various concentrations Nerium oleander leaf extract for mild steel in 3.5 % NaCl. Temperature: 303K and 328K
This behaviour can be explained on the basis that the inhibitor components are firmly held on the metal surface by chemisorption as a result of which a surface film of the reaction product is formed and the surface area of the metal covered by inhibitor molecules increases as temperature rises [19,20]. Hence a chemisorptive mode of adsorption is more likely. Moreover, it is seen from the sample analysis that the mild steel used in this study has minor contaminations of manganese and chromium. The presence of these passivating transition metals in the steel sample probably lead to an increase in the activity of the steel surface to bind with the inhibitor molecule and affect the mechanism of the inhibition process by the inhibitor [21].
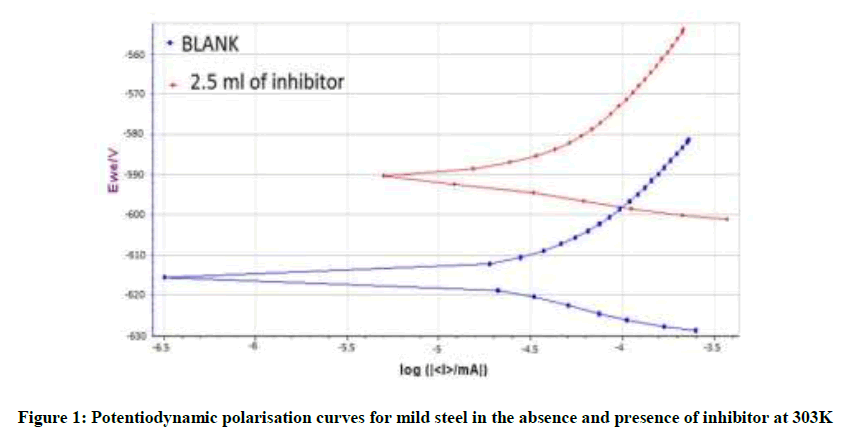
Analysis of potentiodynamic polarisation curves
Potentiodynamic polarisation parameter for mild steel immersed in 3.5% NaCl containing various concentration of N. oleander leaf extract are tabulated in Table 4. The corresponding polarisation curves are shown in Figure 1. Studies for linear polarisation at room temperature were carried out for two systems, blank solution of 3.5% NaCl and 2.5 ml N. oleander in 3.5% NaCl. The corrosion current for aqueous solution containing 3.5% of Cl- was 14.539 μA cm-2 and that for 2.5 ml N. oleander in 3.5% of Cl- was 11.635 μA cm-2. It was found that in the presence of inhibitors, the Icorr decreases. Ecorr values shifted from -616.708 mV to - 591.273 mV. There is small reduction in anodic and cathodic potential values also. The CR was observed to decrease from 0.17078 mm/y to 0.13669 mm/y. Decrease in CR implies increase in IE which can be seen to be 19.97%. The shift in positive side indicates that the inhibitor controls more predominantly the anodic reaction. The displacement in Ecorr value of 2.5 ml inhibitor formulation from the blank is found to be 25 mV which confirms that the inhibitor acts as a mixed type of inhibitor. The decrease in the value of the corrosion current densities decreases the corrosion rate of the metal.
