Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
Effect of Silk Strength by Dietary Supplementation of Silk Worm with 1,3,4-Thiadiazoles, In Silico and In Vitro Bombyx mori DNA Binding Studies
Karteek Rao Amperayani and Uma Devi Parimi*
Department of Chemistry, GIS, GITAM University, Rushikonda, Visakhapatnam, Andhra Pradesh-530045, India
- *Corresponding Author:
- Uma Devi Parimi
Department of Chemistry
GIS, GITAM University
Rushikonda, Visakhapatnam, Andhra Pradesh-530045, India
Abstract
In silico and in vitro DNA binding studies were carried out on silk worm DNA to determine the efficiency of 1,3,4-thiadiazoles (Th1-9) on Bombyx mori larvae during their 5th instar larval stage to increase the tensile strength, quality and quantity of biopolymer silk. These compounds were synthesised using ultrasonication method and supplied in ultra-dose as food supplement along with mulberry leaves to Bombyx mori and recorded the growth and other parameters of silkworm, cocoon parameters and mechanical testing of silk filament. Tensile Strength, elongation and quantity of the silk was higher in larvae fed with synthesised compound in comparison with control larvae. This increase in strength and quality was due to the strong binding interactions of thiadiazoles moiety with silk worm DNA which was confirmed by higher binding energy values showed in In silco studies and DNA binding assay results.
Keywords
1,3,4-thiadiazoles, Bombyx mori, DNA binding assay, Tensile strength
Introduction
Silk biopolymer is a strongest natural fibre with low density, high resistance, good insulator, shimmers, shines and good affinity to dye. Silk is one of the most comfortable fabrics in the world. The silk fibre is chiefly composed of 80% of fibroin and 20% of sericin. It is produced by reeling cocoon formed form silkworm by feeding mulberry leaves as diet. Sericulture is one of the oldest industries in the world and India is the second largest producer of silk in world market. Market value of the Silk biopolymer depends on its quantity, quality and strength produced by the farmer. The silkworm, Bombyx mori, is a monophagous insect which depends only on mulberry leaves for its nutrition and produces silk. The quantity, quality and strength of silk depend on the growth and size of larvae which in turn greatly influenced by the nutritional quality of mulberry leaves. The quality of silk depends on the strength of silk biopolymer which means the extent to which a fibre can be stretched without breaking and it is measured in terms of minimum weight required to break the fibre. To enhance the production of silk fiber and economic sustainability to a farmer, there is a need in enriching the nutritional value of mulberry leaves by supplementing artificial diet along with the mulberry leaves. Many research articles have been published on nutritional enrichment of mulberry leaves with amino acid, alanine and glutamine [1,2], other supplementary nutrients like thiamine or vitamin B1 on the growth of silkworm for silk production [3], Effect of vitamin C deprivation on larval growth and cocoon production has been studied [4], Mulberry leaves enriched with nickel chloride, potassium iodide [5] and their combinations has increased the cocoon weight even at low concentrations.
1,3,4-Thiadiazole is a heterocyclic compound which contains a five member aromatic ring having two nitrogen and one sulfur atom. These are versatile molecule with potential pharmacological activity [6]. This interesting group of compounds possesses diverse biological activities such as antitumour, antioxidant [7], antidiabetic activity [8], antihypertensive agents [9], antitubercular [10], anticonvulsant [11], anti-inflammatory [12], anticancer [13] and antimicrobial [14]. The molecules were shown to have improved efficacy and minimum toxicity. The literature survey reviles that thiadiazoles has growth promoting capability in animals [15,16]. This encouraged us to synthesise amino substituted thadiazoles. The compounds with amine group play an important role in DNA binding [17,18], affect the DNA synthesis by increasing the rate of the DNA replication fork; repair synthesis of DNA was observed [19]. These compounds bind to DNA, affect the DNA synthesis by increasing the movement rate of the DNA replication fork [20]. Thus we have conducted the binding activity assay of the amino substituted thiadiazoles with DNA of silkworm using UV spectrophotometric studies.
As a extension to our study on the effect of biological active heterocyclic compounds [21] on silkworm, the amine derivatives of 1,3,4- thiadiazoles were synthesised by probe sonicator assisted green synthesis. The influence of target compounds (Th1-9) in ultra-low concentrations was studied on the growth and economic parameters of silk. DNA binding studies were also performed. There was no report on increase in tensile strength of silk by food supplement of substituted thiadiazoles to Bombyx mori.
Experimental Section
All the reagents used are of AR grade. The Melting points were determined in open capillary tube. 1H-NMR spectra were run on Brucker spectrometer (300 MHz) using Tetramethylsilane (DMSO) as solvent and Tetramethylsilane (TMS) as internal standard. Mass spectra were recorded on Schimadzu, LC-MS-2010A spectrometer. IR spectra were recorded on Perkin Elmer FTIR spectrophotometer using KBr pellets and UV spectra were recorded on Perkin Elmer Spectrophotometer using double distilled water as blank. The progress of the reaction was monitored by thin layer chromatography on Merck TLC silica gel plates. The solvent system used is Hexane: Ethyl acetate (6:4). The spots are visualized under UV chamber. B. mori (CB Csr2 × Csr4 type) larvae were collected from Sericulture department, Visakhapatnam, Andhra Pradesh. Molecular graphics laboratory (MGL) tools and Software required for docking, AutoDock4.2 was downloaded from www.scripps.edu, Discovery studio visualizer 2.5.5 was downloaded from www.accelerys.com.
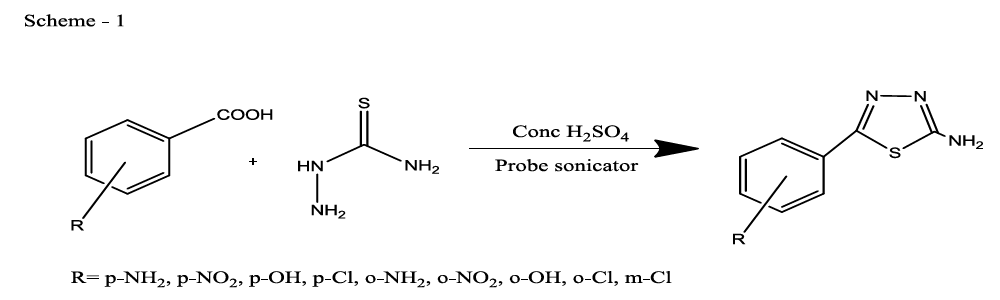
Synthesis of 5-(4-substitutedphenyl)-1,3,4-thiadiazol-2-amine (compounds Th1-9)
Thiadiazoles were synthesised by using both conventional method [22] and green chemistry method. A mixture of thiosemicarbazide (0.01 mol), 4-substituted benzoic acid (0.01 mol) and concentrated sulphuric acid (15 ml) were taken in a beaker and the reaction mixture was subjected to probe sonicator for 15 to 30 min, of reaction was monitored by TLC. After completion of the reaction mixture was poured on to crushed ice, and recrystallized from ethanol to give 5-(4-substituted phenyl)-1,3,4-thiadiazol-2-amine (92% yield) (Scheme 1).
Spectral data
5-(4-aminophenyl)-1,3,4-thiadiazol-2-amine (Th1): The amine was obtained in 92% yield as a brown colored solid. MP: 184-186°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ: 6.58(d, J=4.0 hz, 2H, Ar H), 7.76(d, J=3.3 hz, 2H, Ar H), 6.99 (s, 2H, C-NH2) and 6.27 (s, 2H, Ar-NH2). The 13C-NMR (100 MHz, DMSO) 145.6, 123.5, 115.1, 129.6, 161.6 and 174.1. Mass spectra-m/z: 192.05 (100.0%), 193.05 (9.5%), 194.04 (4.5%), 193.04 (1.5%). The FTIR shows bands at 3314-3336 (νNH2), 1542 (νC=N), 1520 (νC=C) and at 648 (νC-S).
5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (Th2): The amine was obtained in 80% as pale brown compound; mp- 218-220°C. The 1H-NMR (DMSO) aromatic CH protons appeared at δ=8.32 (2H, d, J=3.3 hz, Ar H), 8.05 (2H, d, J=2.5 hz, Ar H) and at 6.99 (2H, s, NH2). The 13C-NMR (100 MHz, DMSO) 147.9, 139.6, 128.8, 124.4, 161.6 and 174.1. Mass spectra-m/z: 222.02 (100.0%), 223.02 (10.9%), 224.02 (4.7%). The IR shows bands at 3339-325 7 (νNH2), 1552 (νC=N), 1535 (νC=C) and at 643 (νC-S).
