Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Decolouring of Synthetic Waste Water by Chemical Oxidation
Rathinakumar Vedachalam1* and Sugumaran Karuppiah22School of Chemical and Biotechnology, SASTRA University, Thanjavur-613401, Tamilnadu, India
Rathinakumar Vedachalam, School of Civil Engineering, SASTRA University, Thanjavur-613401, Tamilnadu, India,
Abstract
Discharge of waste water from the textile industry into the natural water bodies causes serious problem to the aquatic environment. This waste water contains dyes which are mainly made of chemicals in which majority of these chemicals categorized as inorganic. General methods used to treat this waste water can remove organic chemicals, also these methods consumes more time. Hence treating this waste water has become a great challenge to the chemists and environmental engineers. As the dyes which are used in the textile industry are inorganic in nature, the common practices used to treat this is waste water are no longer useful. Hence new methods are required to treat these inorganic chemicals and these methods must be must faster than the common practices. Hence chemical oxidation methods came into existence. The paper deals with chemical oxidation process for the color removal of waste water by using Fenton’s reagent. In this process Hydrogen Peroxide is used as oxidant. Fenton’s reagent uses ferrous ions to make OH- radicals. These OH-oxidizes the chemicals present in waste water and decolorizes it. So, in this paper focuses on preparation of Fenton reagent and optimum time requirement that can remove methylene blue (MB) color.
Keywords
Chemical oxidation, Color removal, Methylene blue, Waste water, Fenton
Introduction
Wastewater emerging from Textile industries includes a large distribution and addition of chemicals and dyes that make the real challenge for the concerned industries in environmental aspects [1,2]. Pollution in textile industry raised in dyeing and finishing processes. These processes demand a wide range of dyestuffs and chemicals which is having highly complied organic chemicals. It is causing very serious problems in their disposal as all of them are not contained in the final product. Apart from the color, some other pollutants found in textile wastewaters are HSS, COD, heat, acidity, and other soluble substances [3]. Despite many test, the procedure prescribed by the American Dye Manufacturers Institute (ADMI) is considered as best experimental system to analysis the color of the waste water [4]. Experimental studies focuses on the removal of color from textile industry and dyestuff manufacturing industry needs more attention with reference to environmental concern [5]. In the last few decades a rather fast evolution of the research activities attributed to the environmental protection has been witnessed as the consequence of the special attention paid to the environment by social, political and legislative international authorities leading in some cases to the delivery of very severe regulations. One of the major steps taken in this process is treating the waste effluent water from the industries before disposing it. The textile industry consumes about 20% of fresh water resources have been developing various process to treat their effluent waters. Some of the methods developed are incineration, chemical adsorption, and advanced oxidation processes. Studies have shown that the variation of initial azo dye concentration produces a variation in kinetics [6]. Over the years there have been many developments in these processes. With increase in chemicals in dye manufacturing, the conventional methods are no longer can be adopted. Hence advanced methods like chemical oxidation processes have been evolved. Exposure of strong oxidizing agent exposed to UV which generates hydroxyl free radicals is the key factor in the Advance Oxidation Process (AOP) [7]. When a water or wastewater containing H2O2 is irradiated with UV, hydroxyl radicals are formed. These hydroxyl radicals were identified as strong oxidants to oxidize organic compounds present in waste water [8]. The organic compounds degraded by radicals by, which lead to either an abstraction of hydrogen atoms or addition to double bonds [9]. Chemical oxidation aims at the mineralization of the contaminants to carbon dioxide, water and inorganic or, at least, at their transformation into harmless products. Apart from this many researchers contribute for the chemical oxidation process [10,11]. Obviously the methods based on chemical destruction, when properly developed, give complete solution to the problem of pollutant abatement differently from those in which only a phase separation is realized with the consequent problem of the final disposal.
Materials and Methods
Methylene blue
Methylene Blue dye (MB) is basic aniline dye, C16H18N3SCl that forms a deep blue solution when dissolved in water. It is utilized in coloring and dyeing industry. The removal of methylene blue from any wastewater is of very important due to its serious threats to the environment.
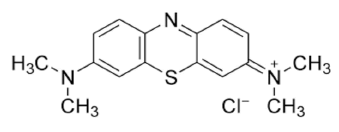
Molecular formula of Methylene blue
Hydrogen peroxide (H2O2)
Hydrogen peroxide is a colorless liquid with bitter taste. Naturally it was presented in mere amounts especially in gaseous form. It is very unstable, having greater affinity to oxygen and water with liberated of enormous heat. It is one of the powerful oxidizing elements when it aligned with organic matters. It is also plays a crucial role in minor concentration (3-9%) especially in hair bleach and clothes. Higher concentration of H2O2 finds its place in textile industry, rubber industry and organic chemical industry. Hydrogen peroxide is a strong oxidizing agent used in aqueous solution as a ripening agent, bleach, and topical anti-infective. It is relatively unstable and solutions deteriorate over time unless stabilized by the addition of acetanilide or similar organic materials. Ferrous sulphate (FeSO4.7H2O)
Ferrous Sulphate is a green colour crystalline compound generally obtained in nature. It plays a significant role in preparation of various chemical compounds and also it is act as a reducing reagent. It is also used a catalyst for hydrogen peroxide.