| Conc. of Nerium oleander leaf extract (ml) |
Ecorr (mV vs SCE) |
Icorr (µA cm-2) |
βa (mV dec-1) |
βc (mV dec-1) |
CR (mm/y) |
IE (%) |
|---|---|---|---|---|---|---|
| 0 | -616.71 | 14.539 | 18.9 | 10.6 | 0.1708 | - |
| 2.5 | -591.27 | 11.635 | 13 | 7.3 | 0.1367 | 19.97 |
Table 4: Potentiodynamic polarisation parameter for mild steel without and with Nerium oleander leaf extract in 3.5% NaCl at 303K
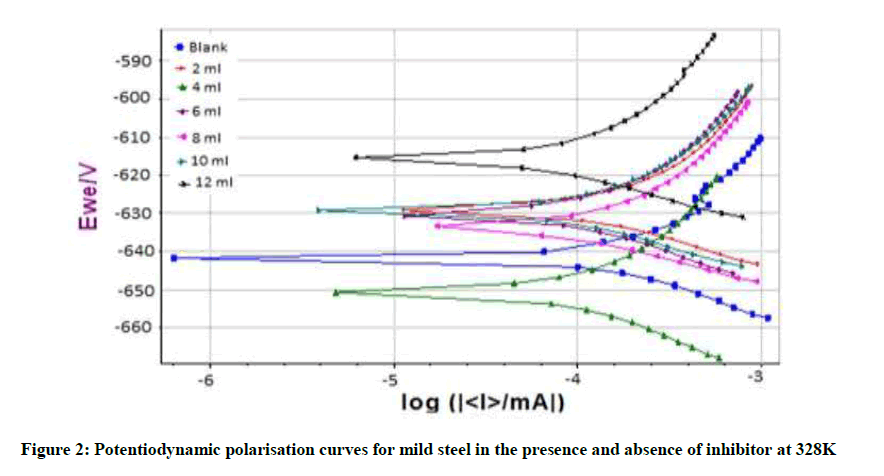
From Table 5 it is observed that the Icorr value for 3.5% NaCl without N. oleander was found to be 103.974 μA cm-2. This value was observed to decrease from 2 ml to 12 ml of N. oleander leaf extract. 12 ml of N. oleander leaf extract showed decrease in Icorr value to 58.636 μA cm-2. The polarisation curves as shown in Figure 2 exhibits a slightly positive shift in the Ecorr value. For example it shifted from -642.692 mV to -616.390 mV confirming the mixed type of inhibitor. Similarly CR was observed to decrease from 1.00213 mm/y to 0.688759 mm/y which implied increase in IE, which was calculated to increase from 8.45% to 43.60%. The reduction in Icorr indicates the efficiency of the inhibitor.
| Conc. of Nerium oleander leaf extract (ml) |
Ecorr (mV vs SCE) |
Icorr (µA cm-2) |
βa (mV) |
βc (mV) |
CR (mm/y) |
IE (%) |
|---|---|---|---|---|---|---|
| 0 | -642.69 | 103.974 | 20.6 | 14.7 | 1.2213 | - |
| 2 | -629.35 | 95.183 | 21.3 | 14.1 | 1.1181 | 8.45 |
| 4 | -651.15 | 89.137 | 30.1 | 21.1 | 1.047 | 14.26 |
| 6 | -630.76 | 85.296 | 24.4 | 16.8 | 1.0019 | 17.96 |
| 8 | -633.57 | 71.889 | 16.6 | 13.4 | 0.8444 | 30.85 |
| 10 | -629.55 | 68.673 | 18.9 | 14.1 | 0.8206 | 33.95 |
| 12 | -616.39 | 58.636 | 21.7 | 13.9 | 0.6888 | 43.6 |
Table 5: Potentiodynamic polarisation parameter for mild steel without and with various concentrations of Nerium oleander leaf extract in 3.5% NaCl at 328K
FTIR analysis
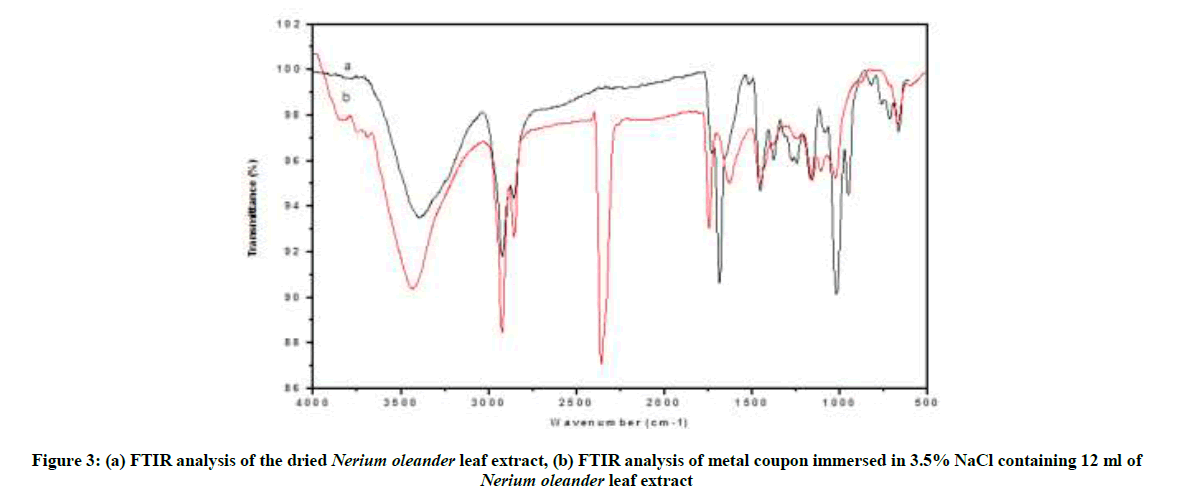