4-(5-amino-1,3,4-thiadiazol-2-yl)phenol (Th3): The amine was obtained in 90% yield as pale yellow crystals; mp: 297°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ=6.86 (2H, d, J=4.5 hz, Ar H), 7.62 (2H, d, J=3.4 hz, Ar H), δ=6.99 (2H, s, NH2) and 5.35 (1H, s, OH). The 13C-NMR (100 MHz, DMSO) at 126.1, 116.4, 128.9, 158.5, 161.5 and 174.1. Mass spectra- m/z is: 193.03 (100.0%), 194.03 (10.6%), 195.03 (4.7%). The IR shows bands at 3171-3296 (νNH), 2900 (νCH) and at 1670 (νC-S).
5-(4-chlorophenyl)-1,3,4-thadiazol-2-amine (Th4): The amine was obtained in 82% yield as colourless compound; mp: 178-182°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ=7.55(d, 2H, J=8.9 hz, Ar H), 8.02 (d, 2H, J=8.4 hz, Ar H), δ=6.99 (s, 2H, NH2). The 13C-NMR (100 MHz, DMSO) at 134.3, 131.6, 128.9, 129.3, 161.6 and 174.1. Mass spectra- m/z is: 211.00 (100.0%), 212.99 (36.5%), 212.00 (9.5%), 214.00 (3.2%), 214.99 (1.5%), 211.99 (1.1%). The IR shows bands at 3314-3336 (νNH2), 1563 (νC=N), 1519 (νC=C) and at 645 (νC-S).
5-(2-aminophenyl)-1,3,4-thiadiazol-2-amine (Th5): The amine was obtained in 87% yield as brown colored compound; mp 184-186°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ: 6.69 (d, 1H, J=8.4 hz, Ar H), 7.16 (t, 1H, J1=7.3 hz, J2=3.4 hz, Ar H), 7.54(d, 1H, J=7.7 hz, Ar H), 6.87(m, 1H, J=7.9 hz, Ar H), 6.99 (s, 2H, C-NH2) and 6.27 (s, 2H, Ar-NH2). The 13C-NMR (100 MHz, DMSO) at 145.2, 116.7, 129.5, 119.2, 128.3, 123.2, 161.6 and 174.1. Mass spectra- m/z: 192.05 (100.0%), 193.05 (9.5%), 194.04 (4.5%), 193.04 (1.5%). The FTIR shows bands at 3314-3336 (νNH2), 1542 (νC=N), 1520 (νC=C) and at 648 (νC-S).
5-(2-nitrophenyl)-1,3,4-thiadiazol-2-amine (Th6): The amine was obtained in 78% yield as pale yellow crystals; mp. 297°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ=8.00 (d, 1H, J=3.4 hz, Ar H), 7.67 (t, 1H, J1=5.1 hz, J2=2.7 hz, Ar H), 7.90 (t, 1H, J1=7.1 hz, J2=2.3 hz, Ar H), 8.05(d, 1H, J=3.4 hz, Ar H) and 6.99 (s, 2H, NH2). The 13C-NMR (100 MHz, DMSO) at 124.4, 129.6, 135.3, 128.4, 131.6, 146.9, 161.6 and 174.1. Mass spectra- m/z: 222.02 (100.0%), 223.02 (10.9%), 224.02 (4.7%). The IR shows bands at 3339-3257 (νNH2), 1552 (νC=N), 1535 (νC=C) and at 643 (νC-S).
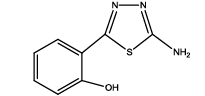
2-(5-amino-1,3,4-thiadiazol-2-yl)phenol (Th7): The amine was obtained in 85% yield as red colored compound; mp. 184-186°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ 7.01(d, 1H, J=3.4 hz, Ar H), 7.24 (t, 1H, J1=3.1 hz, J2=1.1 hz, Ar H), 7.07 (t, 1H, J1=4.1 hz, J2=1.9 hz, Ar H), 7.62 (d, 1H, J=3.4 hz, Ar H), δ=6.99 (s, 2H, NH) and 5.35 (s, 1H, OH). The 13C-NMR (100 MHz, DMSO) at 117.8, 130.1, 121.8, 128.9, 123.7, 155.2, 161.5 and 174.1. Mass spectra- m/z is: 193.03 (100.0%), 194.03 (10.6%), 195.03 (4.7%), The IR shows bands at 3171-3296 (νNH), 2900 (νCH) and at 1670 (νC-S).
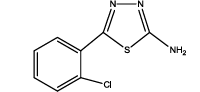
5-(2-chlorophenyl)-1,3,4-thiadiazol-2-amine (Th8): The amine was obtained in 80% yield as pale yellow crystals; mp. 297°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ=7.5 (d, 1H, J=3.2 hz, Ar H), 7.35 (t, 1H, J1=7.4 hz, J2=3.3 hz, Ar H) 7.39 (t, 1H, J1=8.1 hz, J2=3.1 hz, Ar H), 7.73 (d, 1H, J=3.4 hz, Ar H) δ 6.99 (s, 2H, NH). The 13C-NMR (100 MHz, DMSO) benzene carbons appeared at 129.3,130.1, 127.3, 128.9, 136.9, 132.2, 161.6 and 174.1. Mass spectra- m/z is: 211.00 (100.0%), 212.99 (36.5%), 212.00 (9.5%), 214.00 (3.2%), 214.99 (1.5%), 211.99 (1.1%). The IR shows bands at 3212-3342 (νNH), 2900 (νCH) and at 1640 (νC-S).
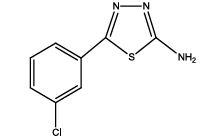
5-(3-chlorophenyl)-1,3,4-thiadiazol-2-amine (Th9): The amine was obtained in 75% yield as pale red colored compound; mp. 184-186°C. The 1H-NMR (300 MHz, DMSO) aromatic CH protons appeared at δ=7.45 (d, 1H, J=7.4 hz, Ar H), 7.45 (t, 1H, J1=8.4 hz, J2=2.4 hz, Ar H) 7.91 (d, 1H, J=5.4 hz, Ar H), 8.01 (s, 1H, Ar H) δ 6.99 (s, 2H, NH). The 13C-NMR (100 MHz, DMSO) at 128.8, 129.5, 127.3, 129.0, 134.9, 127.4, 161.6 and 174.1. Mass spectra-m/z is: 211.00 (100.0%), 212.99 (36.5%), 212.00 (9.5%), 214.00 (3.2%), 214.99 (1.5%), 211.99 (1.1%). The IR shows bands at 3212-3342 (νNH), 2900 (νCH) and at 1640 (νC-S).
Docking studies
The possible docking modes between the thiadiazoles and the silkworm DNA (5D23) was studied using AutoDock software [23]. The lead molecules were designed in ChemDraw software and used to analyze the binding affinity with silk DNA. Crystal structure of Silkworm DNA (5D23) [24] was downloaded from Protein Data Bank website as PDB format and converted to PDBQT format. We have taken Lamarckian genetic algorithm (LGA) for ligand conformations. An extended PDB format, termed as PDBQT file was used for coordinate ligand and macromolecule which includes atomic partial charges, polar bonds and hydrogen bonds. An AutoDock tool was used for creating PDBQT files from traditional PDB files [25]. The docking method employed by us using the protocol given by AutoDock Tools software. AutoDock was run several times to get various docked conformations and used to analyze the predicted docking energy [26]. AutoDock Tools provide various parameters to analyze the results of docking simulations such as, binding energy, ligand efficiency, inhibition constant and intramolecular energy. For each ligand, ten best conformations were generated and scored using AutoDock 4.2 scoring functions [27].
Feeding experiment
Department of Sericulture Visakhapatnam has provided B. mori (CB Csr2 × Csr4 type) larvae for the experiment. These larvae were divided into different batches according to the concentrations taken each group consisting of 45 worms a control batch was also kept. These larvae were fed with mulberry leaves enriched with synthesized compound and control was fed with untreated mulberry leaves. Feeding was provided for four times a day from day one of the 5th instar larva stage. Each compound was separately dissolved in minimum amount of DMSO and diluted with distilled water to obtain 1 mM concentration. From this 0.1 mM, 0.5 mM and 0.01 mM concentrations were achieved by appropriate dilution using distilled water. Mulberry leaves were treated with the above concentrations by swab method and were fed to the silkworm four times a day along with untreated mulberry leaves as control. The treatment was continued until the end of 5th instar larvae stage. Larval weight and mulberry leaves consumption were noted daily. At the end of the 5th instar larva the larvae was bisected and silk gland weight was noted (Figures S1-S10). Percentage gain in weight of silk worm, percentage gain in weight of silk gland, Cocoon weight, shell weight, shell ratio and pupal weight, silk weight, silk length and reliability were measured according to the formulae from standard procedures [28,29].
