Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 12
Electrochemical Analysis of Novel Schiff’s Bases Containing a Coumarin Moiety
Parepalli Y1, Dharmalingam B2, Palanisamy P3*, Tadiboyina R4, Prasada Rao PTSRK5, Sudhakar Reddy P6 and Chavali M7,8*
1Division of Chemistry, Vignan’s University, Guntur- 522 213 Andhra Pradesh, India
2OXALISS International Senior Secondary School, Thatchur, Salem - Chennai Main Road, Kallakurichi- 636213 Tamil Nadu, India
3Department of Physics, Gnanamani College of Engineering, Pachal, Namakkal-637018 Tamilnadu, India
4Aakash Educational Services Ltd., No. 2, AB-Block, 2nd Avenue, Anna Nagar, Chennai-600040, Tamil Nadu, India
5Department of Chemistry, KVSR Siddhartha College of Pharmaceutical Sciences, Siddhartha Nagar, Vijayawada -520010, Andhra Pradesh, India
6Department of Zoology, VSU PG Centre Kavali, Peddapavani Road, Kavali- 524201 Andhra Pradesh, India
7Shree Velagapudi Rama Krishna Memorial College (PG Studies), NAAC 'A' Grade & ISO 9001: 2015 Certified, Affiliated to Acharya Nagarjuna University, Nagaram 522 268 Guntur District, Andhra Pradesh, India
8MCETRC, Tenali, Guntur- 522 201 Andhra Pradesh, India
- *Corresponding Author:
- Palanisamy P
Division of Chemistry
Vignan’s University
Guntur- 522 213 Andhra Pradesh, India
Chavali M
Shree Velagapudi Rama Krishna Memorial College (PG Studies)
NAAC 'A' Grade & ISO 9001: 2015 Certified, Affiliated to Acharya Nagarjuna University
Nagaram 522 268 Guntur District, Andhra Pradesh, India
Abstract
In this work, the electrochemical reduction of some Schiff’s bases contains substituted coumarin moiety and the effects of the substituents have been studied by polarographic technique. Based on the results obtained and previous literature, a tentative mechanism has been proposed. The system investigated in this study was a series of substituted (17Z)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N'-benzylideneacetohydrazides. The system possesses two electrophores; (1) Hydrazone group and (2) Carbonyl group. Thus, the electrochemical reduction of the system, studied with DC polarography, might provide valuable insight into the processes taking place on the surface of the electrode and can be of importance in fundamental electrochemical research.
Keywords
Schiff’s base, Coumarin, Polarographic reduction, Electrochemical.
Introduction
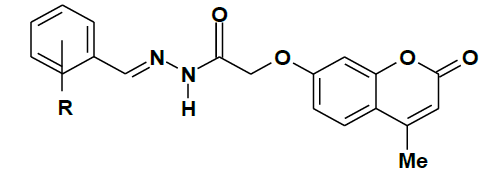
Schiff’s bases are compounds with the azomethine (-C=N-) group formed by the condensation of primary amines and activated carbonyl compounds. The characteristic feature of the compounds containing the (-C=N-) group illustrate the fundamental properties lies in the configuration of complexes with metals. These complexes offer some characteristic chain of coordination compounds, shown to exhibit antibacterial& antifungal [1-6], and antitumour [7,8] activities. These compounds were studied extensively as a ligand that coordinates with metal ions via azomethine nitrogen [9-11] particularly in metal complexation used as oxygen carriers [12,13]. Recently, coumarins gathered a lot of research interest owing to their prominence in synthetic and natural organic chemistry. Structural analogues of coumarin have been shown to exhibit molluscicidal [14], insecticidal properties [15] and as anticoagulants [16]. The system investigated in this study was a series of substituted (17Z)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N'-benzylideneaceto-hydrazides, whose general structure is and moieties are given in Table 1.
 |
|
|---|---|
| R | Code |
| H | 1 |
| 4-OH | 2 |
| 4-Cl | 3 |
| 2,5-OMe | 4 |
Table 1: Substituents and the corresponding numerical codes
The system of our interest contains the hydrazones moiety which possesses a varied range of bioactivities in pharmaceutical and agrochemical field [17-20]. Several of them are considered for their special biological catalyst enzyme activity [21]. Moreover, derivatives of 1, 3, 4-oxadiazole have been displayed to exhibit bioactivities including insecticidal [22], anti-bacterial [23,24], anticancer [25] and anti-inflammatory activities [26]. The bio-activity connected with the hydrazones is ascribed to the existence of the active pharmacophore (-CONH-N=C-).
The proposed system consisting of two electrophores, first one is Hydrazone group and later Carbonyl group. In our study, DC polarography techniques were used to study the electrochemical reduction of the system to acquire the insight about the electrode surface reaction and importance in basic electrochemical research.
Materials and Methods
Polarographic investigations on metal complexes of Schiff’s bases and on coumarins have been reported earlier [27-33]. In this system, (17Z)-2-(4-methyl-2-oxo- 2H-chromen-7-yloxy) N'benzylideneacetohydrazide essentially a Schiff’s base containing coumarin moiety. These compounds have only been synthesized recently and characterization of this compound makes an interesting of polarographic study. The aim of this investigation is to study the electrochemical reduction of the chosen system under polarographic conditions, keeping the effect of pH and substituent’s in view, to propose a possible mechanism for the reduction process.
Applied electrochemical techniques
DC polarography
The techniques employed in this study are DC polarography and differential pulse polarography (DPP). These are, in particular, the former, the simplest and oldest of all electrochemical techniques. Old, nevertheless, polarography has lost neither its charm nor importance in the analysis, qualitative and quantitative both of electroactive analytes, organic as well as inorganic in nature.
