Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 6
Design and Synthesis of Flavonoid Derivatives as Anti-inflammatory Drug
Seema P Rathod1* and Awinash S Chavan22Department of Pharmaceutical Sciences, Raosaheb Patil Danve College of Pharmacy Badnapur, Dr. Babasaheb Ambedkar Technological University, Lonere, India
Seema P Rathod, Department of Pharmaceutical Sciences, Swami Ramanand Teerth Marathwada University, Maharashtra, India, Email: seemaprathod106@gmail.com
Received: 28-Oct-2023, Manuscript No. Dpc-23-120668; Editor assigned: 31-Oct-2023, Pre QC No. Dpc-23-120668 (PQ); Reviewed: 14-Nov-2023, QC No. Dpc-23-120668; Revised: 17-Nov-2023, Manuscript No. Dpc-23-120668 (R); Published: 15-Dec-2023, DOI: 10.4172/0975-413X.15.6.146-150
Abstract
A series of 2-(3’-aminesubstituted)-7-hydroxyl-4H-1-benzopyran-4-one amine derivatives has been synthesized on the basis of molecular docking results. The novel derivatives prepared by condensation of 2,4-Dihydroxyacetophenonee (1.40 ml) and 3-chlorobenzaldehyde (0.01 mole) through Claisen-Schmidt condensation reaction. Novel flavone derivatives were access for anti-inflammatory activity by using carrageenan induced rat paw edema method. Among the synthesized compounds (t, v, w, k) shows good anti-inflammatory activity comparable to reference drug (Celecoxib).
Keywords
Molecular docking; Synthetic flavone; Claisen Schmidt condensation reaction; Anti-inflammatory; Celecoxib
Introduction
Flavonoids based on the backbone of 2-phenyl-4H-chromen-4-one (2-phenyl-1-benzopyran-4-one). The molecular formula of flavone molecule is C15H10O2. It has three-ring skeletons, C6-C3-C6 and the rings are referred to as A, C and B rings, respectively. They are found in seeds, citrus fruits, olive oil, tea and red wine, vegetables, nuts, stems and flowers, honey and are commonly consumed with the human diet. 2-(3’-aminesubstituted)-7- hydroxyl-4H-1-benzopyran-4-one derivatives these are Non-Steroidal Anti Inflammatory drugs (NSAIDs) exhibit their effect by inhibiting COX enzymes and by blocking the synthesis of prostaglandins [1-3].
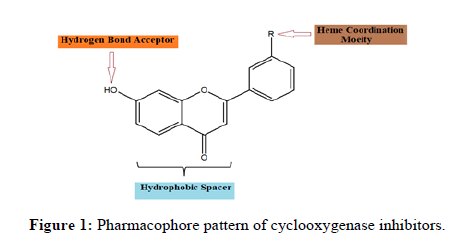
In a literature, revealed that, flavone shows good to moderate anti-inflammatory activity. It is also observed that the flavone moiety should possess following characteristic pharmacophore pattern which is essential for binding to cyclooxygenase enzyme and their by its inhibition. Flavone possess following pharmacophoric pattern (Figure 1).
•The ligand should possess suitably positioned hetero atom, which strongly interact with heme iron.
•The ligand should have a hydrophobic spacer moiety between heme coordinating group and hydrogen bond acceptor moiety.
•Cyclooxygenase inhibitors need a chemical group that is able to accept H-bond from serine 478 present in active site.
On the basis of literature and Pharmacophore pattern of non-steroidal aromatase inhibitors, the aim of present work is, to synthesize novel flavone derivatives, aliphatic/aromatic amines for anti-inflammatory screening i.e., as a fundamental hetero-aliphatic/aromatic system with modification.
Materials and Methods
Molecular docking of flavones studies on COX-2 enzyme
Molecular docking studies of all proposed flavones derivatives were carried using maestro 11.5 Schrodinger software. Glide score is the function, designed to calculate the free energy of binding for a protein ligand complex.
XPGlide score=Ecoul+Evdw+Ebind+Epenalty
Where, Ebind=Ehyd-enclosure+Eh-bond modif+Eh-bond_cc_modif +EPi interaction+Eh-bond pair
Epenalty=Edisolve+Eligand strain
Hydrogen bonds provides stability to the protein-ligand complex, that means more the number of hydrogen bonding with protein, more will be the fitting of ligand molecule in the binding pocket and hence stay more bound or docked with the receptor. These provide stability to the complex [4-6].