The mild steel specimens were immersed in blank, as well as inhibitor solutions, for a period of one day. After one day, the specimens were taken and dried. The nature of the film formed on the surface of the metal specimens was analyzed by various surface analysis techniques. FTIR spectrum of the N. oleander plant extract is shown in Figure 3a. From the spectral analysis it was seen that the main heterocyclic atom that was present in the ethanoic extract of N. oleander was C=O. various functional groups present such as –OH, -C=C, aromaticity, etc. The C=O stretching frequency occurs at 1727 cm-1. The peak at 3394 cm-1 is due to the -OH group. The FTIR spectrum of the film scratched from the surface of the metal immersed in 2.5 ml of Nerium oleander leaf extract is given in Figure 3b. It is seen from the spectrum that the C=O stretching frequency of inhibitor in the free state has shifted from 1727 cm-1 to 1744 cm-1. This shift indicates that the carbonyl oxygen atom was coordinated to Fe2+ resulting in the formation of Fe2+ - complex on the anodic sites of the metal surface. The shift in the peak value from 3394 cm-1 to 3434 cm-1 of the film confirms the adsorption of inhibitor molecule over the metal surface through OH group. The coordination of the metal ion with π-electrons was further confirmed from the shift in the frequency from 1453 cm-1 to 1456 cm-1. Whereas, the alkyl C-H stretching frequency remains the same which had not adsorbed on the metal [3,22,23].
SEM analysis
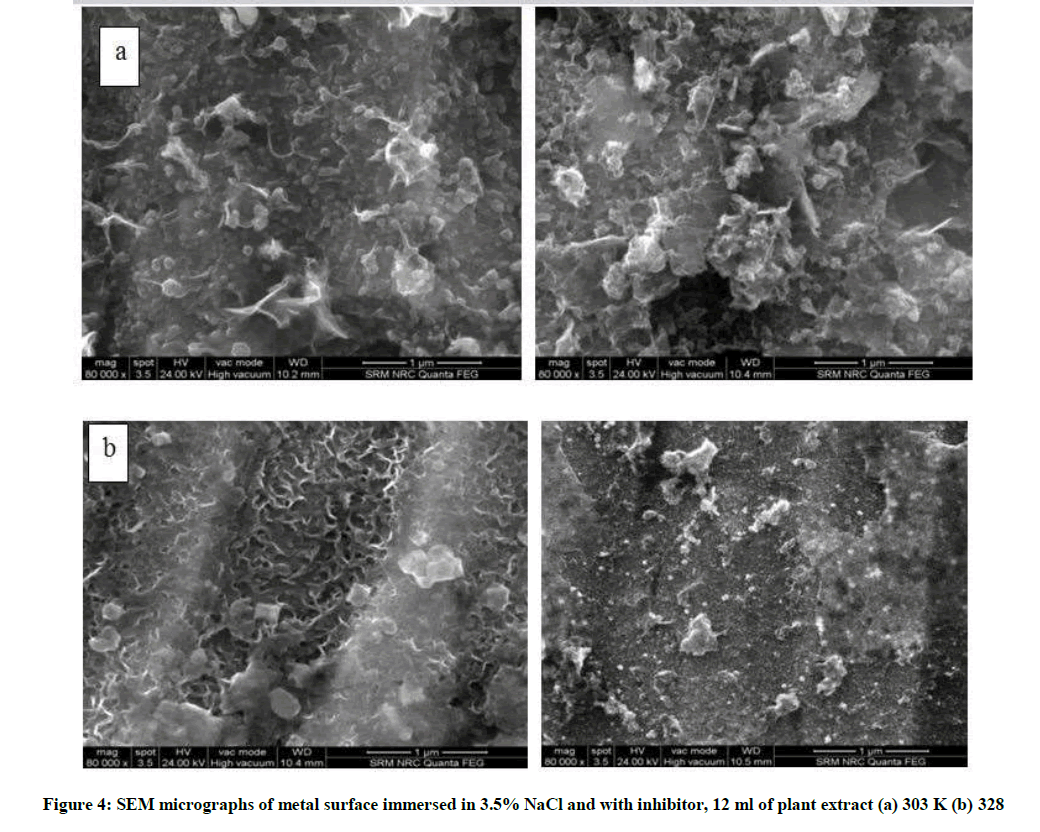
The surface morphology of the metal specimen was examined by SEM analysis. Figure 4a and 4b are the SEM micrographs of mild steel immersed in 3.5% NaCl (blank) and 3.5% NaCl containing 12 ml of N. oleander leaf extract respectively. The micrographs of the metal immersed in 3.5% NaCl shows the roughness of the surface and the adherence of the corrosion products. In support of the weight loss and polarization study the surface morphology of the mild steel specimen in the presence of inhibitor at 328K shows the protective film formed uniformly on the metal surface compared to the micrograph at 300K. Further it supports the fact of chemisorptions of the inhibitor molecule with increase in temperature.