In DC polarography, the mass transport is controlled by diffusion alone. Under these conditions, Ilkovic equation [34] for maximum diffusion current is given as, id=708nCD1/2m 2/3t1/6.
Where, id=diffusion current in μA, m=rate of flow of Hg in mg s-1, t=drop time inseconds, D=diffusion coefficient of the electroactive species in cm2 s-1, C=concentration of the electroactive species and n=number of electrons involved in the process. For mean diffusion current, id=607nCD1/2m 2/3t1/6.
Then, the Ilkovic equation can be rewritten as id=708 nCD1/2h1/2 and hence, id will be directly proportional to √h, provided nCD1/2 is maintained a constant. This is in fact, employed as a test for determining whether the process is diffusion controlled or not.
The following relations have been derived by Heyrovsky, Ilkovic [35] and Kern [36] for the diffusion controlled cathodic reversible and irreversible processes.
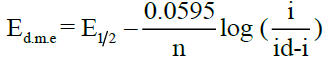 (Reversible)
(Reversible)
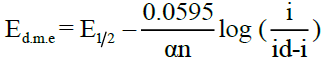 (Irreversible)
(Irreversible)
Where, Ed.m.e = potential at DME vs SCE in Volts
A plot of –E vs 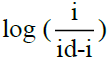 gives a straight line, whose point of intersection with X axis containing –E gives the half wave potential E1/2. The slope (m) of the straight line is used to calculate the ‘Transfer coefficient’ αn using the well-known relation
gives a straight line, whose point of intersection with X axis containing –E gives the half wave potential E1/2. The slope (m) of the straight line is used to calculate the ‘Transfer coefficient’ αn using the well-known relation 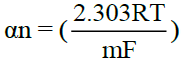 where R is the universal gas constant, T is the absolute temperature, m is the slope of the Tafel plot and F is the Faraday constant. Diffusion coefficient, D is calculated as D = 3.32 × 10-5/η(Vm)1/3 where η is the viscosity of the solution and Vm is the molar volume of the system.
where R is the universal gas constant, T is the absolute temperature, m is the slope of the Tafel plot and F is the Faraday constant. Diffusion coefficient, D is calculated as D = 3.32 × 10-5/η(Vm)1/3 where η is the viscosity of the solution and Vm is the molar volume of the system.
The kinetic parameter ‘k’ is computed at each chosen potential for all polarograms using the relation 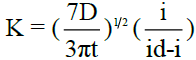 , where t is the drop time in seconds. Plots of -E vs –log (k) are linear and show that the polarograms correspond to the rate determining steps. The reversibility of the system is verified using the famous “Tome’s criterion” which is given by
, where t is the drop time in seconds. Plots of -E vs –log (k) are linear and show that the polarograms correspond to the rate determining steps. The reversibility of the system is verified using the famous “Tome’s criterion” which is given by 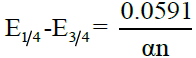 , where n is the number of electrons involved in the rate determining step. Appreciable deviation from this relation indicates irreversibility. In irreversible processes, αna can be calculated as
, where n is the number of electrons involved in the rate determining step. Appreciable deviation from this relation indicates irreversibility. In irreversible processes, αna can be calculated as
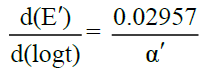 and
and
The familiar relation 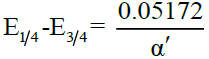
where, E’ = E1/2, the half-wave potential and α’ = αna
Differential pulse polarography (Dpp)
In this variation of DC polarography, square wave pulses of known constant amplitude are applied over the steadily increasing DC potential gradient as in DC polarography. The difference in the current before and after the application of the pulse is plotted as a function of the applied potential at the DME. This shows peaks corresponding to the reduction potentials of the electroactive species. Polarography is used in the present work where no polarographic, in fact, electrochemical data is available i.e., in the reductions of (17Z)-2-(4-methyl-2-oxo-2H-chromen-7- yloxy)-N'-benzylideneacetohydrazides.
Method adopted
The system under investigation was found to be moderately soluble only in dimethyl sulphoxide (DMSO). 10-4 M stock solutions of the system were prepared, assisted by sonication, in DMSO. The Analytical grade (purity >99%) dimethylsulphoxide was procured from chemical suppliers (SD fine Chemicals Pvt., Ltd.) and used without further purification. Each such solution was purged with pure and dry nitrogen (N2) gas for 20 min before every use to remove oxygen (O2) dissolved in the solution.
The pH was maintained using the following five aqueous buffers i) Clark &Lub’s buffer of pH ~1.00, ii) Phosphate buffer of pH ~ 5.00, iii) Phosphate buffer of pH ~7.00, iv) Alkaline borate buffer of pH ~8.00& v) Alkaline borate buffer of pH 10.00, and these solutions were also purged with N2 gas for 15 min before recording polarograms. From the stock solutions, 10-5 M solutions of the system were prepared in each buffer before use. 0.1 mL of a 1% aqueous solution of a non-ionic surfactant, Triton X – 100 was added to all solutions.
The conditions maintained were temperature (291 K), the height of mercury (Hg) reservoir (h=1.21 m), drop time (0.50 s) and sweep rate (12 mV s-1). Both DC polarograms and differential pulse polarograms (DPP) were recorded for each solution using an ELICO Polarographic Analyzer (CL-362, India). A reference electrode (standard calomel electrode) and the auxiliary electrode (platinum wire) were employed. The polarographic analyzer and the three electrode assembly were calibrated using a standard 10-2 M solution of Cd2+. From the polarograms, it was found that the system is reduced in two waves in highly acidic media and in a single wave within highly alkaline media. Diffusion coefficient D was calculated as D=3.32 × 10-5 / η (Vm) 1/3 where η is the viscosity of the solution and Vm is the molar volume of the system.