Results and Discussion
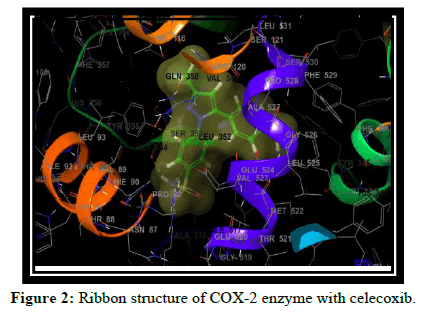
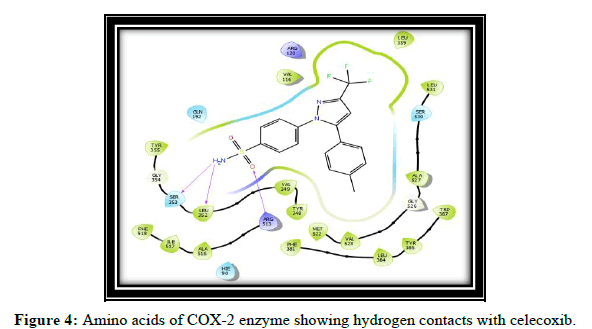
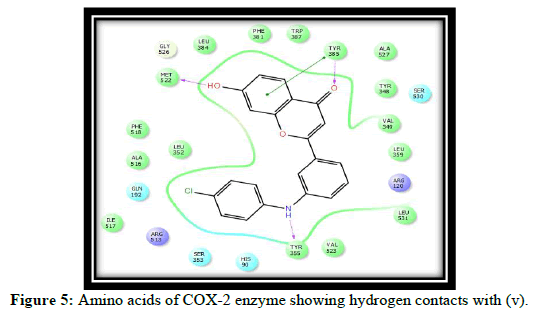
Total 8 molecules of flavones were designed and docked on COX-2 enzyme. Out of these, three molecules with good hydrogen bond interactions and glide score as compared to standard celecoxib are selected. Which includes (t,v,w,k) The Glide score (G-score) and the number of H bonds of all docked ligands and the standard SC-558, Celecoxib are shown in Table 1.
| Compound code | Docking score (Kcal/mole) | Glide score (Kcal/mole) | H-bonds |
|---|---|---|---|
| a | -8.253 | -8.253 | 0 |
| b | -9.077 | -9.089 | 2 |
| c | -8.06 | -8.06 | 0 |
| k | -8.331 | -8.443 | 1 |
| l | -8.574 | -8.594 | 2 |
| t | -10.916 | -10.928 | 3 |
| v | -11.086 | -11.099 | 3 |
| w | -10.525 | -10.538 | 3 |
| Celecoxib | -11.197 | -11.198 | 3 |
Table 1: Docking score, Glide score (G-score) and number of H-bonds for the flavones and celecoxib.
Compound t, v, w possess 3 hydrogen bonding and having glide score of -10.928, -11.099 and -10.538 respectively, compound k has single hydrogen bonding and quiet good dock score. a and c have no hydrogen bonding with COX-2 enzyme [7-10]. It is reported in the literature that good bonding with Tyr355, Arg120, Leu531, Ser353, Ser530 and Tyr 385 is required for good affinity and also for good fitting of the compounds when docked into the enzyme pocket. Therefore from the docking result compound t, v, w, k are considered for the synthesis which may shows good anti-inflammatory activity (Table 2 and Figures 2-5) [11-15].
| S. No. | Compound | Name of amino acids involved in interaction with ligands |
|---|---|---|
| 1 | Celecoxib | SER 353, LEU 352, ARG 513 |
| 2 | t | TYR 355, MET 522 |
| 3 | v | MET 522, TYR 385, TYR 355 |
| 4 | w | MET 522, TYR 385, PHE 518 |
Table 2: Amino acids of 1CX2 enzyme making H-bonding interactions, π-π and π-cation interactions with celecoxib and novel flavones.
From above Table 2 it is clear that flavones showed good hydrogen bonding, π-π stacking and π-cation bonding with COX-2 enzyme.