Preparation of solutions
Stock solution is also known as a base solution for the preparation of the various solutions as for preparing concentrations of 1ppm and smaller concentrations are more difficult. Hence a higher known concentration of the required solution is prepared and by diluting the stock solution we can get the required concentration of the solution Solid methylene blue of 25 mg is correctly weighed in the machine and is added to the 500 ml of distilled water. Hence obtaining 50 ppm stock solution. Similarly, various concentrations of stock solutions can be prepared.
The sample solutions are then undisturbed and lids are closed so that dust and other particles may not mix as this would change its properties. This is how the dye solutions are prepared.
Preparation of FeSO4 solution
It is similar to the preparation of the stock solution i.e., suitable amount of FeSO4 is added to water and dissolved in it. Here 1000ppm, 300 ppm solutions are prepared.
Preparation of Fenton reagent
To prepare Fenton’s reagent take FeSO4 solution, H2O2 solution and water in the ratio of 1:4:4. Addition of water is just to make the solution diluted which is little safer to use. Concentrated Fenton reagent can also be made for better results. Storage of Fenton reagent should be in a dark place as it can react in sunlight.
Experimental methodology
Preparation of stock solution
Stock solution is also known as a base solution for the preparation of the various solutions as for preparing concentrations of 1 ppm and smaller concentrations are more difficult.
Hence a higher known concentration of the required solution is prepared and by diluting the stock solution we can get the required concentration of the solution
Procedure
Solid methylene blue of 25 mg is correctly weighed in the machine and is added to the 500 ml of distilled water. Hence obtaining 50 ppm stock solution. Similarly, various concentrations of stock solutions can be prepared. Note: Stock is to be prepared in higher concentrations than the sample concentrations.
Sample solutions
1) 1 ppm: From the stock solution 2 ml is taken and it is diluted to 100 ml water.
2) 2 ppm: From the stock solution 4 ml is taken and it is diluted to 100 ml water.
3) 3 ppm: From the stock solution 6 ml is taken and it is diluted to 100 ml water.
4) 4 ppm: From the stock solution 8 ml is taken and it is diluted to 100 ml water.
5) 5 ppm: From the stock solution 10 ml is taken and it is diluted to 100 ml water.
The sample solutions are then undisturbed and lids are closed so that dust and other particles may not mix as this would change its properties. This is how the dye solutions are prepared (Figure 1).
Calculation of optimum dosage of Fenton reagent
In order to estimate the optimum dosage of Fenton reagent, 50 ml sample of MB samples are taken of various concentrations of 1-5 ppm. Now Fenton reagent is added to each sample in a calculated amount and changes in the sample water. Dosage of Fenton reagent is decided on the reaction that is taking place. Once the reaction starts, the pH and OD values are calculated for every 10 min duration. This procedure is conducted for each sample of MB. All the final pH and OD values are noted down.
Conclusion
From the investigations we came to the following conclusions are drawn:
1) With the application of Fenton reagent as chemical oxidant it is possible to remove the color even up to 100%.
2) The effect of pH plays a significant role in decolourization as the solution turn to be acidic after the addition of Fenton reagent the process found to be very simple.
3) Fenton reaction is a time dependent process and along with time the color is removed more but the rate of color removal will tend to decrease after certain point.
4) Overall cost evaluations so far available indicate that AOP systems are not more expensive than the well-established technologies for pollutant abatement.
5) Based on the results observed from this experiment, the time plays a crucial role in the colour removal capacity. Among the three difference choices, 20 min found to be optimum for all concentrations.
References
[1] A.L. Adel Kdasi, A. Idris, K. Saed, C. Teong Quan, Global. Nest. Int. J., 2004, 6(3), 222-230.
[2] N. Azbar, T. Yonar, K. Kestioglu, Chemosphere., 2004, 55, 35-43.
[3] E.L. Barnhart, Electric. Power. Res. Institute., 1993, Palo Alto, CA. 94304.
[4] A.Dae-Hee, C. Won-Seok, Y. Tai-Il, Process. Biochem., 1999, 34, 429-439.
[5] U. Pagga, D. Brown, Chemosphere., 1986, 15, 479-491.
[6] H. Shu, C. Huang, M. Chang, Chemosphere., 1994, 29, 2597-2607.
[7] N.H. Ince, D.T. Gonenc, Environ. Technol., 1997, 18, 179-185.
[8] Y. Yang, D.T. Wyatt, M. Bahorshky, Text. Chem. Color., 1998, 30, 27-35.
[9] D.W. Sundstrom, B.A. