Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 2
Evaluation of DNA Binding of Zn (II) Complex Containing Avicularin as a Coligand
IyyamPillai S1, Neeli Parvathi2 and Subramanian S2*2Department of Biochemistry, University of Madras, Guindy Campus, Chennai- 600 025, India
Subramanian S, Department of Biochemistry, University of Madras, Guindy Campus, Chennai- 600 025, India, Email: subbus2020@yahoo.co.in
Received: 25-Jan-2021 Accepted Date: Feb 20, 2021 ; Published: 26-Feb-2021
Abstract
Flavonoids are polyphenolic secondary metabolites produced by the plants. They are extensively studied within drug discovery programs due to their beneficial and pharmacological activities such as antioxidant, antimicrobial, antitumor, antidiabetic and cardio-protective. The ability of flavonoids to coordinate with metal atoms has presented new leads for drug innovation programs, with enhanced pharmacological activities and clinical outputs than the parent flavonoids. Recently, we have reported the synthetic method, spectral analysis and antidiabetic properties of a new Zinc –Avicularin complex. The present effort was focused on the DNA binding of Avicularin and its ternary transition metal complex with Zinc. The nature of the binding interactions between complex and calf thymus DNA (CT-DNA) was studied by electronic absorption spectra, fluorescence spectra, circular dichroic and viscosity measurements. The data obtained evidence that the Avicularin and its Zn (II) complex could interact with CT-DNA via partial/moderate intercalative mode.
Keywords
Avicularin, Zinc-Avicularin complex, DNA binding, Intercalative mode, Viscosity.
Introduction
Currently, cancer is progressively becoming the second most cause of mortality after cardiovascular diseases, causing about a quarter of all deaths around the world. Most of the currently available anticancer drugs are not only costly but also elicit undesirable side effects. Hence, the search for novel therapeutic agents with maximum efficacy and without undesirable side effects continues [1]. Metal-based therapeutics has become a viable field of research in medicinal chemistry after the serendipitous discovery of cisplatin. At present nearly half the number of cancer patients is treated with platinum-based drugs [2]. Nevertheless, their well-known drawbacks, such as general toxicity, multi-factorial drug resistance, low solubility and general toxicity, have restricted their extensive use in chemotherapy [3], which prompted the search for effective non-platinum drugs. Over the years, complexes of Ru, Ir, Cu, Ni, Zn, Co, etc., have been reported to possess comparable anticancer property to cis-platin [4].
A wide range of Schiff’s bases with their reactive azomethine linkage shows interesting inhibitory activity against tumor cells. Schiff’s bases could be hydrolyzed selectively by the tumor cells to act as alkylating agents; at the same time, the active amine becomes free to act as an antimetabolite [5]. A Schiff base acts as a flexible-dentate ligand and often coordinates across the O-atom of the de-protonated phenolic group. The N- atom of the imine group is found to be a competent ligand capable of facilitating the graceful artificial linkage of diverse functional moieties with N-terminal groups to generate a variety of fascinating multidentate metal complexes [6]. Likewise, the metal complexes of Schiff base have diverse applications such as clinical, pharmaceutical, biochemistry, agricultural and in other various fields. The variation of groups or atoms in the Schiff base ligand shows different electronic, geometric and biological properties upon complexation with metal ions [7].
When the metals are chelated with Schiff base ligand, the biological activity of the metal ions was significantly increased based on the geometry, reactivity, and functional group present in the ligand. Coordination of such ligands with metal ions likewise, copper, zinc, cobalt, and nickel, has antimicrobial, antioxidant, and DNA interaction properties [8].