Synthetic work
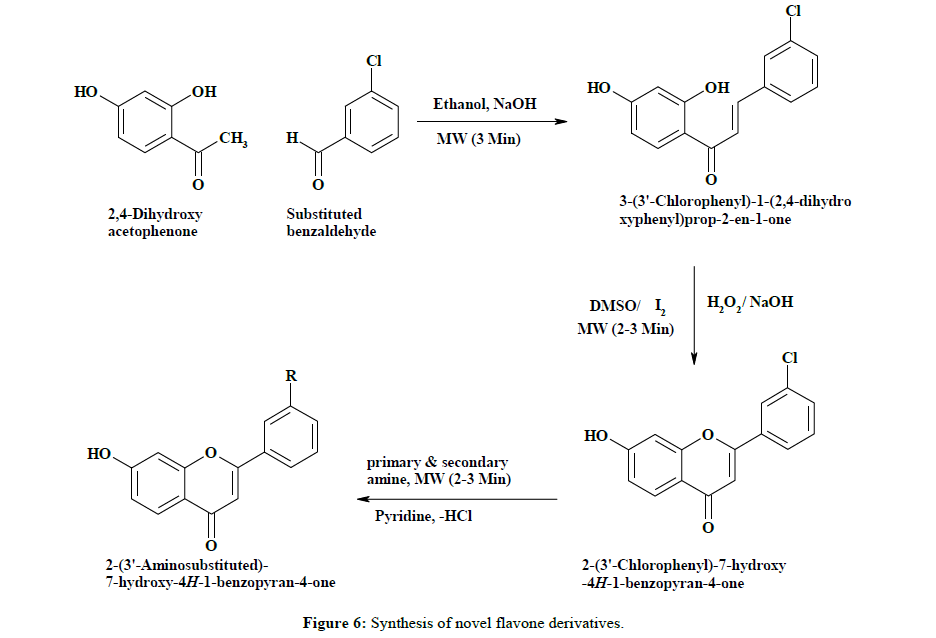
In the present work, synthesis of novel flavone derivatives was carried out by reacting 2, 4-dihydroxyacetophenone with 3-chlorobenzakdehyde in basic medium in microwave for 3 min at level 5 to gives 3-(3’-chlorophenyl)-1-(2,4-dihydroxyphenyl)-prop-2-en-1-one shown in step: I. Further it is, cyclized in microwave for 2-3 min at level 5 to in the presence of DMSO/I2 or H2O2/ NaOH to form 2-(3’-chlorophenyl)-7- hydroxyl-4H-1-benzopyran-4-one step: II. Finally synthesis of all proposed novel flavone derivatives was carried out by adding a aliphatic or aromatic amines to a 2-(3’-chlorophenyl)-7- hydroxyl-4H-1-benzopyran-4-one in pyridine, then mixture was irradiated in microwave for 2-3 min at level 5 to achieve target derivatives step: III. Crude product was then crystallized from methanol (Figure 6) (Tables 3-5) [15-20].
| Sr. No. | Compound | -R/Ar | S. No. | Compound | -R/Ar |
|---|---|---|---|---|---|
| 1 | A | Methylamino | 5 | l | Ethylmethylamino |
| 2 | B | Ethylamino | 6 | t | p-Methylaniline, |
| 3 | C | Propylamino | 7 | v | p-Chloroaniline |
| 4 | K | Diethylamino | 8 | w | p-Nitroaniline |
Table 3: Derivatives of novel flavonoids.
| Sr. No. | Code | Molecular formula | Mol. weight (g/mol) | Yield % | Melting point (°C) | Rf-value | U.V. λmax (nm) |
|---|---|---|---|---|---|---|---|
| 1 | a | C16H13NO3 | 267 | 63.12 | 178-180 | 0.6 | 241.6 |
| 2 | b | C17H14NO3 | 280 | 57 | 184-185 | 0.82 | 309.2 |
| 3 | c | C18H17NO3 | 295 | 57.86 | 187-190 | 0.84 | 297 |
| 4 | k | C19H19NO3 | 309 | 66.21 | 179-182 | 0.62 | 298 |
| 5 | l | C18H17NO3 | 295 | 59.09 | 181-183 | 0.47 | 319 |
| 6 | t | C22H18NO3 | 365 | 72.25 | 190-195 | 0.72 | 340 |
| 7 | v | C21H15NO3CI | 375 | 69.24 | 192-196 | 0.82 | 290 |
| 8 | w | C21H15N2O5 | 344 | 70.16 | 190-194 | 0.68 | 318 |
Table 4: Characterization data for 2-(3’-aminesubstituted)-7- hydroxyl-4H-1-benzopyran-4-one derivatives.