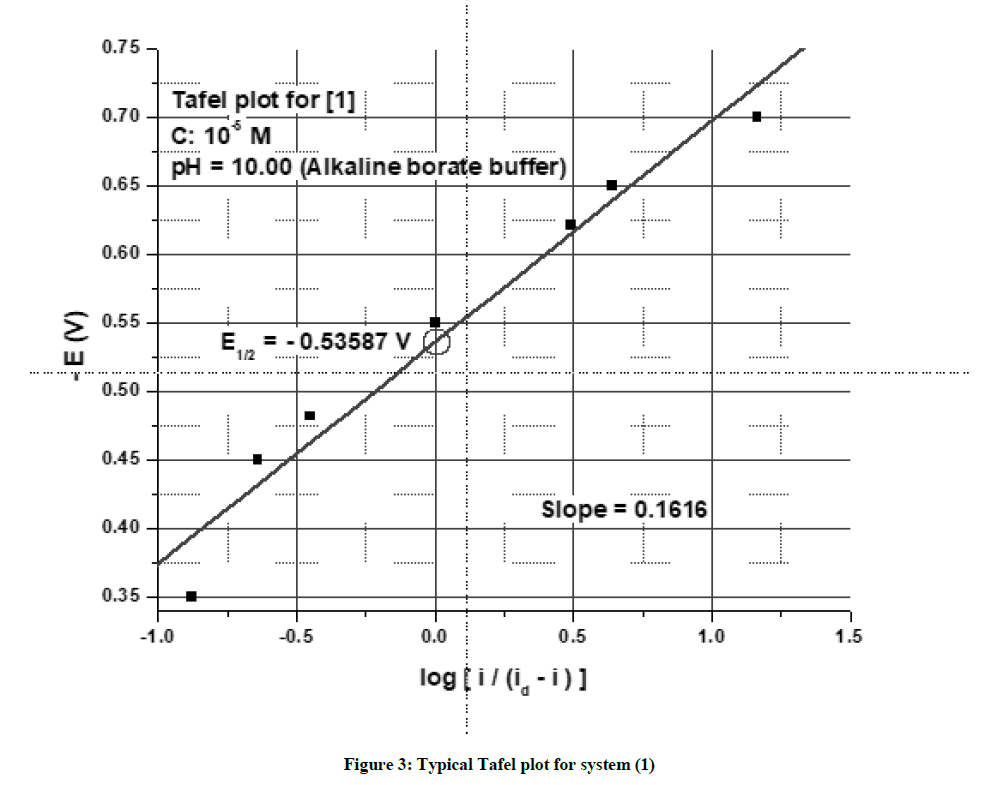
Tafel plots i.e., log (i/id-i) vs -E were plotted or all polarograms. They were linear from them E1/2 was calculated. Transfer coefficient α was calculated from the slope of the Tafel plot, using the well-known relation αn=2.303 RT/mF, where R=universal gas constant, T=absolute temperature, m=slope of the Tafel plot and F = Faraday constant.
The kinetic parameter ‘k’ was computed at each chosen potential as
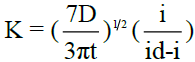 , where t is the drop time
, where t is the drop time
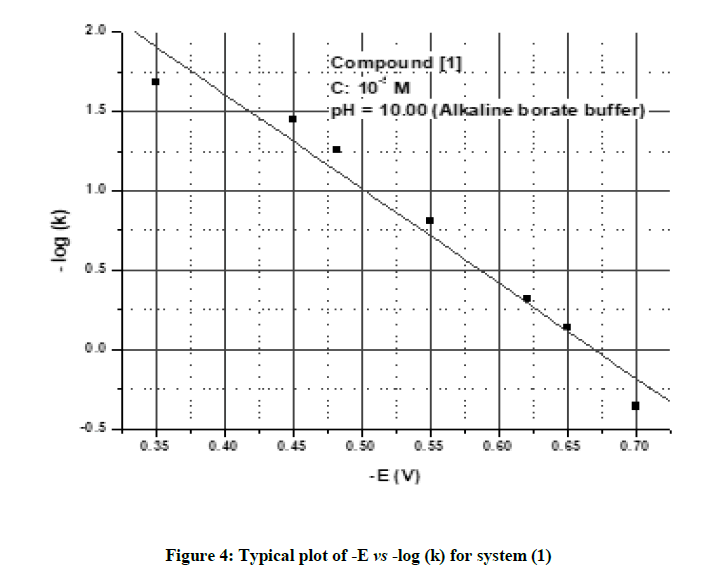
A plot of - E vs – log (k) was linear and showed that the polarogram corresponds to the rate determining step. Based on the observations a plausible mechanism was proposed for the electrochemical reduction of the system under polarographic conditions.