The biological properties of flavonoids witnessed significant progress, with the number of research publications increasing by more than six-fold between the years 1990 to 2017. Flavonoids are polyphenolic plant-derived secondary metabolites comprised of two benzene rings that are linked together with a heterocyclic pyran or pyrone ring [9]. They are synthesized by the phenylpropanoid pathway to protect the plants from environmental stress and microbial invasion in addition to their physiological role. However, flavonoids are known to elicit significant pharmacological actions in the treatment of various chronic human due to the presence of hydroxyl and oxo groups. Flavonoids can act as modulators of cell signaling pathways that are directly involved in cellular functions and survival [10-13]. In addition, they possess significant metal-chelating abilities that can have profound effects on their pharmacokinetic and pharmacological properties [14]. Thus, the flavonoids can be regarded as potential chemo-preventive agents against certain carcinogens.
Over 5000 different flavonoids have been described to date, and they are classified into at least 10 chemical groups such as flavones, flavonols, flavonones, anthocyanins, and isoflavones which are commonly found in plants [15]. Despite their established role of safeguarding against carcinogens, flavonoids are also considered mutagenic and have the ability to damage DNA [16]. Several flavonoids- metal complexes ((M-Fls) bind to DNA more strongly than their flavonoids counterparts. Several reports are available in the literature evidencing that the metal-flavonoids complexes can intercalate into DNA strands, whereas flavonoids alone bind to DNA in other ways or intercalate into DNA to a lesser extent [17]. The nature and dynamics of binding of small molecules (drugs and flavonoids) to biomacromolecules like DNA represent an active area of investigation which can lead to rational drug design [18].
Avicularin, a quercetin-3- O-α-arabinofuranoside, is a flavone glycoside of quercetin widely present in a number of plant species including apple, mango, onions, tea, guava and cranberry [19]. It is known to display diverse pharmacological properties such as antioxidative, anti-inflammatory, anticancer, anti-depressant, anti-allergic, antimicrobial and hepatoprotective [20]. Though Avicularin is a glycoside of quercetin, it is hydrophilic while quercetin is lipophilic in nature and hence they may differ in absorption rate. The pharmacokinetic studies on the concentration-time profiles of Avicularin suggesting that the peak plasma concentration of Avicularin takes place after 30 min and the concentration remained for more than 4 h [21]. A recent study revealed that Avicularin may reverse multidrug resistance in human gastric cancer by increasing the expression levels of B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) and BOK [22].
Zinc is one of the most abundant essential trace elements next to iron in the human body. It is distributed in all parts of the body and its total content is approximately 2–4 gm [23]. Zinc is found to be a crucial element that plays a pivotal role in numerous physiological and pathological processes including cell proliferation, differentiation, and viability including apoptosis [24]. Its catalytic functions in about 1,000 human enzymes of all six enzyme classes are used predominantly in hydrolytic reactions and in some committing steps in intermediary metabolism observed [25]. Zinc complexes also strongly bind DNA and exhibit predominant antitumor activities.
Though zinc is considered to be relatively nontoxic, particularly if taken orally, lower levels of zinc supplementation even closer to the Recommended Dietary Allowance (RDA), (100-300 mg Zn/d vs RDA of 15 mg Zn/d) may interfere with the utilization of copper and iron, adversely affect HDL cholesterol concentrations and impaired immune function [26]. In order to circumvent the adverse effect of zinc at chronic doses, several zinc complexes have been synthesized using biologically active organic ligands. Recently, we have reported the synthesis of a new zinc-Avicularin complex and its antidiabetic properties in experimental type 2 diabetes in rats [27]. In the present study, an attempt has been made to evaluate the binding properties of the transition metal complex with double-stranded calf thymus DNA using electronic absorption spectra, fluorescence spectra, circular dichroic and viscosity measurements to elucidate its binding and pharmacological potential.
Materials and Methods
Commercial solvents were distilled and then used for the preparation of the ligand and its metal complex. DNA was purchased from Bangalore Genei (India). Tris (hydroxymethyl) aminomethane-HCl (Tris–HCl) buffer solution was prepared using deionized and sonicated triple distilled water. UV-Vis spectra were recorded using a Perkin Elmer Lambda 35 spectrophotometer operating in the range of 200-900 nm with quartz cells and ε are given in M-1cm-1. The emission spectra were recorded on a Perkin Elmer LS-45 fluorescence spectrometer. Viscosity measurements were recorded using a Brookfield Programmable LV DVII+ viscometer. Circular dichoric spectra of CT-DNA were obtained using a JASCO J-715 Spectropolarimeter equipped with a Peltier temperature control device at 25 ± 0.1°C with 0.1 cm path length cuvette.