As the entire cocoon including the pupa is sold as part of the raw material, it is essential to quantify the ratio of the weight of the silk shell versus the weight of the cocoon. This is calculated in the formula [29]:
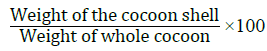
This value gives a satisfactory indication of the amount of raw silk that can be reeled from a given quantity of fresh cocoons.
Processing of cocoons
The following method was used for the extraction of Silk proteins; fibroin and sericin [30,31]. The cocoons were picked from both control and thiadiazole fed groups, the dry weight of shell was noted before processing. The cocoon shells were treated with 0.025% Na2CO3 at 100°C for 1 h followed by washing with hot and cold distilled water, finally with alcohol and then dried in hot air oven at 80 C. After sericin was dissolved the left-over silk fiber was treated with 0.1 N NaOH solutions for 24 h at 40°C with constant stirring. The weights after alkaline treatment were recorded as the fibroin weight. The percentage of sericin and fibroin was calculated as given below.
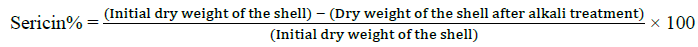
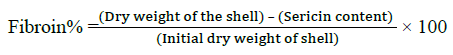
Degumming
Cocoons are boiled in hot water by adding NaOH: 0.05%, NaHCO3: 0.2%, Surfactants: 0.03% for 70 min to remove gum and make them soft.
Reeling
Each cocoon was reeled out using a laboratory wrap reel with the help of sericulture department, Nelimerla. The reelability was calculated with the formula given below. Care was taken during the reeling to ensure that the filament was not stretched and properties did not change during reeling.
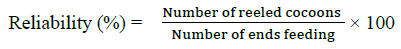
Mechanical testing of silk fibers
Specific gravity: The specific gravity of silk on average of sericin and fibroin measures from 1.32 to 1.40. Generally, the specific gravity of sericin is slightly higher than that of fibroin.
Apparent specific gravity of fabric = W/(1000 × t)
Where, W: Mass per square meter (g/m2) t: Thickness (mm).
Tenacity: The tensile strength when expressed as force per unit linear density is called tenacity. Tenacity indicates the quantity of weight a given fibre can support before breaking. Tenacity is determined by applying a force to a known unit of fiber and measuring the force to break the fiber. That is, tenacity=breaking load/mass per unit length. Tenacity express grams per denier (gd) Degummed silk has greater tenacity than raw silk.
Elongation: Elongation defines the length to which a fibre may be stretched before breaking. Raw silk has an elongation of 18 to 23 percent of its original length. Excess moisture increases the elongation of silk, but decreases its tenacity [29].
Denier: Denier is a unit of measurement that is used to determine the fiber thickness of individual threads:
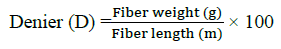
DNA binding assay studies
In silco binding energies of thiadiazoles towards silkworm DNA has motivated us to prove the binding efficiency of target compounds with silkworm DNA and thus performed DNA binding assay by UV spectrophotometric analysis using silkworm DNA and synthesised compounds.
UV-Spectrophotometric analysis
The DNA-binding experiment was performed using UV Spectrophotometry (Mohan Patel et al.,) [32]. Concentration of DNA (silk worm-DNA) was determined from the absorption intensity at 260 nm. Absorption titration experiments were made in a range of 220-400 nm by keeping concentration of silkworm-DNA as constant (100 μg/ml) and adding an increment (10, 30, 50, 70 and 90 μg/ml) concentration of synthesised compound (Th1-9). Compounds of 100 μg/ml concentration without addition of DNA were also taken. After the addition of silkworm-DNA to the target compounds, incubation for 10 min in room temperature has been provided, followed by absorbance. DNA mediated hyperchromisim has been observed. The binding constant, Kb for the synthesized compounds has been determined.