Results and Discussions
The (17Z)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N' benzylideneacetohydrazide system (1)
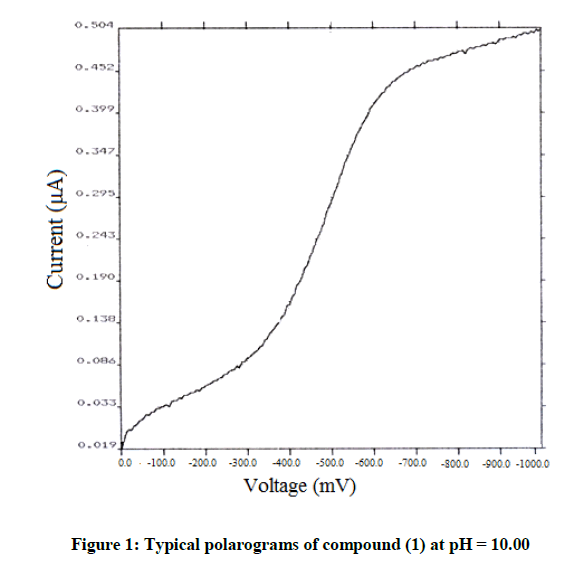
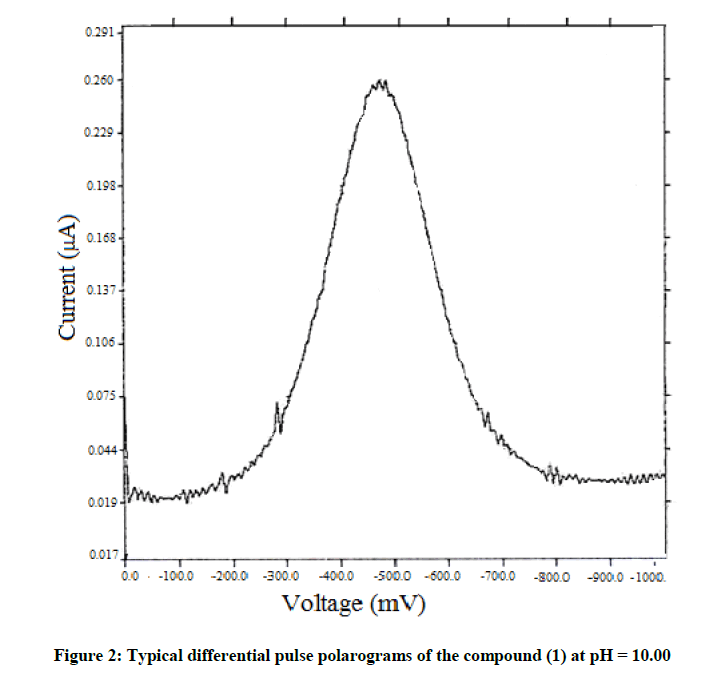
The chosen system and its structural derivatives were studied for their electrochemical reduction at the DME by polarography. The polarographic behaviour of an unsubstituted compound (1) is discussed here. The system (1) exhibited a two-step irreversible reduction in acidic media and a single step irreversible reduction in alkaline and neutral media. Typical DC polarograms and differential pulse polarograms for the compound are shown in Figures 1 and 2 respectively. A typical Tafel plot and a plot of –E vs –log (k) are shown in Figures 3 and 4. The transfer coefficient multiplied by a number of electrons involved in the process was calculated from the slope of Tafel plot. The half wave potentials have been calculated from the point of intersection of the Tafel line with the Y (-E) axis. The complete polarographic data and nature of reduction of (1) in the various pH conditions are presented in Tables 2 and 3 respectively.
| Polarographic reduction of (1) at pH = 1.30 (Clark &Lub’s buffer) | ||||||
|---|---|---|---|---|---|---|
| I Reduction wave of (1 at PH = 1.30) id = 0.065 μA | ||||||
| S. No. | -E vs SCE (V) | I (μA) |
i/(id – i) | Log i/(id – i) | K | -Log (k) |
| 1 | 0.4471 | 0.0164 | 0.3385 | -0.47038 | 0.0531 | 1.2751 |
| 2 | 0.4525 | 0.025 | 0.625 | -0.20412 | 0.078 | 1.0088 |
| 3 | 0.4557 | 0.0325 | 1 | 0 | 0.1568 | 0.8047 |
| 4 | 0.4632 | 0.0493 | 3.1454 | 0.4977 | 0.4931 | 0.3071 |
| 5 | 0.4675 | 0.0585 | 9 | 0.9542 | 1.4111 | 0.1496 |
| II Reduction wave of (1) at pH = 1.30 id = 0.03 μA | ||||||
| 1 | 0.7 | 0.006 | 0.25 | -0.6021 | 0.0392 | 1.4067 |
| 2 | 0.7032 | 0.0078 | 0.3135 | -0.5038 | 0.0492 | 1.3085 |
| 3 | 0.7107 | 0.015 | 0 | 0 | 0.1568 | 0.8047 |
| 4 | 0.7204 | 0.028 | 14 | 1.1461 | 2.1951 | -0.3415 |
| 5 | 0.73 | 0.029 | 20.25 | 1.3064 | 3.175 | -0.5107 |
| Polarographic reduction of (1) at pH = 5.85 (Phosphate buffer) id = 0.528 μA | ||||||
| 1 | 0.175 | 0.00918 | 0.0177 | -1.752 | 0.00276 | 2.5591 |
| 2 | 0.215 | 0.14224 | 0.3691 | -0.4328 | 0.0579 | 1.2375 |
| 3 | 0.3 | 0.26153 | 0.9829 | -0.0075 | 0.1541 | 0.8122 |
| 4 | 0.35 | 0.351 | 1.9875 | 0.2983 | 0.3116 | 0.5064 |
| 5 | 0.39 | 0.403 | 3.2343 | 0.5098 | 0.5071 | 0.2949 |
| Polarographic reduction of (1) at pH = 5.85 (Phosphate buffer) id = 0.528 μA | ||||||
| 1 | 0.175 | 0.00918 | 0.0177 | -1.752 | 0.00276 | 2.5591 |
| 2 | 0.215 | 0.14224 | 0.3691 | -0.4328 | 0.0579 | 1.2375 |
| 3 | 0.3 | 0.26153 | 0.9829 | -0.0075 | 0.1541 | 0.8122 |
| 4 | 0.35 | 0.351 | 1.9875 | 0.2983 | 0.3116 | 0.5064 |
| 5 | 0.39 | 0.403 | 3.2343 | 0.5098 | 0.5071 | 0.2949 |
| Polarographic reduction of (1) at pH = pH = 6.86 (Neutral Phosphate buffer) | ||||||
| 1 | 0.1 | 0.104 | 0.3161 | -0.5002 | 0.04956 | 1.3048 |
| 2 | 0.15 | 0.201 | 0.8645 | -0.0632 | 0.1356 | 0.8679 |
| 3 | 0.2 | 0.251 | 1.3753 | 0.1384 | 0.2156 | 0.6663 |