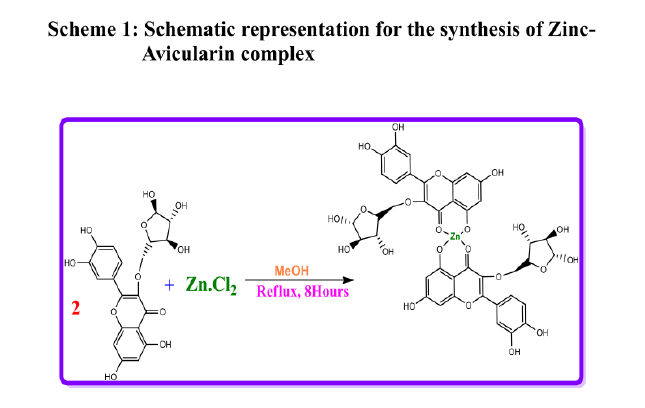
Synthesis of Schiff base Zinc complex of Avicularin.
The zinc-Avicularin complex was synthesized as schematically represented below and previously reported by us [27].
DNA-binding experiments
DNA binding experimentations were carried out in 0.5 mL Tris–HCl/NaCl buffer [50mM Tris–HCl and 5mM NaCl (pH 7.2)] using DMF solution (15μL) of the synthesized Zinc-Avicularin complex. To illustrate the mode and efficiency of our newly synthesized compounds with calf-thymus DNA (CT-DNA), different techniques have been used to study the interaction such as electronic absorption spectra, circular dichroic, viscosity measurements, fluorescence titration, and competitive ethidium bromide (EB) displacement assay. The non-linear least square analysis was performed using Origin lab, version 6.1.
Absorption spectral studies
Absorption titration experiments were performed by using graded concentration of CT-DNA (0, 40, 80, 120, 160, 200, 300 and 400 μM) while maintaining the concentration of the drug and its metal complex constant (40 μM). The corresponding buffer solution was used as a reference. Upon measuring the absorption spectra, an equal amount of CT-DNA solution was added to both the compound solution and the reference solution to eliminate the absorbance of DNA itself. The solutions were allowed to incubate for 10 min at room temperature before the absorption spectra were recorded in the range of 200– 400 nm. The titration processes were repeated until no change in the spectra, indicating binding saturation had been achieved. The equilibrium binding constant (Kb) values for the interaction of the complex with CT-DNA were obtained from absorption spectral titration data using the following equation (1) [28].
[DNA]/ (εa - εf) = [DNA]/ (εb - εf) + 1/Kb (εb-εf) (1)
Where εa is the extinction coefficient observed for the charge transfer absorption at a given DNA concentration, εf the extinction coefficient at the complex free in solution, εb the extinction coefficient of the complex when fully bound to DNA, Kb the equilibrium binding constant, and [DNA] the concentration in nucleotides. A plot of [DNA]/ (εa - εf) versus [DNA] gives Kb as the ratio of the slope to the intercept. The non-linear least square analysis was performed using Origin lab, version 6.1.
Fluorescence emission spectral studies
The emission spectrum is obtained by setting the excitation monochromator at the maximum excitation wavelength and scanning with emission monochromator. Often an excitation spectrum is first made in order to confirm the identity of the substance and to select the optimum excitation wavelength. Further experiments were carried out to gain support for the mode of binding of complexes with CT-DNA. Non-fluorescent or weakly fluorescent compounds can often be reacted with strong fluorophores enabling them to be determined quantitatively. On this basis molecular fluorophore, Ethidium Bromide was used which emits fluorescence in presence of CT-DNA due to its strong intercalation. Quenching of the fluorescence of EthBr bound to DNA were measured with increasing amount of metal complex as a second molecule and Stern–Volmer quenching constant Ksvwas obtained from the following equation[29].