Results and Discussion
In silco DNA binding studies
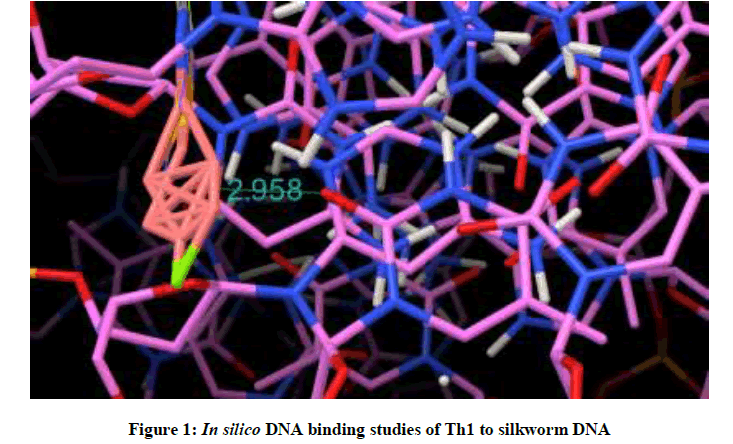
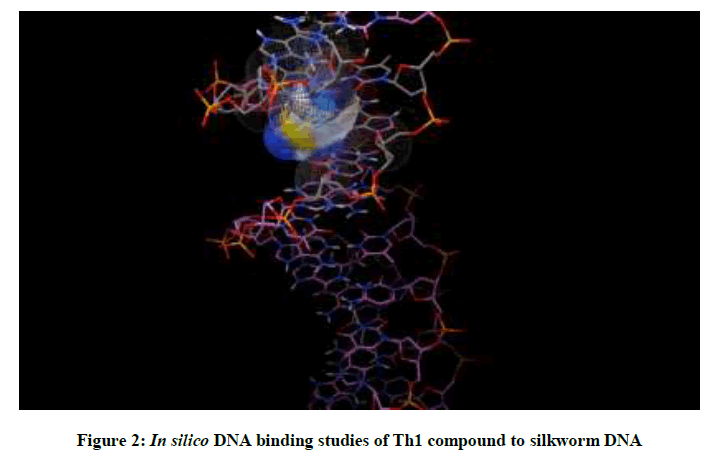
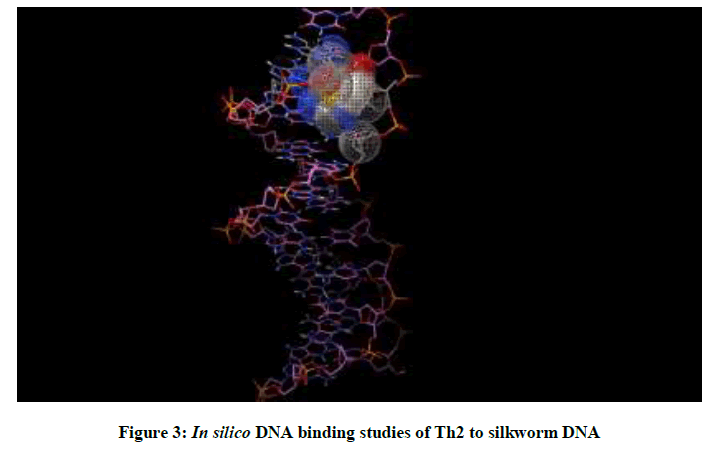
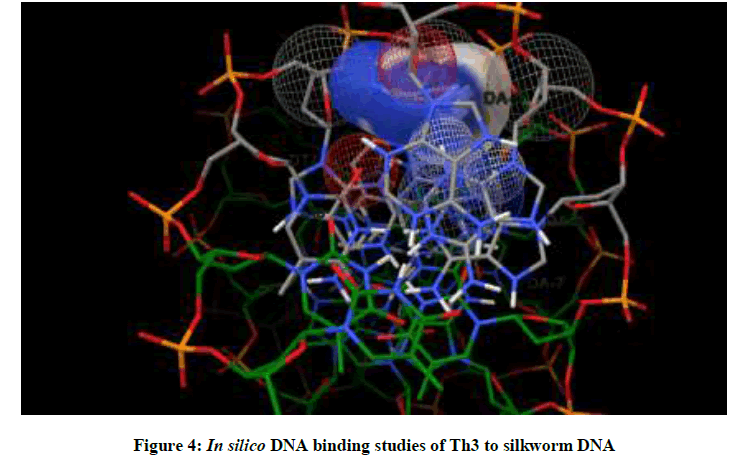
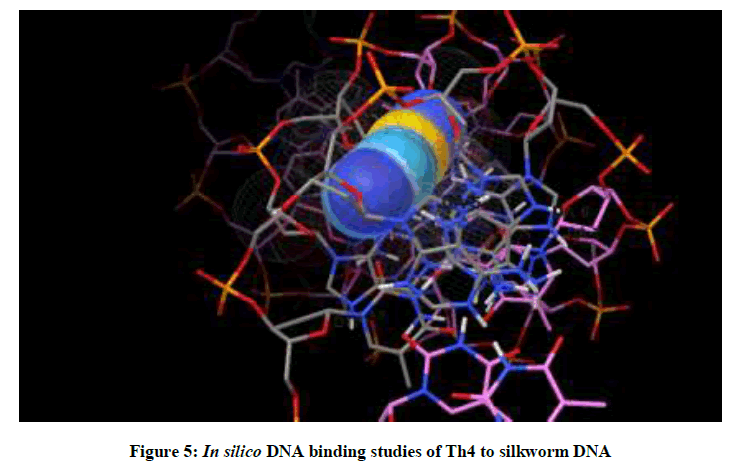
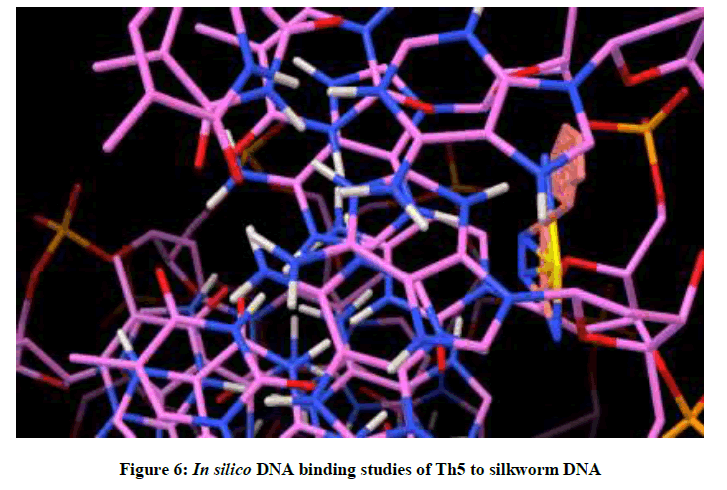
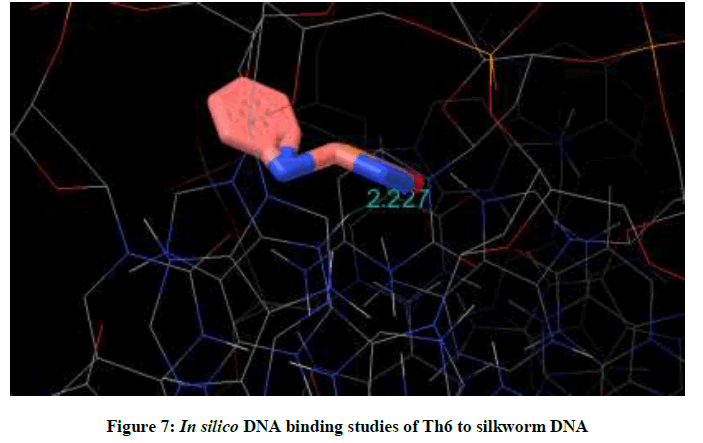
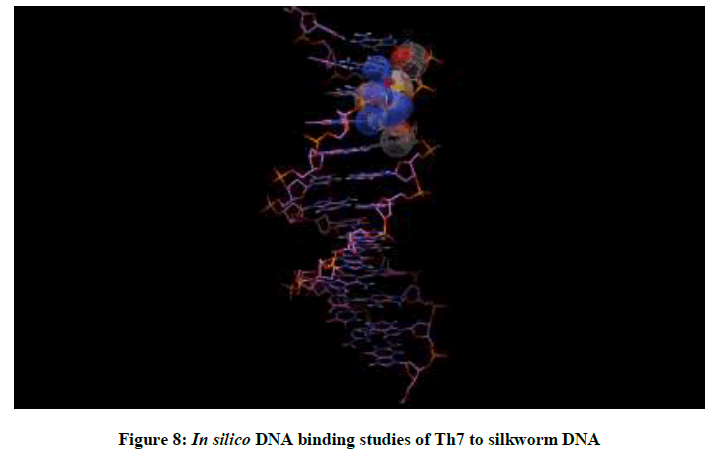
Molecular docking studies on silkworm DNA (5D23) with substituted thiadiazoles were performed by the use of AutoDock4.2 and visualized in Auto dock tools software. For each ligand ten best conformations were taken and various parameters such as, binding energy, ligand efficiency, inhibition constant and intermolecular energy were recorded in Table 1. Docked images of silkworm DNA (5D23) with the ligands substituted thiadiazoles were clearly shown (Figures 1-10). Binding energy of the individual compounds was recorded directly by the software. Substituted thiadiazoles showed binding energy ranges from-7.69 kcal/mol to -5.78 kcal/mol. Among all designed molecules Th1 showed highest binding energy of -5.78. In addition, two other parameters like inhibition constant (Ki) and intermolecular energy were also determined. These two parameters are directly proportional to binding constant. Thus the Inhibition constant ranges from 57.31 μM to 2.3 μM and Th1 shows the highest 57.31 μM as shown in Table 1. The intermolecular energy ranges from -9.19 kcal/mol to -7.87 kcal/mol with Th1 has highest -7.87 kcal/mol. We found a decrease in inhibition constant and intermolecular energy of all the selected compounds with a simultaneous decrease in the binding energy. On the basis of the above study predictions we have designed and synthesized substituted thiadiazoles for enriching the mulberry leaves for enhancing the production and quality of silk biopolymer.
| S. No. | Compound | Binding energy (Kcal/mol) |
Ligand efficiency | Inhibition constant (µM) | Intermol energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | TH1 | -5.79 | -0.45 | 57.31 | -7.87 |
| 2 | TH2 | -7.69 | -0.51 | 2.30 | -8.89 |
| 3 | TH3 | -6.15 | -0.47 | 31.17 | -8.24 |
| 4 | TH4 | -7.34 | -0.56 | 4.18 | -8.23 |
| 5 | TH5 | -6.33 | -0.49 | 22.83 | -8.42 |
| 6 | TH6 | -7.58 | -0.58 | 2.8 | -8.47 |
| 7 | TH7 | -6.5 | -0.43 | 17.06 | -9.19 |
| 8 | TH8 | -6.74 | -0.52 | 11.39 | -7.94 |
| 9 | TH9 | -6.91 | -0.53 | 8.56 | -8.11 |
Table 1: Molecular docking studies on silkworm DNA (5D23) and ligand substituted thiadiazoles
Chemistry
Design of substituted thiadiazoles using In silco studies with highest binding energy have been synthesised using conventional and sonication methods. Conventional method requires reflux of the thiosemicarbazide with different substituted benzoic acids in sulphuric acid at 160°C for 6- 7 h [21]. The above reactants were subjected to sonication for 15-30 min under room temperature using probe sonicator gives desired product. All products obtained were characterized by spectroscopic methods such as 1H-NMR, 13C-NMR, and mass spectrometry. The green synthetic method has substantial effect on the yield of the entire products. In conventional method the yields obtained were very less (60% to 70%) and the time taken for completion of reaction was almost 6-7 h (Table 2) at high temperatures (160°C). However when the synthesis was done using probe sonicator under green synthetic method the time of the reaction was drastically reduced to 15-30 min and the yield of the product was enhanced to 92% (Table 2) and the synthesis was done under room temperature.