| 4 | 0.25 | 0.322 | 2.8879 | 0.4606 | 0.4528 | 0.3441 |
| 5 | 0.3 | 0.344 | 2.8436 | 0.5847 | 0.6026 | 0.22 |
| 6 | 0.35 | 0.367 | 5.5188 | 0.7418 | 0.8653 | 0.0628 |
| 7 | 0.4 | 0.371 | 5.936 | 0.7735 | 0.9307 | 0.0312 |
| Polarographic reduction of (1) at pH = pH = 8.00 (Alkaline Borate buffer) | ||||||
| 1 | 0.24 | 0.0097 | 0.1372 | -0.8627 | 0.0215 | 1.6674 |
| 2 | 0.2643 | 0.0195 | 0.3223 | -0.4917 | 0.0505 | 1.2964 |
| 3 | 0.28 | 0.028 | 0.5385 | -0.2689 | 0.0844 | 1.0735 |
| 4 | 0.3047 | 0.0405 | 1.0253 | 0.0109 | 0.1608 | 0.7938 |
| 5 | 0.32 | 0.05 | 1.6667 | 0.2219 | 0.2613 | 0.5828 |
| 6 | 0.34 | 0.0601 | 3.0242 | 0.4806 | 0.4742 | 0.3241 |
| Polarographic reduction of (1) at pH = pH = 10.00 (Alkaline Borate buffer) | ||||||
| 1 | 0.35 | 0.01 | 0.1316 | -0.8808 | 0.0206 | 1.6855 |
| 2 | 0.45 | 0.016 | 0.2286 | -0.641 | 0.0358 | 1.4457 |
| 3 | 0.4821 | 0.0225 | 0.3543 | -0.4506 | 0.0556 | 1.2553 |
| 4 | 0.55 | 0.043 | 1 | 0 | 0.1568 | 1.8047 |
| 5 | 0.6214 | 0.065 | 30.952 | 0.4907 | 0.4853 | 0.314 |
| 6 | 0.65 | 0.07 | 4.375 | 0.641 | 0.7253 | 0.1395 |
| 7 | 0.7 | 0.0805 | 14.636 | 1.1654 | 2.2948 | -0.3608 |
Table 2: Polarographic data for reduction of (1) in buffered aqueous solutions of varying pH
| The polarographic reduction of (1) | |||
|---|---|---|---|
| pH | -E1/2 (V) | αn (m2 s-1) | Nature of reduction |
| 1.30 (I reduction) | 0.455 | 4.058 | Irreversible |
| 1.30 (II reduction) | 0.71 | 4.3338 | Irreversible |
| 5.85 | 0.3209 | 0.5669 | Irreversible |
| 6.86 | -0.183 | 0.2603 | Irreversible |
| 8 | -0.3014 | 0.813 | Irreversible |
| 10.3 | 0.536 | 0.3574 | Irreversible |
| The polarographic reduction of (2) | |||
| 1.30 (I reduction) | 1.213 | 0.598 | Irreversible |
| 1.30 (II reduction) | 1.2168 | 0.791 | Irreversible |
| 5.85 (I reduction) | 2.8061 | 0.513 | Irreversible |
| 5.85 (II reduction) | 2.3983 | 0.6168 | Irreversible |
| 6.86 | 0.76 | 0.2536 | Irreversible |
| 8 | 0.3501 | 0.6359 | Irreversible |
| 10.3 | 0.5471 | 0.5268 | Irreversible |
| The polarographic reduction of (3) | |||
| 1.30 (I reduction) | 0.7278 | 1.699 | Irreversible |
| 1.30 (II reduction) | 0.8125 | 0.9805 | Irreversible |
| 5.75 | 0.479 | 0.2301 | Irreversible |
| 6.86 | 0.3625 | 0.2934 | Irreversible |
| 10.3 | 0.3121 | 0.7105 | Irreversible |
| The polarographic reduction of (4) | |||
| 1.30 (I reduction) | 0.712 | 2.021 | Irreversible |
| 1.30 (II reduction) | 0.7925 | 2.3362 | Irreversible |
| 5.75 | 0.3082 | 0.2547 | Irreversible |
| 8.61 | 0.4643 | 0.2084 | Irreversible |
| 10.3 | 0.4643 | 0.939 | Irreversible |
Table 3: Summary of polarographic reductions of (1), (2), (3) and (4)
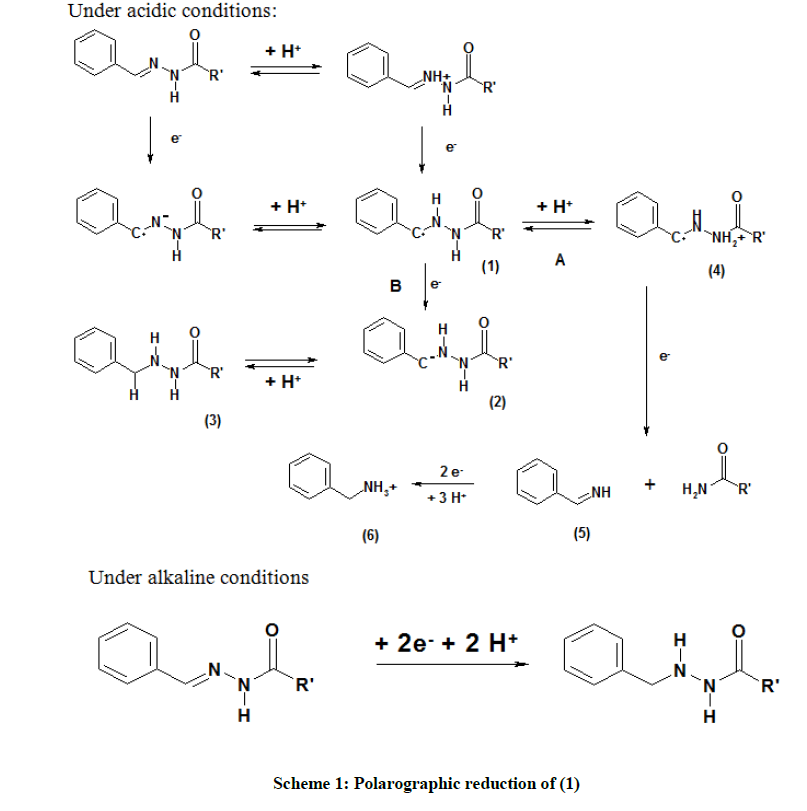
From this data, is seen that the system is reduced in two irreversible steps under highly acidic conditions and in a single irreversible step in alkaline media. Based on the observations, a plausible mechanism has been proposed as shown in Scheme 1. Under acidic conditions, the compound is reduced in two waves.