Conclusion
Weight loss study reveals that N. oleander is a potential inhibitor in aqueous medium. Satisfactory inhibition efficiencies were observed. It was also seen that the IE increases with increase in temperature, indicating chemisorption as the corrosion mechanism. Linear polarisation studies coincided with the weight loss study. The formation of a protective film was confirmed by FTIR spectroscopy and SEM micrographs. Potentiodynamic polarisation study reveals the inhibitor as the mixed type of inhibitor.
References
- R.L. Twite, G.P. Bierwagen, Progress in Organic Coatings.,1998, 2(33), 91-100.
- B.E. Amitha Rani, Bharathi Bai J. Basu, Int. J. Corros.,2012, 1-15.
- A. Pradityana, Sulistijono, A. Shahab, L. Noerochim, D. Susanti, Int. J. Corros.,2016, 1-6.
- M. Mahdavian, R. Naderi, Corros. Sci., 2011, 53(4), 1194-1200.
- A.Y. El-Etre, Corros. Sci.,1998, 40(11), 1845-1850.
- A.Y. El-Etre, M. Abdallah, Corros. Sci.,2000, 42(4), 731-738.
- S. Gudić, E.E. Oguzie, A. Radonić, L. Vrsalović, I. Smoljko, M. Kliškić, Macedonian J. Chem. Chem. Engin.,2014, 33(1), 13-25.
- A. Singh, E.E. Ebenso, M.A. Quraishi, Int. J. Corros.,2011, 2012.
- P.B. Raja, M.G. Sethuraman, Science Direct.,2007, 62, 113-116.
- H.A. Fetouh, T.M. Abdel-Fattah, M.S. El-Tantawy, Int. J. Electrochem. Sci., 2014,9, 1565-1582.
- H.J. Chen, T. Hong, W. Paul Jepson, Corrosion., 2000.
- E.E. Oguzie, Corros. Science.,2007, 49(3), 1527-1539.
- N.I. Kairi, J, Kassim, Int. J. Electrochem. Sci.,2013, 8, 7138-7155.
- V.O. Njoku, E.E. Oguzie, C. Obi, A.A. Ayuk, Adv. Chem.,2014.
- F. Bentiss, M. Lebrini, M. Lagrene´e, Corros. Sci., 2005, 47, 2915-2931.
- G. Moretti, F. Guidi, G. Grion, Corros. Sci.,2004, 46, 387-403.
- E.I. Ating, S.A. Umoren, I.I. Udousoro, E.E. Ebenso, A.P. Udoh, Green Chem. Lett. Rev., 2010, 3(2), 61-68.
- P.B Raja, M. Ismail, S. Ghoreishiamiri, J. Mirza, M.C. Ismail, S. Kakooei, A. Abdul Rahim, Chem. Engin. Commun., 2016, 1563-5201.
- B.I. Ita, O.E. Offiong, Mater. Chem. Phys., 2001, 70(3), 330-335.
- A. Popova, E. Sokolova, S. Raicheva, M. Christov, Corros. Sci., 2003, 45(1), 33-58.
- F.M. Mahgoub, F.M. AlNowaiser, A.M. AlSudairi, Protection of Metals and Physical Chemistry of Surfaces.,2011, 47(3), 381-394.
- A. Rajendran, Int. J. PharmTech. Res., 2011, 3(2), 1005-1013.
- B.S Siddiqui, N. Khatoon, S. Begum, A.D. Farooq, K. Qamar, H.A. Bhatti, S.K. Ali, Phytochemistry., 2012, 77, 238-244.