| Compound | Substituted benzoic acid | Product | Time | Yield (%) | ||
|---|---|---|---|---|---|---|
| Conventional (h) | Green (min) |
Conventional | Green | |||
| Th1 | 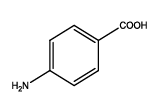 |
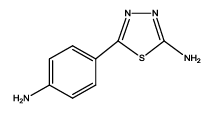 |
6 | 15-20 | 70 | 92 |
| Th2 | 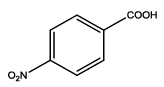 |
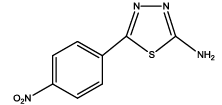 |
7 | 20-30 | 65 | 80 |
| Th3 | 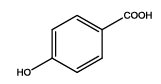 |
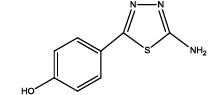 |
6 | 15-20 | 68 | 90 |
| Th4 | 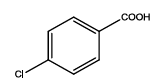 |
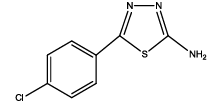 |
6 | 20-25 | 68 | 82 |
| Th5 | 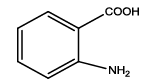 |
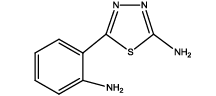 |
6 | 15-20 | 65 | 87 |
| Th6 | 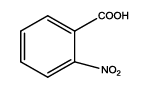 |
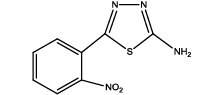 |
7 | 20-30 | 60 | 78 |
| Th7 | 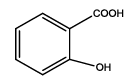 |
 |
6 | 15-20 | 65 | 85 |
| Th8 | 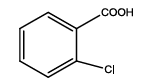 |
 |
7 | 20-25 | 62 | 80 |
| Th9 | 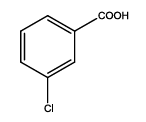 |
 |
7 | 20-30 | 60 | 75 |
Table 2: Reaction time and yields of synthesised thiadiazoles using conventional and green synthesis
Growth and economic parameters
The change in the growth of larvae, cocoon and the characteristics of silk biopolymer were studied by feeding mulberry leaves enriched with synthesised compound three times a day during the 5th instar larvae. The initial weights of larvae and gain in weight after consuming mulberry leaves were recorded daily. There was an increase in growth as well as weight of enriched larvae in comparison with control larvae. After completion of 5th instar stage some of the larvae were dissected, silk glands were separated and weighed. The remaining larvae were left for the formation of cocoon for further studies.
Cocoons are sold in the marketplace based on weight as this index signals the approximate quantity of raw silk that can be reeled. Thus post cocoon parameters are very important for the economic feasibility of silk production. Weight of cocoon, weight of pupa, weight of shell, length and weight of silk filament, shell %, reelability, fibronin% and sericin% were calculated and tabulated (Table 3).
| S. No. | compound Concentrations in mM | Avg. wt of cocoon (g) | Avg. wt of pupa (g) | Avg. wt of empty shell (g) | Avg. wt of silk (g) | Raw silk % | Avg. silk filament length (m) | shell % | reliability % | Fibronin % | Sericin % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | control | 0.96 ± 0.04 | 0.77 ± 0.06 | 0.2 ± 0.04 | 0.107 ± 0.005 | 65 | 600 ± 30 | 19.79 | 40 | 65.4 ± 1.2 | 30.12 ± 1.2 |
| 2 | Th1 | ||||||||||
| 0.01 | 1.2 ± 0.04 | 1.12 ± 0.06 | 0.25 ± 0.04 | 0.233 ± 0.008 | 73 | 730 ± 70 | 25.64 | 100 | 68.1 ± 1.2 | 31.16 ± 1.9 | |
| 0.05 | 1.35 ± 0.04 | 0.96 ± 0.07 | 0.2 ± 0.04 | 0.157 ± 0.010 | 70 | 720 ± 60 | 23.66 | 75 | 67.4 ± 1.6 | 29.17 ± 1.6 | |
| 0.1 | 1.5 ± 0.02 | 1.15 ± 0.04 | 0.35 ± 0.02 | 0.257 ± 0.012 | 77 | 760 ± 20 | 29.64 | 100 | 69.6 ± 1.1 | 30.19 ± 1.3 | |
| 3 | Th2 | ||||||||||
| 0.01 | 0.92 ± 0.09 | 0.75 ± 0.06 | 0.1 ± 0.06 | 0.106 ± 0.004 | 60 | 610 ± 40 | 15.27 | 40 | 63.5 ± 1.4 | 31.14 ± 1.5 | |
| 0.05 | 0.98 ± 0.07 | 0.82 ± 0.07 | 0.1 ± 0.02 | 0.114 ± 0.010 | 62 | 620 ± 20 | 17.56 | 50 | 62.6 ± 1.9 | 33.18 ± 1.6 | |
| 0.1 | 1.05 ± 0.04 | 0.96 ± 0.06 | 0.1 ± 0.02 | 0.119 ± 0.008 | 63 | 620 ± 20 | 17.69 | 40 | 62.9 ± 1.1 | 32.16 ± 1.2 | |
| 4 | Th3 | ||||||||||
| 0.01 | 1.21 ± 0.07 | 0.94 ± 0.03 | 0.27 ± 0.02 | 0.179 ± 0.010 | 73 | 700 ± 60 | 24.1 | 100 | 67.2 ± 1.6 | 31.14 ± 1.5 | |
| 0.05 | 1.43 ± 0.02 | 1.13 ± 0.04 | 0.30 ± 0.04 | 0.225 ± 0.012 | 70 | 720 ± 40 | 20.97 | 50 | 66.4 ± 1.2 | 30.15 ± 1.6 | |