Io/I=1+Ksvr. (2)
Where I0, is the ratio of fluorescence intensities of the complex alone, I is the ratio of fluorescence intensities of the complex in the presence of CT-DNA. Ksvis a linear Stern-Volmer quenching constant and r is the ratio of the total concentration of quencher to that of DNA, [M] / [DNA]. A plot of I0 / Ivs. [complex]/ [DNA], Ksvis given by the ratio of the slope to the intercept. The apparent binding constant (Kapp) was calculated using the equation KEB[EB] / Kapp[complex], where the complex concentration was the value at a 50% reduction of the fluorescence intensity of EB and KEB = 1.0 x 107M-1([EB] = 3.3μM).
CD spectral studies
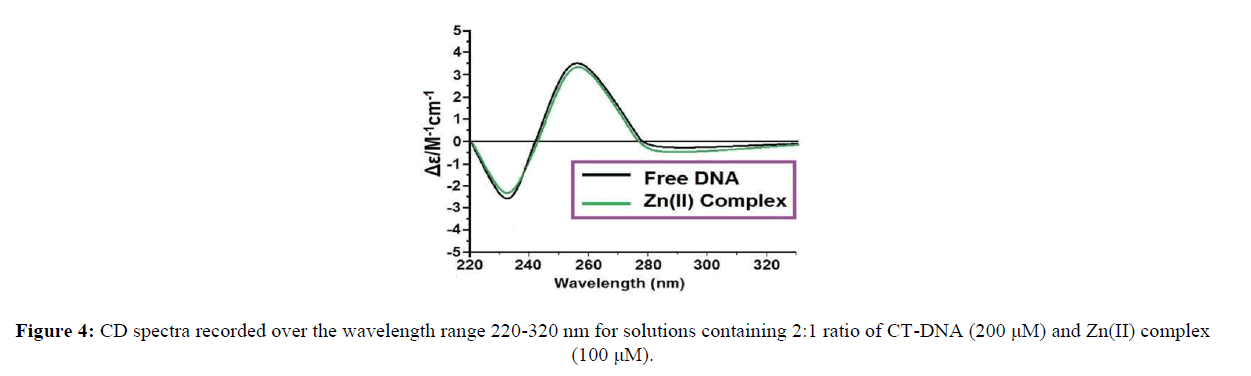
Circular dichroic spectra of CT-DNA in the presence and absence of metal complex were obtained by using a JASCO J-715 Spectropolarimeter equipped with a Peltier temperature control device at 25 ± 0.1°C with a 0.1 cm path length cuvette. The spectra were recorded in the region of 220–320 nm for 200 μM DNA in the presence of 100 μM of the complexes.
Viscosity measurements
To find the binding mode of the complexes towards CT-DNA, viscosity measurements were carried out on CT-DNA (0.5 mM) by varying the concentration of the complexes (0.01 mM, 0.02 mM, 0.03 mM, 0.04 mM, 0.05 mM). Data were presented as (η/ηo) versus binding ratio of concentration of complex to that of concentration of CT-DNA , where η is the viscosity of DNA in the presence of complex and ηo is the viscosity of DNA alone.
Results and Discussions
DNA binding studies are a commonly used preliminary testing method for antitumor activity of compounds. It is essential to know the type of interaction between small molecules with double helix to demonstrate the underlying mechanism of binding mode. In general, the molecules bind with DNA through three different binding modes, electrostatic, groove and intercalative. Most of the heteroaromatic molecules display intercalative mode of binding with DNA because of the larger planarity of the compound with extended conjugation and the greater stacking interaction between the compound and base pairs of DNA [30].