Mechanism
The proposed mechanism is elucidated in Scheme 1. After the uptake of a proton and an electron, the radical (1) thus formed may accept either a proton (route A) or an electron (route B). In route A, the protonated radical (4) accepts an electron, which results in the cleavage of the nitrogen-nitrogen bond with an amide as the leaving group, which may be further hydrolyzed to an acid. The imine (5) thus formed is protonated and reduces further. In route B, the protonation in alkaline solution is relatively slow [37] and the radical may be reduced to the carbanion (2), followed by its protonation. Under alkaline conditions, it proposed that saturation of C=N bond takes place [38-41].
Here, and through the rest of this communication, R’ is the coumarin moiety. It has been shown previously that R’ is reduced at potentials ~-1.2 V to -1.6 V, which lies outside the voltage range 0.00 V to -1.00 V used for the present investigation. Therefore, the reduction of the same has not been considered for all the compounds discussed in this communication. The same procedure of analysis was applied to all the systems studied.
The polarographic behaviour of (4E)-N'-(4-hydroxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (2)
The nature of reduction of (2) in various buffered aqueous solutions is listed in Table 3. Compound (2) gets reduced in two irreversible steps under acidic conditions with relative ease and in a single irreversible step under alkaline conditions. The mechanism proposed Scheme 1 is similar to the one given for (1).
The polarographic Behaviour of (4E)-N'-(4-chlorobenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (3)
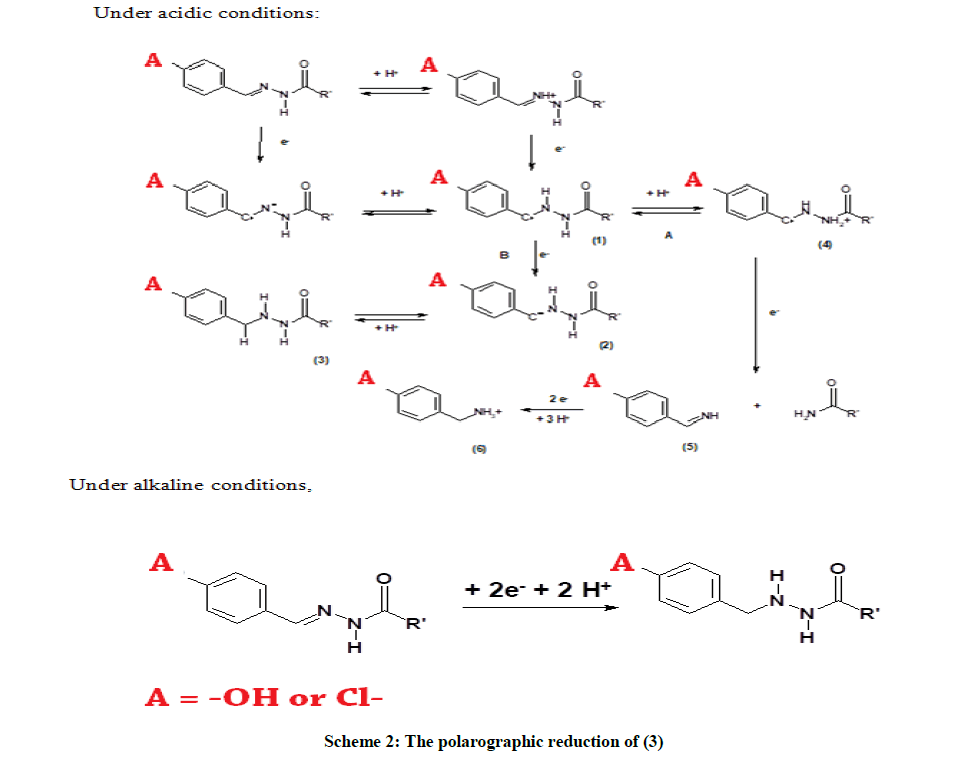
It is observed that (3) is reduced in two irreversible steps under acidic conditions and in a single irreversible step under alkaline conditions, which is similar to (1) and (2). Table 3 lists the nature of reduction of (3) under each chosen pH. It is proposed that (3), like (1) and (2), undergoes an irreversible reduction in two steps under acidic conditions and in a single irreversible step under alkaline conditions. The plausible mechanism given in Scheme 2 is similar to the ones proposed for earlier systems. The reduction of the chlorobenzene moiety cannot be ignored, as has been shown in earlier works [42-55]. This has not been studied in the present investigation as it is out of the scope of the study.