| 0.1 | 1.45 ± 0.04 | 1.11 ± 0.06 | 0.20 ± 0.04 | 0.253 ± 0.008 | 75 | 740 ± 70 | 25.9 | 100 | 66.9 ± 1.6 | 31.19 ± 1.5 | |
| 5 | Th4 | ||||||||||
| 0.01 | 1.11 ± 0.01 | 0.71 ± 0.01 | 0.14 ± 0.06 | 0.193 ± 0.010 | 65 | 620 ± 40 | 17.75 | 40 | 68.4 ± 1.2 | 29.16 ± 1.2 | |
| 0.05 | 1.21 ± 0.09 | 0.83 ± 0.04 | 0.21 ± 0.02 | 0.175 ± 0.008 | 67 | 650 ± 30 | 20.22 | 50 | 69.4 ± 1.2 | 31.10 ± 1.4 | |
| 0.1 | 1.3 ± 0.02 | 1.05 ± 0.04 | 0.23 ± 0.02 | 0.259 ± 0.012 | 70 | 680 ± 20 | 22.3 | 100 | 65.4 ± 1.2 | 28.15 ± 1.6 | |
| 6 | Th5 | ||||||||||
| 0.01 | 1.17 ± 0.01 | 0.87 ± 0.01 | 0.28 ± 0.06 | 0.193 ± 0.010 | 70 | 700 ± 40 | 22.75 | 100 | 65.4 ± 1.1 | 28.13 ± 1.5 | |
| 0.05 | 1.26 ± 0.09 | 0.98 ± 0.04 | 0.32 ± 0.02 | 0.175 ± 0.008 | 72 | 670 ± 30 | 22.22 | 50 | 63.9 ± 1.8 | 26.15 ± 1.2 | |
| 0.1 | 1.3 ± 0.02 | 1.13 ± 0.04 | 0.39 ± 0.02 | 0.259 ± 0.012 | 75 | 720 ± 20 | 24.3 | 100 | 63.4 ± 1.6 | 28.19 ± 1.6 | |
| 7 | Th6 | ||||||||||
| 0.01 | 0.97 ± 0.09 | 0.79 ± 0.06 | 0.1 ± 0.07 | 0.103 ± 0.004 | 60 | 600 ± 40 | 16.85 | 40 | 68.8 ± 1.1 | 29.13 ± 1.8 | |
| 0.05 | 1.03 ± 0.07 | 0.85 ± 0.07 | 0.2 ± 0.01 | 0.111 ± 0.010 | 68 | 630 ± 20 | 20.58 | 50 | 69.9 ± 1.5 | 30.19 ± 1.7 | |
| 0.1 | 1.09 ± 0.04 | 0.89 ± 0.06 | 0.2 ± 0.01 | 0.117 ± 0.008 | 68 | 650 ± 20 | 20.99 | 50 | 68.5 ± 1.6 | 29.12 ± 1.5 | |
| 8 | Th7 | ||||||||||
| 0.01 | 1.07 ± 0.20 | 0.89 ± 0.18 | 0.21 ± 0.04 | 0.152 ± 0.008 | 66 | 680 ± 40 | 19.09 | 40 | 66.4 ± 1.1 | 30.18 ± 1.5 | |
| 0.05 | 1.26 ± 0.04 | 1.02 ± 0.07 | 0.25 ± 0.04 | 0.165 ± 0.006 | 68 | 753 ± 70 | 20.63 | 100 | 67.7 ± 1.3 | 31.18 ± 1.6 | |
| 0.1 | 1.32 ± 0.12 | 1.06 ± 0.12 | 0.26 ± 0.01 | 0.171 ± 0.008 | 66 | 753 ± 20 | 19.4 | 50 | 66.5 ± 1.7 | 32.13 ± 1.2 | |
| 9 | Th8 | ||||||||||
| 0.01 | 1.12 ± 0.06 | 0.9 ± 0.02 | 0.22 ± 0.05 | 0.166 ± 0.006 | 66 | 660 ± 50 | 19.64 | 50 | 65.8 ± 1.2 | 29.11 ± 1.4 | |
| 0.05 | 1.33 ± 0.13 | 1.09 ± 0.11 | 0.24 ± 0.02 | 0.183 ± 0.012 | 70 | 750 ± 10 | 21.05 | 40 | 68.4 ± 1.9 | 30.19 ± 1.5 | |
| 0.1 | 1.16 ± 0.05 | 0.91 ± 0.05 | 0.26 ± 0.02 | 0.151 ± 0.010 | 69 | 670 ± 60 | 20.35 | 50 | 66.8 ± 1.3 | 31.18 ± 1.6 | |
| 10 | Th9 | ||||||||||
| 0.01 | 0.91 ± 0.09 | 0.79 ± 0.06 | 0.1 ± 0.03 | 0.110 ± 0.004 | 60 | 610 ± 40 | 15.33 | 40 | 66.8 ± 1.1 | 31.19 ± 1.5 | |
| 0.05 | 0.99 ± 0.07 | 0.71 ± 0.07 | 0.22 ± 0.01 | 0.115 ± 0.010 | 62 | 600 ± 20 | 17.16 | 40 | 67.9 ± 1.7 | 35.12 ± 1.3 | |
| 0.1 | 1.02 ± 0.04 | 0.72 ± 0.06 | 0.22 ± 0.01 | 0.124 ± 0.008 | 65 | 620 ± 20 | 19.89 | 50 | 66.2 ± 1.5 | 36.11 ± 1.2 | |
Table 3: Economic parameters of Bombyx mori
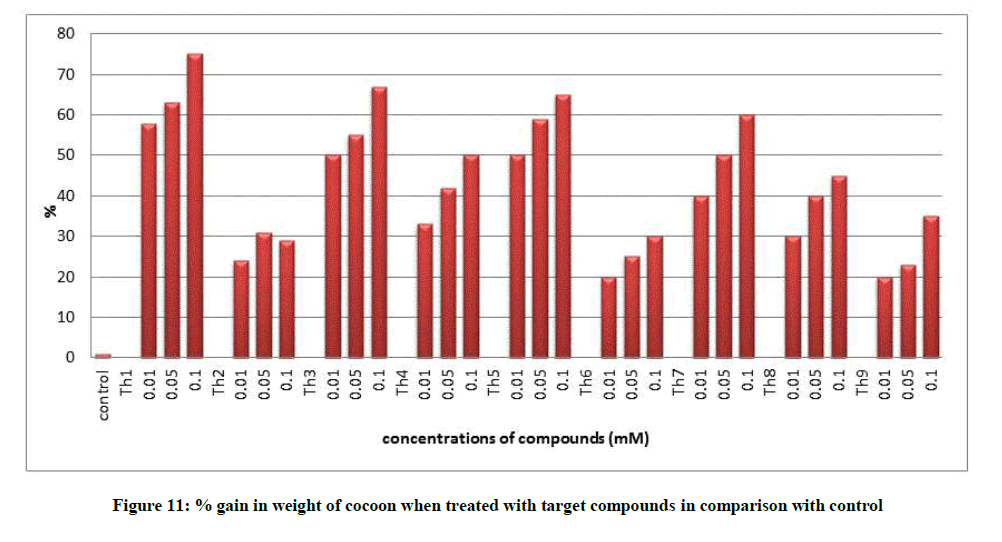
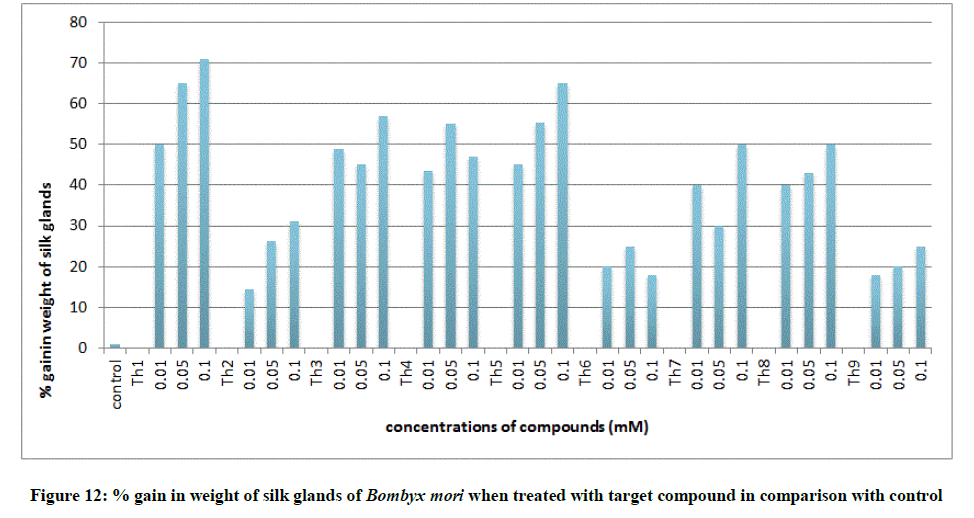
All the growth and economic parameters were improved and greater than the control maintained. The percentage increase in weight of cocoon and percentage increase in weight of silk glands compared with control after feeding during 5th instar larvae was plotted in Figures 11 and 12. Among all the synthesised compounds; 6 compounds with electron donating group (Th1, Th3, Th4, Th5, Th7 and Th8) has much more effect on the growth of silkworm compared with electron withdrawing group (Th2, Th6 and Th9). Compound with diamine group has highest cocoon weight (1.5 g), highest silk filament length (760 m) and 100% reliability than the others (Table 3). The mechanical properties like specific gravity, diner, tenacity and elongation percentage of silk biopolymer has been estimated (Table 4) which show that the thiadiazole enriched larvae produced stronger silk fiber with good diner tenacity and elongation percentage than the control larvae. The increase in strength of the silk fiber is due to the strong binding interactions of diamines to silkworm DNA.