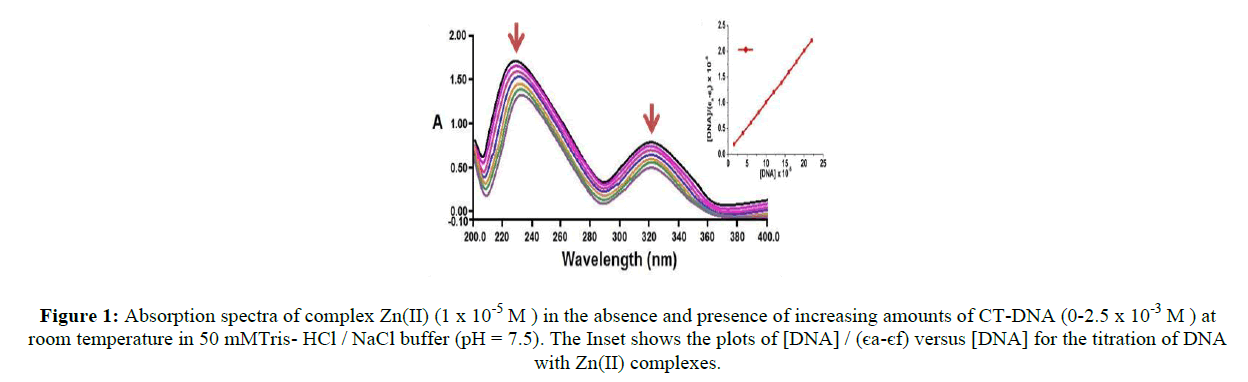
UV-Vis spectroscopy is an effective technique to detect interactions between DNA and small molecules. The mode of the interactions is reflected from the changes in the position of the peak and absorptive intensity [31]. The absorption spectra of tested compound (Figure 1), in the absence and presence of CT-DNA have shown that increase in the concentration of CT-DNA to the synthesized metal complex causes hypochromism, due to the intercalative mode involving a strong stacking interaction between an aromatic chromophore and the base pairs of DNA of various percentage. The appreciable decrease in absorption intensity and significant red shift of the π–π* band of complex is similar to that observed for its interaction with DNA in DMSO solution, suggesting that the complex bind to DNA strongly. The extent of the hypochromism commonly parallels the intercalative binding strength [32]. In order to attain the binding strength of the synthesized Zinc-Avicularin complex, the intrinsic binding constants (Kb) was calculated from the ratio of the slope to intercept in the plot of [DNA]/ (εa - εf) = [DNA]/ (εb - εf) + 1/Kb (εb - εf).
Figure 1: Absorption spectra of complex Zn(II) (1 x 10-5 M ) in the absence and presence of increasing amounts of CT-DNA (0-2.5 x 10-3 M ) at room temperature in 50 mMTris- HCl / NaCl buffer (pH = 7.5). The Inset shows the plots of [DNA] / (єa-єf) versus [DNA] for the titration of DNA with Zn(II) complexes.
Intrinsic binding constants Kb of the synthesized Zn(II) complex obtained is 5.11 x 104 M-1 respectively from the decay of the absorbance at 372 nm with increasing concentrations of DNA by using equation (1). So, it is obvious that the present complexes are involved in intercalative interactions with CT-DNA. Our attained outcomes are consistent with earlier reports on preferential binding to CT-DNA in the metal complex [33]. Viscosity and circular dichroism measurements could have been supportive to us to confirm the intercalative binding mode.
