Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 4
Electrochemical Behavior of Reinforcement Steel in Simulate Concrete Pore Solution with and without Chloride Ions
Ehteram A Noor*, Aisha Al-Moubaraki H and Dalal I Al-MasoudiEhteram A Noor, Science Faculty, Chemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia, Email: aatallha@kau.edu.sa
Abstract
Reinforcement steel working electrode was used in electrochemical tests (EIS and PDP) after 0 h and 24 h immersion in SCP solutions (saturated Ca(OH)2) maintained at 30°C with and without various [Cl-]. EIS data obtained both at 0 h and 24 h immersion revealed that at 0.01 M<[Cl-]<0.10 M, steel resists corrosion more than the Cl- free solution, while at [Cl-] ≥ 0.10 M steel corrosion rate increases appreciably. PDP data showed that at (i) [Cl-]<0.05 M for 0 h immersion and (ii) [Cl-]<0.2 5 M for 24 h immersion, steel resists pitting corrosion even if it was polarized to the oxygen evolution potential, indicating film formation of corrosion products on the steel surface which becomes more denser with increasing immersion time. Accordingly, the CTL and the corresponding ratio ([OH]/[Cl-]) of steel in SCP solution varied with the immersion period as well as with the techniques used.
Keywords
Corrosion, Steel, Concrete, Pore solution, Chloride ions, Potentiodynamic, Imepdance
Introduction
Steel-reinforced concrete is considered the most important material used extensively in the construction due to its economical and durable properties [1,2]. Reinforcing steel bars (rebar) embedded in the concrete not only to give the structure with best possible strength but also to create a protective surroundings for the rebar. As a result of the high alkalinity environment of concrete and low diffusion rate of both oxygen and water from atmosphere to the concrete pores, the rebar will be protected by a stable passive film and hence insignificant level of corrosion rate is maintained [3-5]. The corrosion rate of rebar becomes significant when some environmental factors cause deterioration of the cover concrete and enhance the diffusivity of aggressive species [6]. Carbon dioxide (CO2) and chloride ions (Cl-) are of the major aggressive species responsible for the corrosion problems of reinforcement steel in concrete structures. The ingress of CO2 will reduce the pore solution alkalinity and eventually passivity breakdown and corrosion of rebar take place. While, the Cl- ions either present initially or by diffusion from outside environment play an important role for the most damage in concrete structures by the so called chloride-induced corrosion [6,7]. The main characteristic of chloride-induced corrosion is as follows [6]: (i) Separation between the anodic and cathodic areas is occurred, and corrosion rate is significant and localized. (ii) Once the localized (pitting) corrosion is initiated, it is far more difficult to remedy than carbonation.
For corrosion to be initiated, the passive film must be penetrated by the Cl- ions and excite the surface of the steel to form an anode as follows [8]:
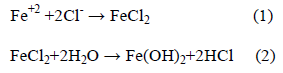
The activated (i.e., depassivated) area becomes an anode, while the passivated surface becomes a cathode. At the anode of the cell, the reaction is described by:
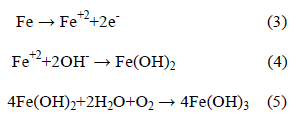
In contrast, the cathodic reaction is given by:
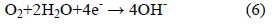
According to Equation 2, Cl- ion is regenerated so that the rust (Eqn. 5) contains no chloride, although the iron chloride is formed as the intermediate stages (Eqn. 1). So, Cl- ions are not consumed in the process but facilitate to break down the passive film of oxide on the steel and permit the corrosion process to proceed rapidly.
The field of chloride-induced reinforcement steel corrosion has been covered extensively by various investigations either in concrete or in Simulated Concrete Pore (SCP) [7,9-12]. Generally, the existence of critical chloride concentration called Chloride Threshold Level (CTL) which result in a serious corrosion rate is clear cut. However, no unified conclusion about the value of CTL was stated. This may be due to the variance in experimental conditions and methods used to predict corrosion rates.