The polarographic Behaviour of (4E)-N'-(2, 5-dimethoxybenzylidene)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy) acetohydrazide (4)
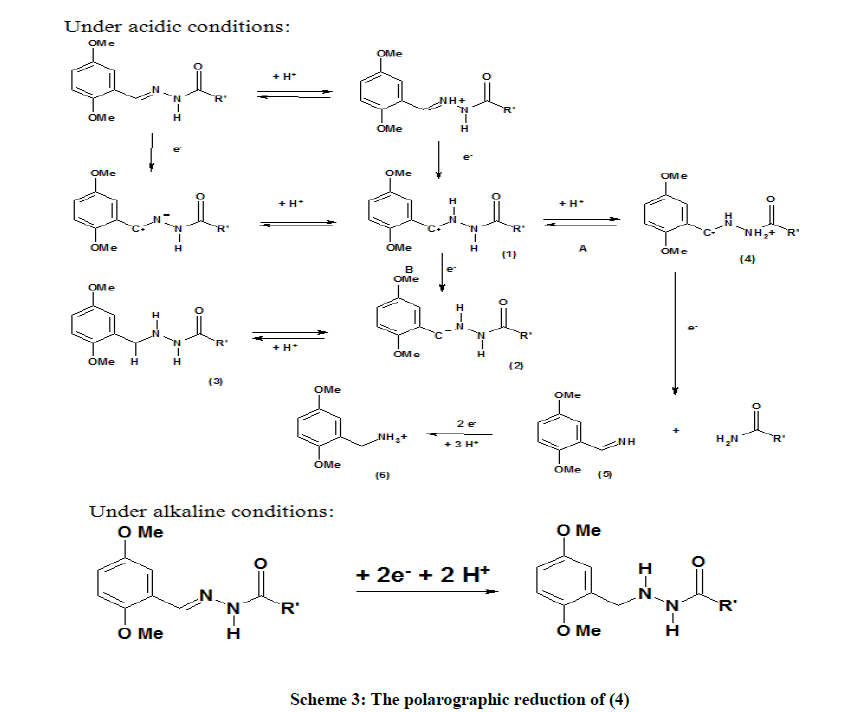
It was found that (4) was, just as (1), (2) and (3), reduced in two irreversible steps in highly acidic conditions and in a single irreversible step under alkaline conditions. Table 3 lists the nature of reduction of (4) under the chosen values of pH. From the above observations, it is proposed that the reduction of (4) follows a mechanistic pathway given in Scheme 3.
Scope for further studies
Further studies on the effect of more substituents are being performed on the same system. The authors are conscious of the limitations of the present investigation and proposes that further work has to be carried out using techniques like cyclic voltammetry, controlled potential coulometry and controlled potential stripping along with in-situ vibrational spectroscopic methods in order to enable one to be supremely confident of the nature of intermediates formed if any, and the number of electrons involved in each step, which would lead to a better understanding of the mechanism of the reduction process and thus give a good insight into the details of the reduction product.
Conclusion
In electrochemical reduction, at the dropping mercury electrode (DME), substituted (17Z)-2-(4-methyl-2-oxo-2H-chromen-7-yloxy)-N'-benzylideneacetohydrazides were studied using DC polarography and differential pulse polarography. The system in general showed two irreversible reduction waves in highly acidic conditions and one irreversible reduction wave in alkaline conditions, the exceptions being the 2,5-dimethoxy, N,N-dimethyl and 3-nitro-substituted systems. A general mechanism has been proposed for the reduction of (1), (2), (3) and (4) in acid and alkaline media, the former leading to the formation of an amine (ammonium salt) and the latter to the saturation of carbon-nitrogen double bond in the hydrazone group.
References
- A.A.A. Abu-Hussen, J. Coord. Chem., 2006, 59, 157-176.
- M.S. Karthikeyan, D.J. Prasad, B.P.S. Bhat, K.B. Shivarama, H.S. Kumari, Bioorg. Med. Chem., 2006, 14, 7482-7489.
- K. Singh, M.S. Barwa, P. Tyagi, Eur. J. Med. Chem., 2006, 41(1), 147-153.
- P. Panneerselvam, R.R. Nair, G. Vijayalakshmi, E.H. Subramanian, S.K. Sridhar, Eur. J. Med. Chem., 2005, 40, 225-229.
- S.K. Sridhar, M. Saravanan, A. Ramesh, Eur. J. Med. Chem., 2001, 36(7-8), 615-625.
- S.N. Pandeya, D. Sriram, G. Nath, E. De Clercq, Eur. J. Pharmacol., 1999, 9, 25-31.
- R. Mladenova, M. Ignatova, N. Manolova, T. Petrova, Eur. Polym. J., 2002, 38, 989-999.
- O.M. Walsh, M.J. Meegan, R.M. Prendergast, Eur. J. Med. Chem., 1996,31, 989-1000.
- K. Arora, K.P. Sharma, Synth. React. Inorg. Met. Org. Chem.,2003, 32, 913-922.
- P.A. Vitago, S. Tamburini, Coord. Chem. Rev., 2004, 248, 1717-2128.
- T. Katsuki, Coord. Chem. Rev., 1995, 140, 189-214.
- T.D. Thangadurai, M. Gowri, K. Natarajan, Synth. React. Inorg. Met. Org. Chem., 2002, 32, 329-343.
- R. Ramesh, Inorg. Chem. Comm., 2004, 7, 274-276.
- M.E. Speeter, W.C. Anthony, J. Am. Chem. Soc., 1954, 76(23), 6208-6210.
- A.K. Mitra, S.K. Misra, A. Patra, Synth. Commin., 1980, 10, 915-919.
- L.A. Singer, N.P. Kong, J. Am. Chem. Soc., 1966, 88(22), 5213-5219.
- Y. Sawadaet, Pest. Manag. Sci., 2003, 59, 49-57.
- A.M.R. Bernardino, A.O. Gomes, K.S. Charret, A.C.C. Freitas, V.F. Amaral, Eur. J. Med. Chem., 2006,41(1), 80-87.
- Küçükgüzel, Ş.G. Rollas, S. Synthesis, Farmaco., 2002, 57, 583-587.
- J. Patole, U. Sandbhor. S. Padhye, D.N.Deobagkar, C.E. Anson, A. Powell, Bioorg. Med. Chem. Lett., 2003, 13, 51-55.
- Z.H. Chohan, H. Pervez, K.M. Khan, C.T. Supuran, J. Enzyme. Inhib. Med. Chem., 2005, 20(1), 81-89.
- B. Chai, S. Cao, H. Liu, G. Song, X. Qian, Heterocycl. Commun., 2002, 8, 601-606.
- A. Al-Rahman A.H. Farghaly, J. Chin. Chem. Soc., 2001, 51(1), 147-156.
- Z.M. Liu, G. Yang, X. Qin, J. Chem. Technol. Biotechnol., 2001, 76(11), 1154-1158.
- A.K. Mansour, M.M. Eid, N.S.A.M. Khalil, Molecules., 2003, 8(10), 744-755.
- I. Borza, Bioorg. Med. Chem. Lett., 2006, 16(17), 4638-4640.
- M.A. Vazquez, F. Muñoz, J. Donoso, F. García Blanco, Intl. J. of Chem. Kin., 1990, 22(9),905-914.
- M. Nath, N. Chaudhary, Synth. React. Inorg. Met-Org. Chem., 1998, 28(1), 121-133.
- S.K. Freeman, Anal. Chem., 1953, 25(11), 1750-1751.
- S. Ibrahim, El-Hallag, G.B. El-Hefnawy, Y.I. Moharram, E.M. Ghoneim, Can. J. Chem.,78(9), 2000, 1170-1177.
- M. Thirumavalavan, P. Akilan, M. Kandaswamy, Electrochemical and kinetic response of heterobinuclear Ni(II)Zn(II) complexes derived from unsymmetrical lateral macrobicyclictricompartmental ligands, InorganicaChimicaActa, vol.359, no.8, 2006 p.2555-2560
- F. Faridbod, Md. R. Ganjali, R. Dinarvand, P. Norouzi, S. Riahi, Sens., 2008, 8(3), 1645-1703.
- I.F. Makarevich, Chem. of Nat.Comp.,1972, 8(2), 184-190.
- D. Ilkovic, Collect. Czech. Commun., 1934, 6, 498-513.
- J. Heyrovsky, D. Ilkovic, Collect. Czech. Chem. Commun., 1935, 7, 198-214.
- M.M.H. Kern, J. Am. Chem. Soc., 1954, 76, 4234-4236.
- H. Lund, Electrochemica. Acta.,1983, 28(3), 395-396.
- H. Lund, Acta Chem. Scand., 1959, 13, 249-267.
- M. Masuiand, H. Ohmori, Chem. Pharm. Bull. (Tokyo),1964, 12, 877-879.
- H. Lund, Tedrahedron Lett., 1968, 9(33), 3651-3654.
- Y. Asahi, Topics in Organic Polarography, (Edi.) P. Zuman, Four electron anodic wave, Chem. Pharm. Bull. (Tokyo), 1964,11, 930-939.
- C.P. Andrieux, J.M. Savaent, K.B. Su, J. Phys. Chem., 1986,90(16), 3815-3823.
- C.P. Andrieux, C. Blocman, J. Am. Chem. Soc.,101, 1979, 3431.
- T. Matsue, S. Kitahara, T. Osa, Denki Kagaku,50, 1982, 732.
- C. Saboureau, M. Troupel, S. Sibille, E.d'Incan, J. Périchon, J. Chem. Soc. Chem. Commun.,1989, 895-896.
- N.S. Murcia, D.G. Peters, J. Electroanal. Chem.,1992, 326(1-2), 69-79.
- E.K. Miller, Z. Vajtner, J. Org. Chem.,1985, 50, 9, 1394-1399.
- Z. Chami, M. Gareil, J. Pinson, J-M Saveant, A. Thiebault, Tedrahedron Lett., 1988,29, 6, 639-642.
- Z. Chami, M. Gareil, J. Pinson, J-M Saveant, A. Thiebault, J. Org. Chem.,1991,56, 2, 586-595.
- S. Donnelly, J. Grimshaw, J.T. Grimshaw, J. Chem. Soc. Perkin. Trans.,1993, 1, 1557-1562.
- S. Donnelly, J. Grimshaw, J.T. Grimshaw, Electrochim. Acta.,1996, 41, 4, 489-492.
- E. Lojou, M. Devaud, M. Heintz, M. Troupel, J. Perichon, Electrochim. Acta.,1993, 38, 613-617.
- R. Barhdadi, J. Gal, M. Heintz and M. Troupel, J. Chem. Soc. Chem. Commun.,1992, 50, 50-51.
- R. Barhdadi, J. Gal, M. Heintz, M. Troupel, J. Périchon, Tedrahedron., 1993,49, 5091-5098.
- M. Bordeau, C. Biran, P. Pons, M. Pierre Leger-Lambert, J. Dunogues, J. Org. Chem., 1992,57, 4705-4711.