| S. No. | Specific gravity | Denier | Tenacity (g/denier) | Elongation (%) |
|---|---|---|---|---|
| Control | 1.33 | 1.605 | 2.6 | 18 |
| Th1 | 1.45 | 1.911 | 3.5 | 20 |
Table 4: Specific gravity and tensile strength of silk fibre
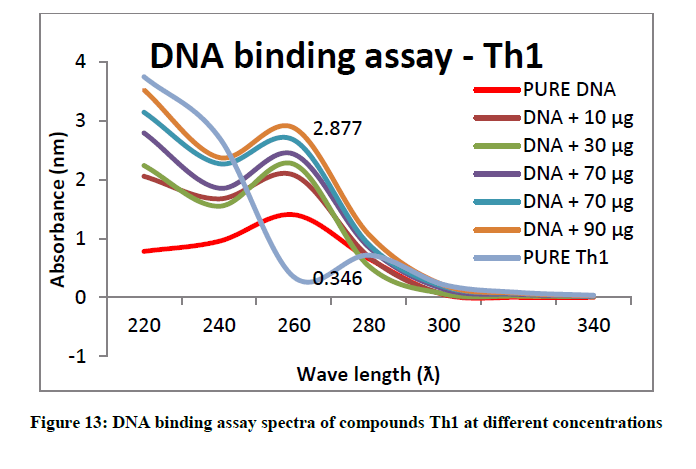
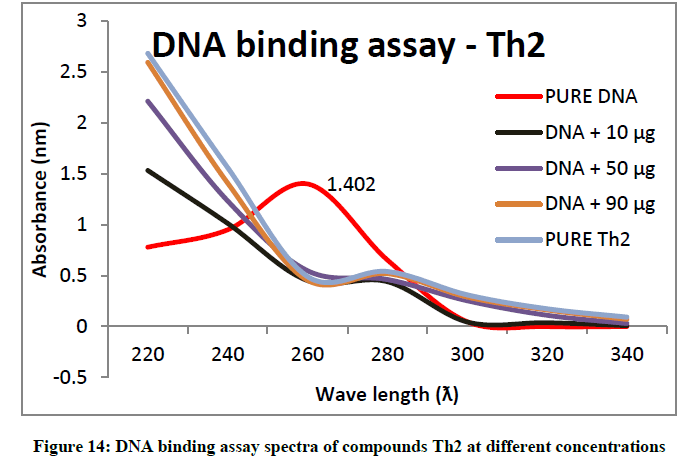
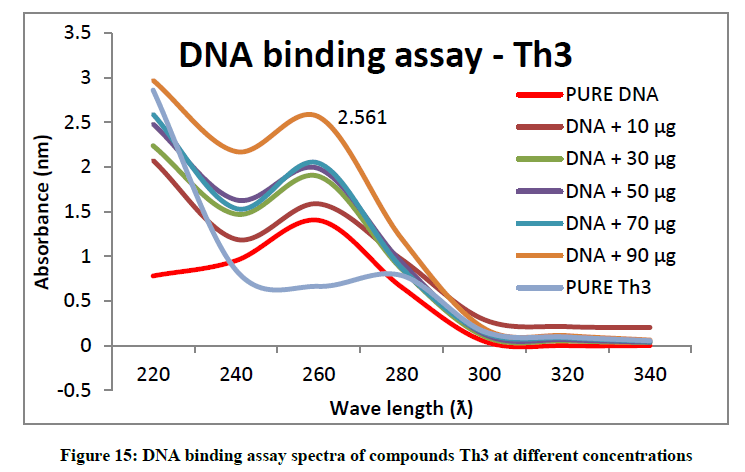
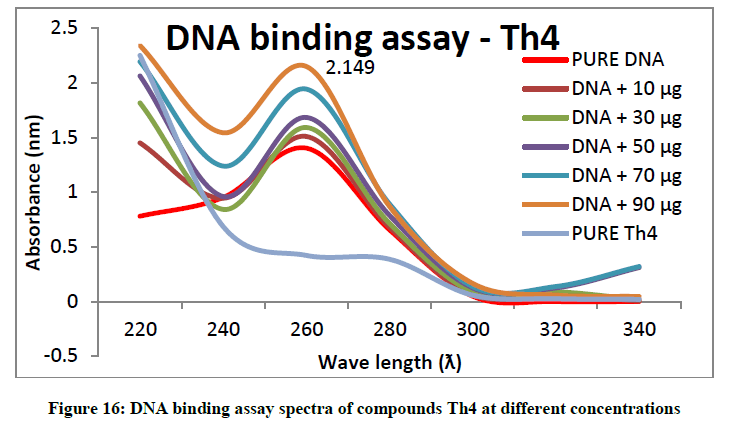
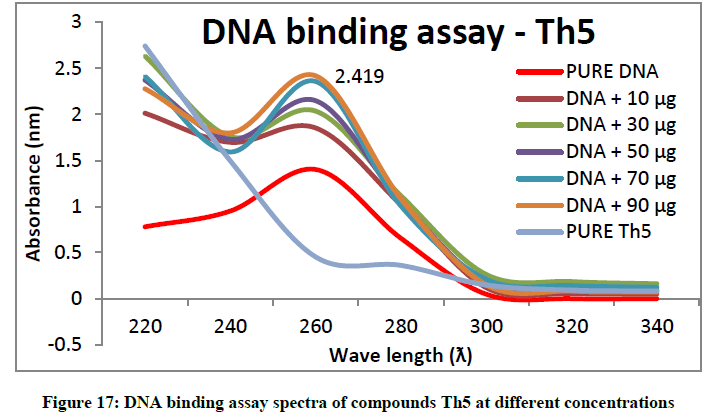
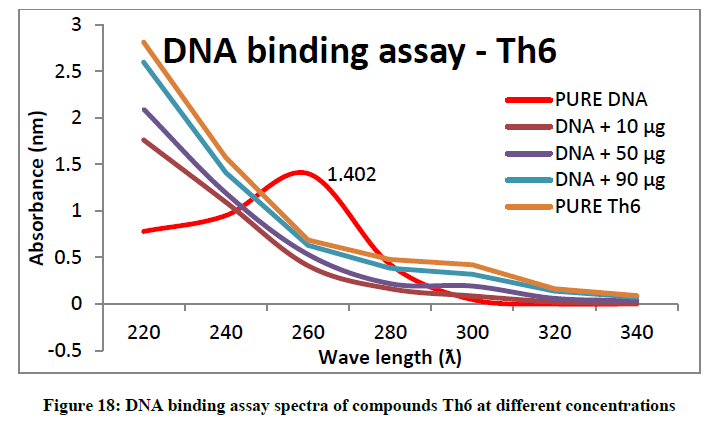
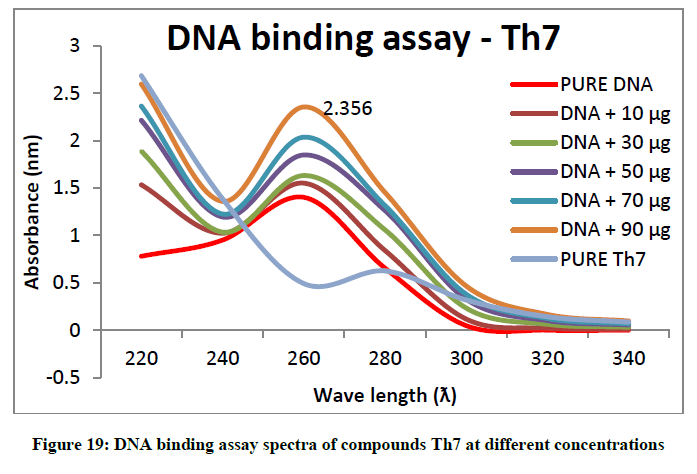
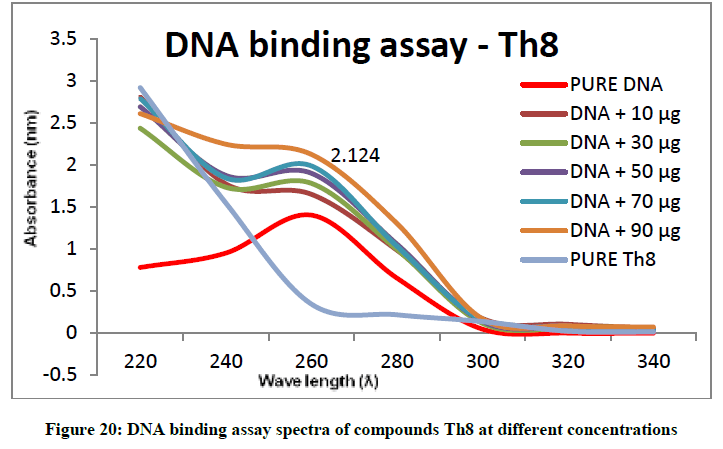
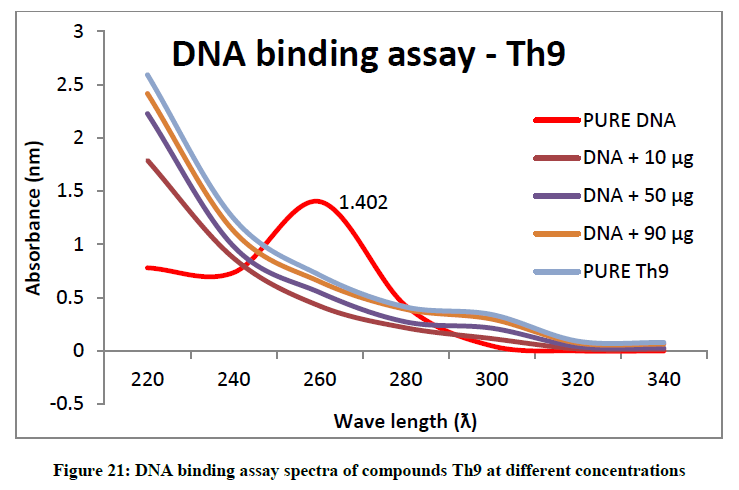
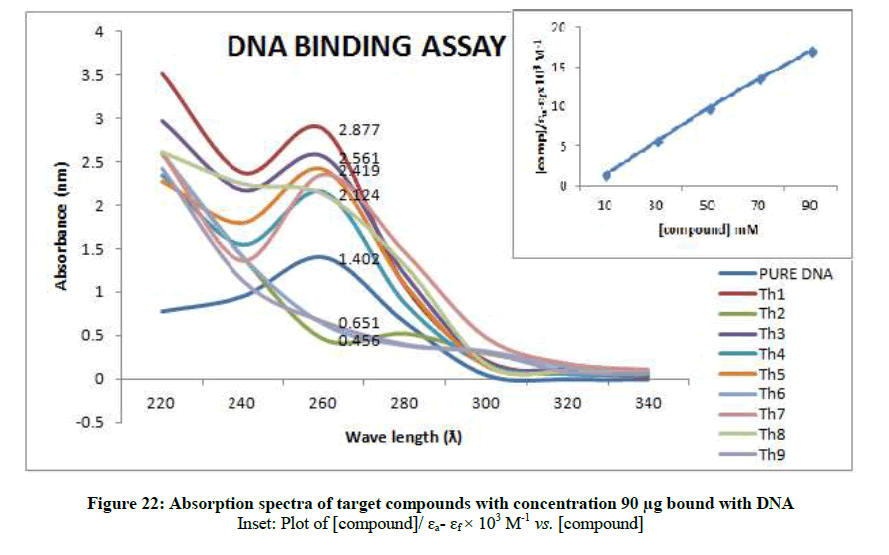
To support the experimental findings and In silco studies DNA binding assay studies using UV spectrophotometry has been performed on silkworm DNA. DNA binding assay results were plotted on graph by taking wavelength (λ) on X-axis and absorption (Ao) on Y-axis and found that six compounds with electron donating group (Th1, Th3, Th4, Th5, Th7, and Th8) show good binding efficiency to the silkworm-DNA (Figures 13-20). The other three compounds with electron withdrawing group (Th2, Th6 and Th9) do not show absorption maxima at 260 nm and thus we concluded that these compounds are not binding to silkworm-DNA (Figures 14, 18, and Figure 21). Compounds (Th1-9) with concentration of 90 μg and DNA at 260 nm was plotted and found to have a hyperchromic shift (Figure 22). Binding constant for target compounds was taken from the graph (Figure 22) and tabulated (Table 5). The results are in correlation with the experimental findings that the compounds (Th1, Th3, Th4, Th5, Th7, and Th8) which are binding to the DNA showed better growth in terms of cocoon weight, pupa weight, silk gland weight, silk production and reliability whereas the other three compounds (Th2, Th6 and Th9) which are not binding to the DNA are inferior in terms of growth and economic parameters. The binding constant for Th1 is highest with 1.7 × 103 M-1 which shows that the Thiadiazoles with diamine binds to silkworm DNA more effectively and increases the strength of silk fiber. All the growth parameters are in correlation with the DNA binding assay. We conclude that DNA binding plays an important role in the growth and economic parameters of Bombyx mori; however a molecular mechanism is yet to be studied.