The technology of corrosion rate measurements has been advanced for lab or field studies in order to give accurate and timely corrosion measurements. Electrochemical techniques have been used widely to study fundamental phenomenological corrosion reactions of steel in concrete or SCP solutions [7,10-12]. The general methods being used include electrical resistance, Potentiodynamic Polarization (PDP), Linear Polarization Resistance (LPR) and Electrochemical Impedance Spectroscopy (EIS). In The present study the influence of Cl- ions concentration, [Cl-], on the corrosion behavior of reinforcement steel in SCP solutions for 0 h (once immersion) and 24 h immersion at 30°C was investigated by applying PDP and EIS measurements. The upper limit of CTL was determined based on the electrochemical parameters such as polarization resistance (Rp), pitting potential (Epit) and corrosion current density (Icorr).
Experimental
Saturated calcium hydroxide (Ca(OH)2) was used in this work to simulate concrete pore solutions with pH value of 12.6. A set of SCP solutions containing various chloride levels in the range from 0.01 to 1.00 M were used to simulate chloride contamination. All solutions were prepared using analytical reagent chemicals and deionized water.
The material of working electrode was commercial reinforcement steel rebar which had the following composition by percent weight: C: 0.280; Si: 0.220; Mn: 0.730; P: 0.015; S: 0.006; N: 0.007; Fe: balance. The steel was cut into cylindrical rod of 5 cm in length and 1 cm in diameter. The steel rod was preserved with epoxy resin except the working surface of 0.785 cm2 which exposed to the tested solutions. Before each experiment, the working electrode was polished with different grades of emery paper up to 1200, washed with deionized water and degreased with ethanol and finally dried with stream of air.
EIS and PDP tests were performed in an electrochemical cell of three electrodes using ACM instrument version 5 (Gill AC serial no. 1649). The steel working electrode, which was explained in the previous paragraph. A Coiled platinum wire (0.2 mm diameter) was used as an auxiliary electrode. The reference electrode was Ag/AgCl, KClsat.. All measurements were done after 0 h and 24 h immersion in stagnant SCP solutions with and without various [Cl-] and at 30°C. The EIS spectra were collected over the frequency range from 30 kHz to 0.1 Hz using 30 mV sinusoidal perturbation. The PDP curves were recorded in the potential range from -1000 to 800 mV with a scan rate of 2.5 mV.s-1. The impedance spectra were fitted to appropriate equivalent circuit using ZsimDemo 3.20, while the polarization curves were analyzed by using ACM instrument software version 5.
Conclusion
The following points give the most important results obtained from this investigation:
• The resistance of SCP solution (Rs) decreased with increasing [Cl-] which attributed to the increase of solution conductivity with the addition of Cl- ions.
• Both 0 h and 24 h immersion recorded a decrease in Rp -1 (i.e., corrosion rate) values with [Cl-] in the range from 0.01 M to 0.05 M, while a continuous increase was observed with [Cl-] ≥0.10 M.
• Ecorr value shifted to more active potentials with [Cl-] and this behavior is more pronounced in the case of 24 h immersion.
• At [Cl-]<1.00 M, 24 h immersion condition showed less values for Icorr as compared with 0 h immersion, indicating that the corrosion products becomes more denser on the steel surface with increasing immersion time which increase the energy barrier for metal dissolution.
• Rp, Epit and Icorr are good indicative parameters for the CTL of steel in SCP solutions contaminated with Cl- ions.
• The results revealed that the value of CTL varied with immersion period and the technique used. The obtained CTL from EIS and PDP measurements are respectively (i) 0.1 M (for both 0 h and 24 h immersion) and (ii) 0.05 and 0.25 M (at zero and 24 h immersion, respectively).
Acknowledgments
The authors are grateful to King AbdulAziz City for Science and Technology (KACST) for funding this study by grand number AT-36-008.
References
[1] A.A. Gürten, K. Kayakirilmaz, M. Erbil, Constr. Build. Mater., 2007, 21, 669.
[2] S. Fajardo, D.M. Bastidas, M. Criado, M. Romero, J.M. Bastidas, Constr. Build. Mater., 2011, 25(11), 4190.