Emission spectral studies
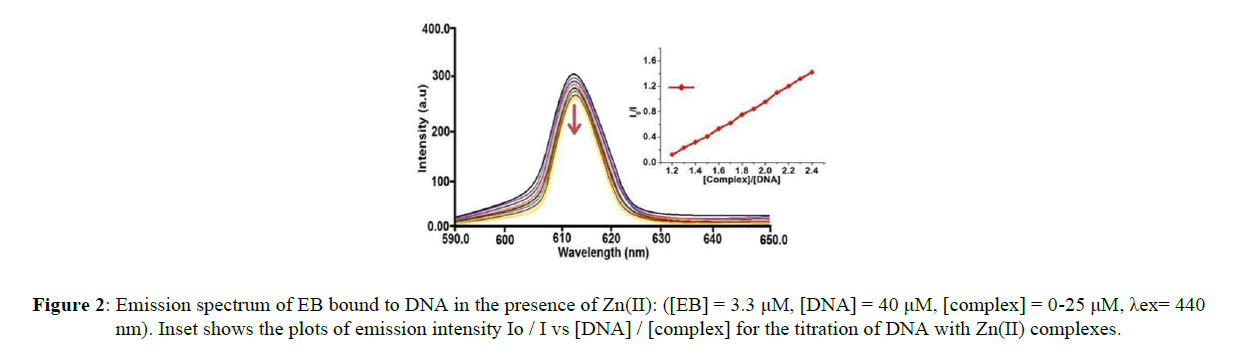
The results derived from the absorption studies prompted us to get an insight into the mechanism by which Zn-Avicularin complex binds towards DNA. Owing to the fact that the synthesized complex contains planar rings in its structure, there is a possible that the synthesized Zn (II) complex may interact towards CT-DNA by the formation of intercalation complex. For compounds that do not display luminescence either alone or in aqueous buffer, ethidium bromide is commonly used. Ethidium bromide is an intercalating molecule emitting intense fluorescence when bound to DNA [34]. Relative binding activity of the complexes towards CT-DNA was studied by the fluorescence spectral method using ethidium bromide (EB) bound CT-DNA solution in Tris–HCl/NaCl buffer (pH = 7.2).
Ethidium bromide is non-emissive in aqueous buffer due to fluorescence quenching of the free ethidium bromide by the solvent molecules. In the presence of DNA, ethidium bromide shows enhanced emission intensity due to its intercalative binding to DNA. If competitive binding of a compound to DNA resulted in the displacement of the DNA-bound ethidium bromide and considerable decrease in ethidium bromide emission intensity is observed, then the mode of binding of the complex to DNA is by intercalation [35]. The Zinc-Avicularin complex does not fluoresce with an excitation at 612 nm. The EB competition assay results are shown in Figure 2.
The fluorescence intensity of DNA- bound EB around 610 nm decreased outstandingly with an increase of the complex concentration while there was no change in the position and shape of the emission peaks. This decrease in fluorescence intensity may be due to the quenching of some EB molecules that were released from DNA into the solution after being substituted by the synthesized Zn(II) complex. The phenomenon that the fluorescence of DNA-bound EB was quenched as a result of DNA and compound interactions is a typical sign of intercalation [36]. The quenching plots (insets in Figure 2) illustrate that the fluorescence quenching of EB bound to DNA Zn(II) complex in linear agreement with the Stern–Volmer equation, which confirms thatthe complexes bound to DNA. The Kapp values for Zn(II) complex are found to be 6.04 x 105 M-1 respectively. The attained results are in consistent with that of absorption spectroscopic studies.
Viscosity studies
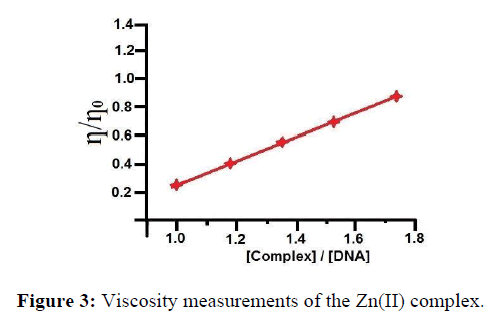
Binding modes of the complexes were further investigated by viscosity measurements. Though photophysical experiments provide information about binding modes of metal complex with DNA, they do not afford conclusive evidences for the exact mode of binding. Hydrodynamic measurements such as viscosity which is sensitive to length changes are regarded as the least uncertain and the most critical tests of binding modes in solution. Viscosity of DNA is increased in the case of classical intercalator due to increase in the length of DNA helix, as base pairs are separated to accommodate the intercalator [37]. Further suitable conditions intercalation causes significant increase in the viscosity of DNA solution due to the disjointing of base pairs at intercalation spots. The results of the viscosity measurements for the synthesized complex which bound to DNA show increase in relative viscosities with an increase in the [complex]/[DNA] ratio (where [complex] is 50,100,150 and 200 μl) as shown in Figure 3.
Thus, the increase in the viscosity has been attributed to the enlargement of the separation between base pairs, which are pushed apart to accommodate the intercalating molecule. These results suggested an intercalative binding mode of the complexes with DNA and also hold up the results obtained from electronic absorption studies [38].
Circular dichoric spectral studies
Circular dichroism spectroscopy is a useful tool to study the change in DNA conformation during interaction. In DNA spectrum, there are two specific bands, the positive band (273 nm) related to base stacking and the negative one (264 nm) related to right-handed helicity. These bands are highly sensitive to the interaction between DNA and molecules [39]. Due to simple groove binding and hydrophobic interactions, very little changes appeared in the base stacking and helicity bands. This is while intercalation causes a noteworthy change in the intensity of both bands. The CD profile of right handed B-DNA exhibits two positive (220 nm and 268 nm) and two negative (210 nm and 246 nm) elliptical signals and slight variations arise when the %GC content is varied. CD is typically used to determine the preferred binding mode (e.g. minor groove, major groove, and intercalation) within the asymmetric DNA environment by observing the CD signal relative to untreated DNA at wavelengths of ~ 210, 220, 246 and 268 nm [40]. As shown in Figure 4, the CD spectrum of DNA exhibits a positive absorption at 277 nm due to the base stacking and a negative band at 240 nm due to the helicity of B-DNA.
In the presence of the complex, both the positive and negative peak intensities of the CD spectra of DNA were increased. The changes in the CD spectra in the presence of the complex show stabilization of the right handed B form of CT-DNA. So, the key interactions of the complex with CT - DNA can be ascribed to the intercalative mode.
Conclusion
In conclusion, in the present study a zinc-Avicularin complex was designed to reduce the toxicity of the zinc with improved efficacy. The data obtained through systematic studies demonstrated that the Zinc-Avicularin complex as synthesized and reported by us earlier exhibits high binding affinity to CT-DNA. Various instrumental methods were used for finding the interaction mechanism. The subsequent findings sustained the fact that the complex can bound towards CT-DNA viz intercalation binding mode.
In absorption spectrum, the absorption intensity of the complex increased (hypochromism) evidently after incremental addition of DNA, which indicated the interactions between DNA and the complex.
1) The intrinsic binding constant (Kb = 5.01 x 104 M-1) is comparable to intercalative binding complexes.
2) The Zn (II) complex binding to CT DNA via intercalation is given through the emission quenchingexperiment.
3) The Kapp values for Zn (II) complex are found to be 6.04 x 105M-1respectively.
4) The increase in the viscosity has been attributed to the enlargement of the separation between base pairs, which are pushed apart to accommodate the intercalatingmolecule.
5) The changes in the CT-CD spectra of DNA in the presence of increasing amounts of the complex show stabilization of the right-handed B form ofCT-DNA.
References
- M Barcelo-Oliver, A Garcia-Raso, A Terrón et al., J Inorg Biochem. 2007, 101: p. 649-659.
- S Dilruba and GV Kalayda. Cancer Chemother Pharmacol. 2016, 77: p. 1103-1124.
- L Kelland. Nat Rev Cancer. 2007, 7: p. 573–584.
- JL Qin, WY Shen, ZF Chen et al., Sci Rep. 2017, 7: p. 1-18.
- MM Kamel, HI Ali, MM Anwar et al., Eur J Med Chem. 2010, 45(2): p. 572-580.
- RL Yan, H Yan, C Ma et al., J Org Chem. 2012, 77(4), 2024-2028.
