Research Article - Der Pharma Chemica ( 2018) Volume 0, Issue 0
Pithecellobium dulce Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium
Sivakumar PR and Srikanth AP*
PG & Research Department of Chemistry, Government Arts College, Coimbatore, TN, India
- *Corresponding Author:
- Srikanth AP
PG & Research Department of Chemistry
Government Arts College
Coimbatore, TN, India
Abstract
An aqueous extract of Pithecellobium dulce has been studied as a corrosion inhibitor in controlling corrosion of mild steel in 1 N HCl medium by weight loss, electrochemical impedance spectroscopy and potentiodynamic polarization techniques. Polarization study revealed that the inhibitor acts as mixed type of inhibitor. The electrochemical impedance study further confirms the formation of an adsorbed film on the mild steel. The adsorbed film over the mild steel surface has been confirmed by Scanning Electron Microscope (SEM) analysis. The adsorption character of plants extract on mild steel surface obey Temkin isotherm.
Keywords
Mild Steel, EIS, SEM, Polarization, Acid corrosion
Introduction
Mild steel is the most common form of steel because of its relatively low cost and material properties and are extensively used in numerous applications in various industries. Most of the acid solutions, especially HCl is used to remove undesirable scale or rust [1-5].
In order to protect mild steel from corrosion, corrosion inhibitors are widely used in industry to reduce the corrosion rate. Most inhibitors are organic compounds containing N, S and O in their molecule. Paints coating, galvanic and cathodic protection are main methods to protect steel against corrosion. A large number of researcher have been dedicated to the corrosion of mild steel and use of inorganic, organic, polymers and hetero cyclic compounds as corrosion inhibitors in acidic media. The most of these artificial organic inhibitors are not only expensive but also toxic for human health and environment. Many researchers examined various naturally occurring substances as corrosion inhibitors for different metals in various environments [6-12]. Investigation and evaluation of naturally occurring substances (Organic inhibitors) are eco-friendly, compatible, non-polluting, less toxic, easily available, biodegradable and economic has continued due to the presence of hetero atoms like nitrogen, sulfur and oxygen in their structure to be used as corrosion inhibitor. The present study seeks to investigate the potential of using Pithecellobium dulce green corrosion inhibitors as a cheap and environmentally safe corrosion control agent for mild steel in 1N HCl acidic medium (Figure 1).
Specimen preparation
Mild Steel (MS) specimens containing C=0.01%, Mn=0.34%, P=0.08% and Fe=99.51% were used for the study. MS of size 3 × 2 × 0.1 cm was used for weight loss study; specimens with an exposed area of 1 cm2 were used for electrochemical study. The surface preparation of the mechanically polished specimens were carried out using different grades of emery paper and then degreased with acetone.
Preparation of the corrosive media
The green inhibitor was prepared by refluxing a 20 g powder of P. dulce leaves in double distilled water for 5 h and kept overnight. The aqueous solution extract was filtered and volume was made up to 500 ml using double distilled water. The inhibitor showed positive test for alkaloid content. Various concentrations of the plant extracts were prepared by dissolving the known quantity of the resultant powder in acid media.
Weight loss method
The polished and pre weighed MS specimens of uniform size were suspended in 100 ml test solutions with and without the inhibitor at different concentrations for a period of 24 h. Then the specimens were washed, dried and weighed. The weight loss was calculated. From this data, Inhibition Efficiency (IE) was calculated as follows.
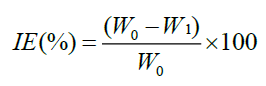
Where, W0-Weight loss of MS without inhibitor and W1-Weight loss of MS with inhibitor.
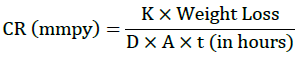
Where, K=8.76 × 104 (constant), D is density in g/cm3 (7.86), W is weight loss in grams and A is area in cm2.
Electrochemical studies [13-24]
CHI electrochemical analyzer model 608D was used to record Tafel polarization curve and Nyquist impedance curve. A conventional three electrode system was used for this purpose. MS specimen of exposed area of 1 cm2 with stem was used as a working electrode. Pt electrode and calomel electrode served as axillary and reference electrodes respectively. The polarization measurements were recorded at the end of 30 min immersion at 30 ± 1°C by changing the electrode potential automatically from -200 mV to -800 mV. IE values were calculated from the Icorr values using following equation.
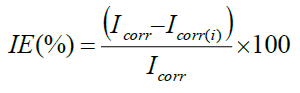
Where, Icorr -Corrosion current density of MS without inhibitor and Icorr(i)-Corrosion current density of MS with inhibitor.
AC impedance measurements were carried out at Ecorr immersion on standing in the atmosphere of air at the range from 0.1 Hz to 10000 Hz at amplitude of 10 mV. The impedance diagrams are given in Nyquist representation. IE was calculated from the charge transfer resistance (Rct) values by using the following equation.
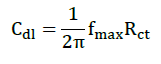
Where, Rct is charge transfer resistance, and Cdl is double layer capacitance.
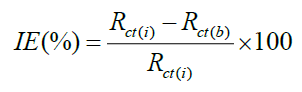
Where, Rct(b)-charge transfer resistance of MS without inhibitor and Rct (i)-Charge transfer resistance of MS with inhibitor [25-30].
Scanning Electron Microscope (SEM) analysis
The specimens used for surface morphology examination were immersed in acid containing optimum concentration of inhibitor and blank for 24 h immersion. Then, they were removed, rinsed quickly with distilled water and dried. The analysis was performed on JOEL model SEM to examine the surface morphology.
Results and Discussion
The weight loss method is a form of gravimetric method of studying the anti-corrosion activity of the inhibitor. Weight loss technique was complete for mild steel in 1 N HCl with several concentrations of P. dulce leaves extract ranging from 10 to 60 ppm. The results obtained from the varying concentration of the inhibitor are depicted in Table 1. It is detected from the table that the highest inhibition efficiency of about 96.40% was achieved at 40 ppm of P. dulce leaves extract.
| Concentration of Pithecellobium dulce extract (ppm) |
Weight loss (g) |
Corrosion rate (mmpy) |
Inhibition efficiency (%) |
|---|---|---|---|
| Blank | 0.3557 | 206.47 | * |
| 10 | 0.0455 | 026.41 | 88.04 |
| 20 | 0.0380 | 022.57 | 90.01 |
| 30 | 0.0346 | 020.00 | 90.91 |
| 40 | 0.0134 | 007.77 | 96.48 |
| 50 | 0.0215 | 012.48 | 94.35 |
| 60 | 0.0218 | 012.65 | 94.39 |
Table 1: Data from weight loss method for MS corroding in 1 N HCl solutions at various concentrations of Pithecellobium dulce leaves extract
Fourier Transform Infra-Red (FTIR) Spectroscopy measurement [37-42]
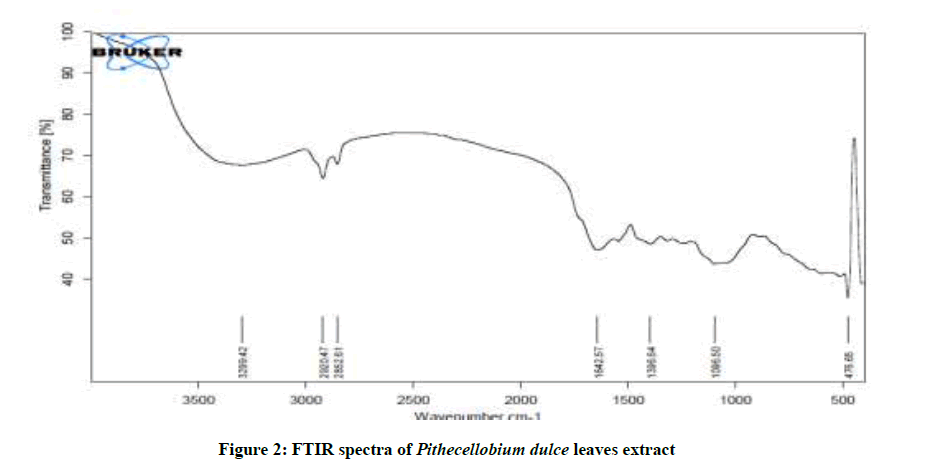
Figure 2 shows the FTIR spectra of P. dulce leaves extracts. The O-H stretching frequency appears at 3299 cm-1. The peak at 2920 cm-1 is attributed to C-H stretching. The C=O stretching appears at 1642 cm-1. The peak at 1096 cm-1 is due to the oxygen atom present in the aromatic ring. This conform the presence of mild steel P. dulce leaves extract complex on the metal surface. Mild steel has coordinated with the O–atom of the OH group, -C = O group and the ring oxygen atom.
Potentiodynamic polarization studies [43-48]
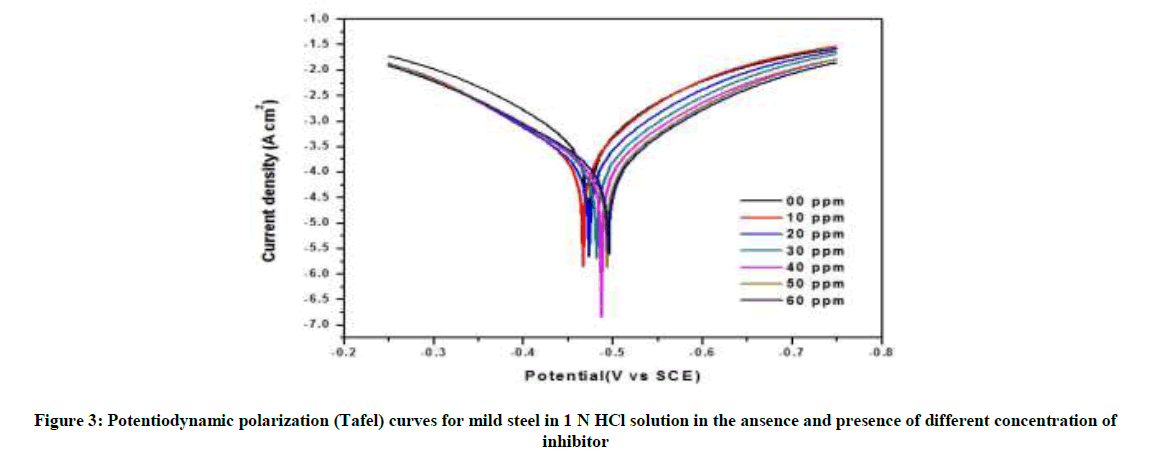
The anodic and cathodic polarization curves of mild steel in 1 N HCl solution without and with numerous concentrations of P. dulce leaves extract at room temperature are revealed in Figure 3 and parameter values are summarized in Table 2. It is apparent that Icorr decrease significantly with increasing concentration of inhibitor. The optimum inhibition efficiency of P. dulce extracts was 96.89% at 50 ppm. This suggests that P. dulce leaves extract act as mixed type of corrosion inhibitor.
| Conc. (Ppm) |
Ecorr/ (mV/ SCE) |
Icorr/ (mA/cm2) |
bc (mV/dec.) |
ba (mV/dec.) | LPR Ohm*2 |
IE (%) |
|---|---|---|---|---|---|---|
| Blank | -0.474 | 3.161 × 10-4 | 108.51 | 101.84 | 72.30 | * |
| 10 | -0.473 | 2.087 × 10-4 | 127.74 | 093.46 | 112.4 | 33.97 |
| 20 | -0.497 | 1.842 × 10-4 | 121.81 | 095.47 | 126.3 | 41.72 |
| 30 | -0.502 | 1.212 × 10-4 | 116.03 | 090.88 | 182.9 | 61.65 |
| 40 | -0.510 | 7.524 × 10-5 | 112.19 | 092.22 | 292.4 | 76.19 |
| 50 | -0.516 | 5.645 × 10-5 | 110.87 | 093.15 | 389.9 | 82.14 |
| 60 | -0.521 | 4.715 × 10-5 | 108.80 | 095.19 | 468.2 | 85.08 |
Table 2: Electrochemical parameters from polarization measurement, calculated values of inhibition efficiency
Electrochemical impedance studies [49-51]
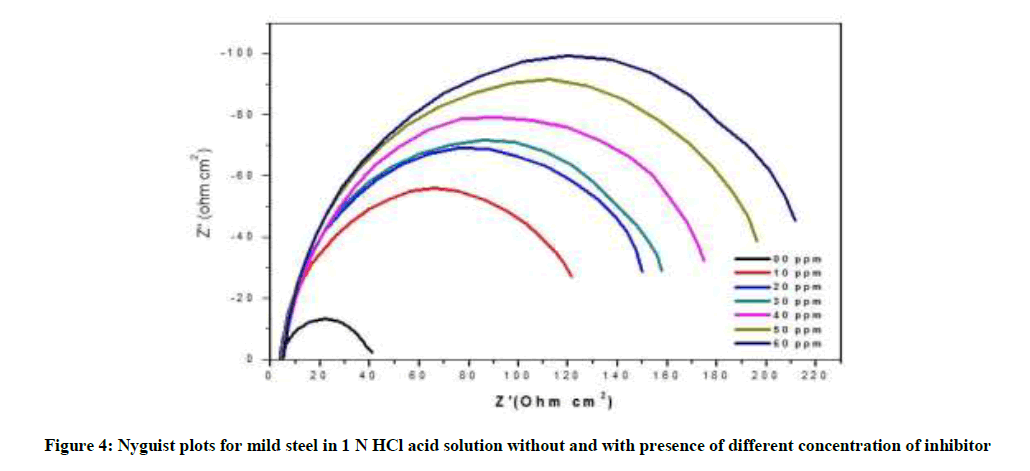
The electrochemical impedance diagram for mild steel in 1 N HCl with different concentration of P. dulce leaves extract are shows in Figure 4 and the impedance parameters are derived in Table 3. From the Table 3, it is clear that the Rct values increased and that the Cdl values decreased with increasing inhibitor concentration. Nyquist plots are showed almost in a semi-circular shape, showing that the adsorption of inhibitor on the metal surface.
| Conc. (Ppm) |
Rct (ohmcm2) |
Cdl (µF/cm2) |
IE (%) |
|---|---|---|---|
| Blank | 043.76 | 8.312 | * |
| 10 | 115.40 | 3.667 | 62.08 |
| 20 | 150.10 | 2.424 | 70.84 |
| 30 | 249.80 | 2.220 | 82.48 |
| 40 | 407.30 | 2.111 | 89.26 |
| 50 | 521.80 | 1.930 | 91.61 |
| 60 | 616.70 | 1.818 | 92.90 |
Table 3: Impedance parameter for mild steel in 1 N HCl acid solution in the absence and presence of varied concentration of inhibitor
Phytochemical analysis of the plant extracts [52]
Preliminary phytochemical analysis of P. dulce leaves extract (aqueous) showed the presence of various chemical constituent included triterpinoids, tannins, saponins, anthraquinones, sugar, alkaloids, carbohydrates, proteins, the outcomes are scheduled in Table 4.
| Phytochemical test | Aqueous extract |
|---|---|
| Alkaloids | + |
| Carbohydrates | + |
| Diterpens | + |
| Saponins | + |
| Phytosterols | - |
| Tannins | + |
| Flavanoids | + |
| Phenol | + |
| Glycosides | - |
| Amino acids | + |
(+).. Presence (-)... Absence
Table 4: Phytochemical screening test of extract of Pithecellobium dulce leaves
SEM [53]
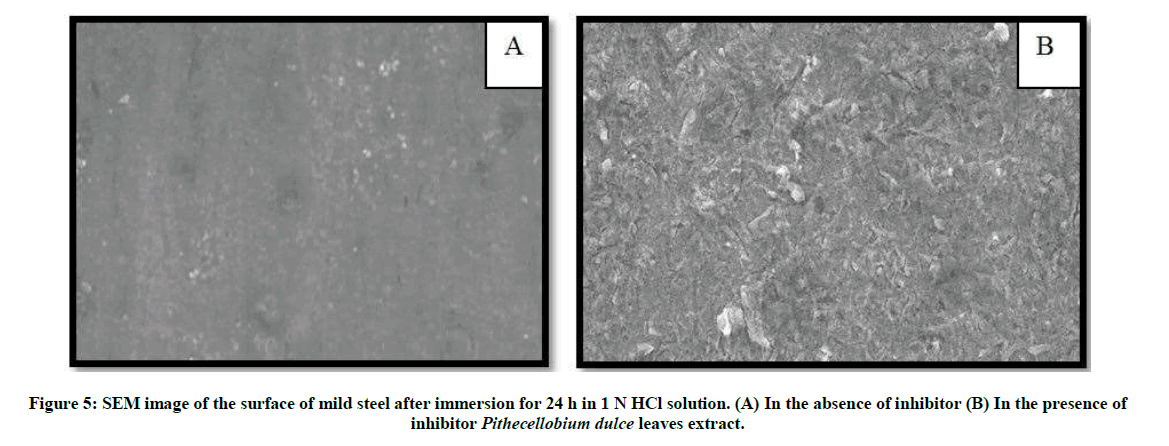
The SEM images were recorded to establish the interaction of inhibitor molecules with metal surfaces. Figure 5a represent as plain mild steel immersed in 1 N HCl, rough surface was notified. But in inhibited solution (Figure 5b), the rate of corrosion is suppressed, as the electrode surface is nearly free from corrosion due to the adsorption of the inhibitor on the mild steel surface.
Effect of immersion time [54]
The variation of weight loss with time for the corrosion of mild steel in acid solution with and without of different concentration of inhibitor data are summarized in Table 5.
| Conc. (Ppm) |
1 h | 3 h | 5 h | 7 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| Blank | * | * | * | * | * | * |
| 10 | 78.29 | 68.23 | 64.34 | 54.06 | 45.45 | 33.97 |
| 20 | 79.08 | 72.34 | 74.90 | 57.12 | 49.99 | 41.72 |
| 30 | 87.99 | 76.06 | 82.45 | 64.45 | 59.12 | 61.65 |
| 40 | 88.12 | 79.89 | 86.71 | 71.22 | 68.23 | 76.19 |
| 50 | 90.30 | 87.45 | 92.63 | 79.78 | 74.33 | 82.14 |
| 60 | 90.45 | 91.09 | 94.00 | 90.12 | 89.22 | 85.08 |
Table 5: IE at various concentration and different immersion periods
Effect of temperature [55]
In order to investigate of temperature on the anti-corrosion inhibition properties of P. dulce leaves extract in the temperature range of 303-323K and the data are depicted in Table 6.
| Conc. (Ppm) |
303K | 313K | 323K |
|---|---|---|---|
| Blank | * | * | * |
| 10 | 44.17 | 49.23 | 39.13 |
| 20 | 57.66 | 59.67 | 49.37 |
| 30 | 62.22 | 63.78 | 53.73 |
| 40 | 71.11 | 77.34 | 67.24 |
| 50 | 76.75 | 82.09 | 72.99 |
| 60 | 85.43 | 87.54 | 85.64 |
Table 6: The Percentage inhibition efficiency of Pithecellobium dulce plants at various temperatures
Adsorption isotherms
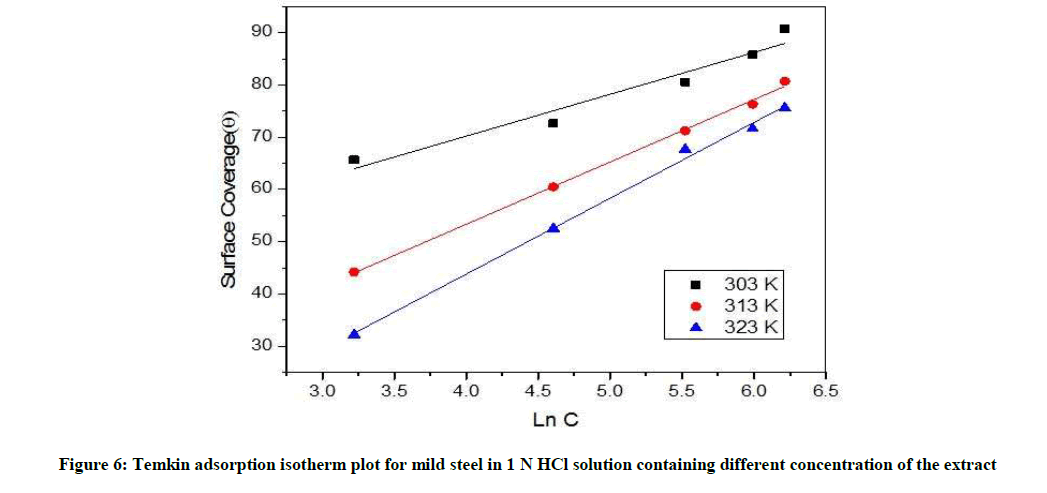
Weight loss data (Table 6) are quite useful in determining inhibitor adsorption characteristics. The most used isotherm is bockris-swinkless, deboer, temkin, langumir, frumkins, flory-hyggins. For Temkin isotherm, surface coverage (θ) was plotted against Ln C (Figure 6). A straight line was found for the PD leaves extract obey Temkin adsorption isotherms.
Conclusion
PD leaves extract can acts as an excellent eco-friendly green inhibitor for the corrosion of mild steel in 1 N hydrochloric acid. The inhibition efficiency values obtained from both chemical and electrochemical methods (Weight loss, polarization and impedance measurement) are in good agreement with each other. Protective film formed on the metal surface is confirmed by using electrochemical studies such as polarization and impedance technique. The plant extract fit the values by Temkin isotherm.
References
- A.P. Srikanth, S. Nanjundan, N. Rajendran, Prog. Org. Coat., 2007, 60, 320.
- D.L. Lake, Corrosion Prevention and Control.,, 1988, 35(4), 113.
- A. Ostovari, S.M. Hoseinieh, M. Peikari, S.R. Shadizadeh, S.J. Hashemi, Corros. Sci., 2009, 51, 1935.
- J.O.M. Bockris, B. Yang, J. Electrochem. Soc., 1991, 138, 2237-2252.
- G. Schmitt, Institute of Materials, London, 1994, 64.
- V.S. Sastri, J.R. Perumareddi, Corros., 1997, 53, 617.
- K. Babić-Samardžija, K.F. Khaled, N. Hackerman, Anti Corros. Method Mat., 2005, 52, 11.
- B.G. Clubley, Royal Society of Chemistry, Cambridge., 1990.
- M.A. Quraishi, M. Wajid Khan, M. Ajmal, S. Muralidharan, S. Venkatakrishna Iyer, Anti-Corros. Method. Mater., 1996, 43, 5.
- S. Muralidharan S. Venkatakrishna Iyer, Anti-Corros. Method. Mater., 1997, 44, 100.
- I.B. Obot, N.O. Obi-Egbedi, S.A. Umoren, Int. J. Electrochem. Sci., 2009, 4, 863.
- J. Sinko, Prog. Org Coat., 2001, 42, 267.
- L.A. Nnanna, B.N. Onwuagba, I.M. Mejeha, K.B. Okeoma, Afr. J. Pure Appl. Chem., 2010, 4, 11.
- P. Nagarajana, J. Morris Princya, J. Christy Ezhilarasia, D. Kavithaa, N. Sulochana, J. Ind. Council Chem., 2009, 26, 153.
- P. Deepa Rani, S. Selvaraj, J. Phytol., 2010, 2, 58.
- A.M. Al-Turkustani,S.T.Arab, R.H. Al-Dahiri,Modern Appl.Sci., 2010, 4(5).
- E. Emeka Oguzie, Portugaliae Electrochimica Acta., 2008, 26, 303.
- M. Sangeetha, S. Rajendran, J. Sathiyabama, A. Krishnaveni, P. Shanthy, N. Manimaran, B. Shyamala devi, Portugaliae Electrochimica Acta., 2011, 29(6), 429.
- A.K. Satapathy, G. Gunasekaran, S.C. Sahoo, K. Amit, P.V. Rodrigues, Corros. Sci., 2009, 51, 2848.
- E.E. Oguzie, Corro. Sci., 2007, 49(3), 1527.
- M.B.M. Ali, K. Kannan, J. Appl. Sci. Environ. Manage., 2009, 13, 27.
- S.A. Verma, M.N. Mehta, Transact. Socie. Advan. Electrochem. Sci. Technol., 1997, 32, 4, 89.
- A. Mesbah, C. Juers, F. Lacouture, S. Mathieu, E. Rocca, M. Francois, J. Steinmetz, Solid State Sci., 2007, 9, 322.
- P.C. Okafor, V.I. Osabor, E.E. Ebenso, Pigm. Resin Technol., 2007, 36, 299-305.
- K. Anuradha, R. Vimala, B. Narayanansamy, J. Arockia Selvi, S. Rajendran, Chem. Eng. Commun., 2008, 195, 352.
- C.O. Peter, E.E. Eno, J.E. Udofot, Int. J. Electrochem. Sci., 2010, 5, 978.
- M. Lebrini, F. Robert, P.A. Blandinières, C. Roos, Int. J. Electrochem. Sci., 2011, 6, 2443.
- M.K. Sharma, P. Arora, S. Kumar, S.P. Mathur, R. Ratnani, Corros. Eng. Sci. Technol., 2008, 43, 213.
- M. Gopiraman, P. Sakunthala, R. Kanmani, R.V. Alex, N. Sulochana, Int. J. Ionics., 2011, 17, 843.
- M. Sangeetha, S. Rajendran, J. Sathiyabama, A. Krishnaveni, P. Shanthy, N. Manimaran, B. Shyamaladevi, Port. Electrochim. Acta., 2011, 29, 429.
- L. Xiang-Hong, D. Shu-Duan, F. Hui, J. Appl. Electrochem., 2010, 40, 1641.
- M. Sivaraju, K. Kannan, Int. J. ChemTech. Res., 2010, 2, 1243.
- A. Chetouani, B. Hammouti, Bull. Electrochem., 2001, 19, 23.
- P.C. Okafor, E.E. Ebenso, Pigm. Resin Technol., 2007, 36, 134.
- A. Bouyanzer, B. Hammouti, L. Majidi, Mat. Lett., 2006, 60, 2840.
- E.E. Oguzie, Mater. Chem. Phys., 2006, 99, 441.
- S.S. Shivakumar, K.N. Mohana, Adv. Appl. Sci. Res., 2012, 3, 3097.
- O. Benali, H. Benmehdi, O. Hasnaoui, C. Selles, R. Salghi, J. Mater. Environ. Sci., 2013, 4, 127.
- M. Ramananda Singh, J. Mater. Environ. Sci., 2013, 4, 119.
- R.A. Mohammed, M. Abdulwahab, I.A. Madugu, J.O. Gaminana, F. Asuke, J. Mater. Environ. Sci., 2013, 4, 93.
- A.M. Badiea, K.N. Mohana, J. Mat. Eng. Perform., 2009, 18, 1264.
- P. Bothi Raja, M.G. Sethuraman, Mater. Lett., 2008, 62, 113.
- S. Kabir Khanzada, A. Kabir Khanzada, W. Sheikh, S. Abid Ali, pak. J. Bot., 2013, 45(2), 557.
- A. Lalitha, S. Ramesh, S. Rajeshwari, Electrochim. Acta., 2005, 51, 47.
- M. Guannan, L. Xianghong, Q. Qing, Z. Jun, Corros. Sci., 2006, 48, 445.
- M.G. Hosseini, M. Ehteshamazadeh, T. Shahrabi, Electrochim. Acta., 2007, 495, 3680.
- E.S. Ferreira, C. Giacomelli, F.C. Giacomelli, A. Spinelli, Mater. Chem. Phys., 2004, 83, 129.
- W.H. Li, Q. He, S.T. Zhang, C.L. Pei, B.R. Hou, J. Appl. Electrochem., 2008, 38, 289.
- J. Angelin Thangakani, S. Rajendran, J. Sathiyabama, J. Lydia Christy, A. Surya Prabha, M. Pandiarajan, Eur. Chem. Bull., 2013, 1(5), 265.
- N. Vijan, A.P.P. Regis, S. Rajendran, M. Pandiarajan, R. Nagalakshmi, Eur. Chem. Bull., 2013, 2(5), 275.
- S. Agila Devi, S.J. Rajendran, M. Pandiarajan, Eur. Chem. Bull., 2011, 2(2), 84.
- S. Rajendran, K. Anuradha, K. Kavipriya, A. Krishnavei, J. Angelin Thangakani, Eur. Chem. Bull., 2012, 1(12), 503.
- M. Pandiarajan, P. Prabhakar, S. Rajendran, Eur. Chem. Bull., 2012, 1(7), 238.
- T. Poongodi, R. Hemalatha, World J. Pharm. Pharm. Sci., 2015, 4, 1266.
- R.N. Nair, S. Sharma, I.K. Sharma, P.S. Verma, A. Sharma, Rasay. J. Chem., 2010, 3(4), 783.