| S. No. | Compounds | Binding constant (Kb) |
|---|---|---|
| 1 | Th1 | 1.7 × 103 M-1 |
| 2 | Th3 | 1.1 × 103 M-1 |
| 3 | Th4 | 0.09 × x 103 M-1 |
| 4 | Th5 | 0.9 × 103 M-1 |
| 5 | Th7 | 0.4 × 103 M-1 |
| 6 | Th8 | 0.05 × 103 M-1 |
Table 5: Binding constants (Kb) of synthesised compounds
Conclusion
A series of 9 amine derivatives of 1,3,4-thiadiazoles were designed by In silco studies and synthesised using probe sonicator assisted green synthesis. The synthesised compounds (Th1-9) in ultralow concentrations were given as diet supplement for silkworms and studied the growth and economic parameters of B. mori. The mechanical testing to find out the strength of silk biopolymer is performed and observed that the silk produced by thiadiazoles enriched worms is much stronger than the control worms. In order to confirm the results in silico and in vitro DNA binding assay was performed and proved that the synthesised compounds which are having strong binding affinity to silkworm DNA showed highest growth rate and strong silk filament. This was the first study on the growth and economic parameters of Bombyx mori by thiadiazoles synthesised using green synthesis.
Acknowledgements
The authors wish to thank GITAM University for providing the necessary lab facilities. We also sincerely thank department of Sericulture, Visakhapatnam for providing us larvae and mulberry leaves. This project work was supported and funded by UGC-MRP project UGC project (No. 42-295/2013(SR) letter dated 25 -03 -2013 sanctioned to Dr. P. Uma Devi.
References
- R. Radjabi, Academic. J. Entomol., 2010, 3, 45-51.
- M.D. Khan, B.N. Saha, Sericolgia., 1995, 35, 657-663.
- R.B. Nirwana, B.B. Kaliwal, Sericolgia., 1998, 38, 639-646.
- L. Cappellozza, S. Cappellozza, A. Saviane, G. Sbrenna, Appl. Entomol. Zool., 2005, 40, 405-412.
- R. Islam, A.O. Ali, D.K. Paul, S. Sultana, N.K. Banu, R. Islam, J. Biol. Sci., 2004, 4, 170-172.
- A. Tanveer, Aivind, K. Singh, N. Jaiswal, D. Singh, Int. Res. J. Pharm., 2012, 3, 70-82.
- S. Wafaa, A. Hamama Moustafa, H. Gouda Marwa, H. Badr Hanafi, Zoorob. Med. Chem. Res., 2013, 22, 3556-3565.
- S.R. Pattan, B.S. Kittur, B.S Sastry, Indian J. Chem., 2011, 50B, 615-618.
- B. Amarish, Samel, R. Nandini, J. Chin. Chem. Soc., 2010, 57, 1327-1330.
- A. Martin, Indian J. Pharm. Biol. Res., 2013, 1, 12-15.
- S.K. Srivatsava, S. Srivatsava, S. D. Srivatsava, Indian J. Chem., 1999, 38B, 183-187.
- A. Almasirad, N. Vousooghi, S.A. Tabatabai, A. Kebriaeezadeh, A. Shafiee, Acta. Chim. Slov., 2007, 54, 317-324.
- A.A. Kadi, N.R. El-Brollosy, O.A. Al-Deeb, E.E. Habib, T.M. Ibrahim, A.A. El-Emam, Eur. J. Med. Chem., 2007, 42, 235-242.
- S. Aboraia, A. Rahman-abdel, M. Hamdy, M. Mahouz, Nadia, Bioorg. Med. Chem., 2006, 14, 1236-1246.
- G. Asato, G. Berkelhammer, U.S. Patent, US3830924 A, 1974.
- J. Dorothy U.S. Patent, US4543357 A, 1985.
- X. Ping Hui, Z. Lin-Mei, Indian J. Chem., 1999, 38B, 1066-1069.
- K.M. Konigsfeld, M. Lee, S.M. Urata, J.A. Aguilera, J.R. Milligan, Int. J. Radiant. Biol., 2012, 88, 230-238.
- K. Kleppe, A. Osland, V. Fosse, R. Male, I. Lossius, D. Helland, J.R. Lillehaug, A.J. Raae, R.K. Kleppe, I.F. Nes, Med. Biol.,1981, 59, 374-380.
- L.E. Geiger, D.R. Morris, Nature., 1978, 272, 730-732.
- A. Karteek Rao, M. Anita, P. Uma Devi, Int. J. ChemTech Res., 2016, 9, 332-341.
- R. Pattan Shashikant, S. kekare, D. Prajact, S.A. Nirmal, S. Musmade Deepak, K. Parjane Smita, V. Daithankar Aarti, J. Chem. Pharm. Res., 2009, 1, 191-198.
- A. Madeswaran, M. Umamaheswari, K. Asokkumar, T. Sivashanmugam, V. Subhadradevi, P. Jagannath, Asian J. Pharm. Life Sci., 2012, 2, 174-181.
- L.Y. Yu, W. Cheng, K. Zhou, W.F. Li, H.M. Yu, X. Gao, X. Shen, Q. Wu, Y. Chen, C.Z. Zhou, Nucleic Acids Res., 2016, 44, 3936-3945.
- P. Khodade, R. Prabhu, N. Chandra, J. Appl. Cryst., 2007, 40, 598-599.
- G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, J.A. Olson, J. Comput. Chem., 2009, 30, 2785-2791.
- H. Park, J. Lee, S. Lee, Proteins., 2006, 65, 549-554.
- H. Akai, Int. J. Wild Silkmoths Silk., 2000, 5, 255-259.
- FAO, Manual on sericulture, Silk reeling, Agricultural Services Bulletin., 1972, 3, 72/3.
- Y. Tashiro, T. Morimoto, S. Matsura, S. Nagata, J. Cell Biol., 1968, 38, 574-588.
- A. Sabina, A. Taseem, F. Mokhdomi Malik, A.R. Trag, A. Raies, Qadrie, Trends in Life Sciences., 2012, 1, 12-16.
- N. Mohan Patel, N. Hardik Joshi, R. Chintan Patel, J. Chem. Sci., 2014, 126, 739-749.