| Sr. No. | Groups | Mean of paw volume (mL) | ± SEM | % Inhibition of paw edema | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 0 h | 1 h | 2 h | 3 h | 0 h | 1 h | 2 h | 3 h | ||
| 1 | Control | 0.51 | 0.57 | 0.56 | 0.55 | 0.087 | 0.098 | 0.095 | 0.098 | -- | -- | -- | -- |
| 2 | A | 0.56 | 0.47 | 0.46 | 0.45 | 0.098 | 0.142 | 0.096 | 0.122 | -- | 17.55 | 17.86 | 18.19 |
| 3 | B | 0.56 | 0.47 | 0.46 | 0.45 | 0.098 | 0.142 | 0.096 | 0.122 | -- | 16.2 | 16.8 | 17.02 |
| 4 | C | 0.57 | 0.48 | 0.46 | 0.46 | 0.098 | 0.102 | 0.096 | 0.096 | -- | 16.79 | 16.04 | 17.37 |
| 5 | K | 0.57 | 0.43 | 0.38 | 0.32 | 0.08 | 0.128 | 0.124 | 0.107 | -- | 23.7 | 33.14 | 44.03 |
| 6 | L | 0.51 | 0.43 | 0.46 | 0.4 | 0.102 | 0.143 | 0.149 | 0.145 | -- | 24.57 | 25 | 27.28 |
| 7 | T | 0.57 | 0.43 | 0.38 | 0.3 | 0.098 | 0.128 | 0.128 | 0.109 | -- | 30.7 | 39.14 | 40.03 |
| 8 | V | 0.57 | 0.43 | 0.42 | 0.41 | 0.098 | 0.143 | 0.149 | 0.16 | -- | 21.46 | 24 | 24.46 |
| 9 | W | 0.51 | 0.36 | 0.3 | 0.2 | 0.102 | 0.092 | 0.088 | 0.052 | -- | 43.26 | 43.75 | 45.08 |
| 10 | Celecoxib | 0.54 | 0.38 | 0.38 | 0.27 | 0.096 | 0.104 | 0.104 | 0.087 | -- | 33..34 | 32.15 | 45.91 |
Table 5: Anti-inflammatory activity obtained by carrageenan induced rat paw edema method and comparative study of inhibition (%I) for anti-inflammatory activity by carrageenan induced rat paw edema method.
Conclusion
From present work it is prove that novel series of 2-(3’-aminesubstituted)-7- hydroxyl-4H-1-benzopyran-4-one derivatives has been synthesized, physical characteristics followed by the anti-inflammatory screening occurs. Derivatives (t, v, w, k) shows the good anti-inflammatory activity when compared against vehicle treated control and standard celecoxib.
Acknowledgment
Authors gratefully acknowledge to Dr. Raghu Rangaswamy, vice president Schrodingerinc for providing me one month free trial license to do my project work. Authors are also thankful to university department of pharmaceutical sciences, Rashtrasant Tukadoji Maharaj Nagpur university, Nagpur and Dr. Prafulla M. Sabale director of board of examination and evaluation Rashtrasant Tukadoji Maharaj Nagpur university, Nagpur for providing necessary facilities.
References
- Sourav DE, Babu NM, Babu ST, et al. Mintage J Pharma Med Sci. 2016; 5(3): p. 18-27.
- Al-Mulla A. Der Pharma Chem. 2017; 9(13): p. 141-147.
- Arora P, Arora V, Lamba HS, Wadhwa D. Int J Pharm Sci Res. 2012; 3(9): p. 2947.
- Gupta R. Int J Comput Appl. 2015; 975: p. 8887.
- Gupta M. Int J Phys Chem Math Sci. 2015; 4(1): p. 21-24.
- Pearce S. Drug Discov. 2017; 67(41): p. 77-79.
- Martins P, Jesus J, Santos S, et al. Molecules. 2015; 20(9): p. 16852-16891.
- Haider S. J Phytochemistry Biochem. 2017; 5(1): p. 1-4.
- Veeresham C. J Adv Pharm Technol Res. 2012; 3(4): p. 200-201.
[Crossref] [Google Scholar] [PubMed]
- Hughes JP, Rees S, Kalindjian SB, et al. Br J Pharmacol. 2011; 162(6): p. 1239-1249.
[Crossref] [Google Scholar] [PubMed]
- Kumar N, Hendriks BS, Janes KA, et al. Drug Discov Today. 2006; 11(17-18): 806-811.
[Crossref] [Google Scholar] [PubMed]
- Surabhi S, Singh BK. J Drug Deliv Ther. 2018; 8(5): p. 504-509.
- Kore PP. 1. Int J Med Chem. 2012; 34(7): 16-19.
- Van Drie JH. J Comput Aided Mol Des. 2007; 21(10-11): p. 591-601.
[Crossref] [Google Scholar] [PubMed]
- Sliwoski G, Kothiwale S, Meiler J, et al. Pharmacol Rev. 2014; 66(1): p. 334-395.
[Crossref] [Google Scholar] [PubMed]
- Vijayakrishnan R. J Postgrad Med. 2009; 55(4): p. 301.
[Crossref] [Google Scholar] [PubMed]
- Talele TT, Khedkar SA, Rigby AC. Curr Top Med Chem. 2010; 10(1): p. 127-141.
[Crossref] [Google Scholar] [PubMed]
- Gradman AH, Schmieder RE, Lins RL, et al. Circulation. 2005; 111(8): p. 1012-1018.
[Crossref] [Google Scholar] [PubMed]
- Singh J, Chuaqui CE, Boriack-Sjodin PA, et al. Bioorganic Med Chem Lett. 2003; 13(24): p. 4355-4359.
[Crossref] [Google Scholar] [PubMed]
- Ripphausen P, Nisius B, Peltason L, et al. J Med Chem. 2010; 53(24): p. 8461-8467.
[Crossref] [Google Scholar] [PubMed]