- HA Pramanik , PC Paul, P Mondal et al., J Mol Struct. 2015, 1100: p. 496-505.
- T Arun, R Subramanian and N Raman., J Photochem Photobiol B: Biology. 2016, 154: p. 67-76.
- G Yoo, SJ Park, TH Lee et al., Pharmacogn Mag. 2015, 11(43): p. 651-656.
- HY Kim, JY Ko, SB Song et al., J Korean Soc Food Sci Nutr. 2012, 41: p. 1508-1514.
- JS Nam, SY Park, HL Jang et al., Appl Biol Chem. 2017, 60: p. 535-543.
- J Lee, JP Rodriguez, KH Lee et al., Appl Biol Chem. 2017, 60: p. 487-496.
- RJ Williams, JP Spencer and CR Evans., Free Radic Biol Med. 2014, 36: p. 838-849.
- AKPandey, National Academy Science Letters. 2007, 30 (12): p. 383-386.
- K Robards and M Antolovich, Analyst 1997. 122 (2): p. 11-34.
- H Arif, ASohail, MFarhan et al., Int J Biol Macromol. 2018, 106: p. 569-578.
- MM Kasprzak, A Erxlebenb and SJ Ochockia. RSC Adv. 2015, 5: p. 45853-45877.
- MJWaring. J Mol Biol. 1970, 54: p. 247-279.
- G Carullo, AR Cappello, L Frattaruolo et al., Fut Med Chem. 2016, 9(1): p. 79-93.
- ZShen , Y Xu, X Jiang et al., J Hou Med Sci Monit. 2019, 15(25): p. 2777-2784.
- F Xu, H Guan, G Li et al., Chromatographia. 2009, 69: p. 1251.
- XF Guo, JP Liu, SQ Ma et al., Biomed Pharmacother. 2018, 103: p. 67-74.
- J Jansen, W Karges, L Rink et al., J Nutr Biochem. 2009, 20(6): p. 399-417.
- P Babula, V Kohoutkova, R Opatrilova et al., Chimicoggi/Chemistry Today. 2010, 28: p. 18-21.
- W Maret. Prev Nutr Food Sci. 2017, 22(1): p. 1-8.
- ]GJ Fosmire. Zinc toxicity. 1990, 51(2): p. 225-227.
- N Parvathi, S Iyyampillai and SP Subramanian. Diabesity. 2020, 6(2): p. 9-18.
- A Wolfe, GH Shimer and T Mechan. Biochem. 1987, 26: p. 6392-6396.
- JR Lakowiez and G Webber, Biochem.,1973, 12(21): p. 4161-4170.
- T Indumathi, FR Fronczek and KJ Rajendra Prasad. J Mol Str. 2012, 1016: p. 134-139
- J Jaumot and R Gargallo, Curr Pharm Design. 2012, 18: p. 1900-1916.
- JK Barton, AT Danishefsky and JM Goldberg. J Am Chem Soc. 1984, 106: p. 2172-2176.
- DD Li, JL Tian, WGu et al., J Inorg Biochem. 2010, 104: p. 171-179.
- H Li, XY Le, DW Pang et al., J. Inorg. Biochem.,2005, 99: p. 2240 - 2247.
- BC Baguley and ML Bret, Biochem. 1984, 23: p. 937-943.
- NC Garbett, NB Hammond and DE Graves, Biophys J. 2004, 87(6): p. 3974-3981.
- G Yang, JZ Wu, L Wang et al., J Inorg Biochem. 1997, 66: p. 141-144.
- SSatyanarayana, JCDabrowiak and JB Chaires. Biochem. 1992, 31: p. 9319-9324.
- J Kypr, I Kejnovska, D Renciuk et al., Nucleic Acids Res. 2009, 37(6): p. 1713-1725.
- A Kellett, Z Molphy, CSlator et al., Chem Soc Rev. 2019, 48(4): p. 971-988.