[3] K. Kobayashi, K. Shuttoh, Cem. Concr. Res., 1991, 21, 273.
[4] M. Raupach, Mater. Struct., 1996, 29, 174.
[5] H. Kahyaoğlu, M. Erbil, B. Yazici, A.B. Yilmaz, Turk. J. Chem., 2002, 26, 759-769.
[6] J.P. Broomfield, Corrosion of steel in concrete, E & FN Spon, London, UK, 1997.
[7] C.Q. Ye, R.G. Hu, S.G. Dong, X.J. Zhang, R.Q. Hou, R.G. Du, C.J. Lin, J.S. Pan, J. Electroanal. Chem., 2013, 688, 275-281.
[8] A.M. Neville, Properties of concrete, Pearson Education Limited., Harlow, UK, 1995, 4.
[9] G.K. Glass, N.R. Buenfeld, Corros. Sci., 1997, 39(5), 1001-1013.
[10] M. Saremi, E. Mahallati, Cem. Concr. Res., 2002, 32(12), 1915-1921.
[11] R. Liu, L. Jiang, J. Xu, C. Xiong, Z. Song, Constr. Build. Mater., 2014, 56, 16-20.
[12] G. Liu, Y. Zhang, Z. Ni, R. Huang, Constr. Build. Mater., 2016, 115, 1-5.
[13] X. Jing, Y. wu, Constr. Build. Mater., 2011, 25(5), 2655-2662.
[14] F. Mansfeld, M.W. Kendig, S. Tsai, Corros., 1982, 38(11), 570-579.
[15] M.G. Hosseini, M. Ehteshamzadeh, T. Shahrabi, Electrochim. Acta., 2007, 52(11), 3680-3685.
[16] J. Shi, W. Sun, J. Jiang, Y. Zhang, Constr. Build. Mater., 2016, 111, 805-813.
[17] G. Blanco, A. Bautista, H. Takenouti, Cem. Concr. Compos., 2006, 28, 212-219.
[18] M. Criado, S. Fajardo, J.M. Bastidas, Int. J. Corros., 2012, 2012.
[19] Y. Guo, X.P. Wang, Y.F. Zhu, J. Zhang, Y.B. Gao, Z.Y. Yang, R.G. Du, C.J. Lin, Int. J. Electrochem. Sci., 2013, 8, 12769-12779.
[20] G. Sahoo, R. Balasubramaniam, Corros. Sci., 2008, 50(1), 131-143.
[21] D.A. Jones, Prentice Hall, USA, 1996, 2.
[22] G.J. Verbeck, Am. Concr. Inst., 1975, SP-49, 21-38.
[23] S. Angappan, S. Sathiyanarayanan, G. Rajagobal, K. Balakrishnan, Bull. Electrochem., 1996, 12(1-2), 48-50.
[24] D.A. Haussman, Mater. Perf., 1998, 37(10), 64-68.
[25] G.T. Burstein, C. Liu, R.M. Souto, S.P. Vines, Corros. Eng. Sci. Technol., 2004, 39(1), 25-30.
[26] M. Pourbaix, NACE Int., Houston, 1974, 2.
[27] Y.T. Tan, S.L. Wijesinghe, D.J. Blackwood, Corros. Sci., 2014, 88, 152-160.
[28] R.T. Loto, J. Mater. Environ. Sci., 2013, 4(4), 448-459.
[29] A.S. Hamdy, E. El-Shenawy and T. El-Bitar, Int. J. Electrochem. Sci., 2006, 1, 171-180.
[30] C. Andrade, C. Alonso, Mater. Struct., 2004, 37, 623-643.
[31] G.D. Eyu, G. Will, W. Dekkers, J. MacLeod, Materials., 2016, 9(9), 748.
[32] L. Jiang, G. Huang, J. Xu, Y. Zhu, L. Mo, Constr. Build. Mater., 2012, 30, 516-2521.
[33] M.D. Asaduzzaman, C.M. Mustafa, M. Islam, Chem. Ind. Chem. Eng. Q., 2011, 17(4), 477-483.



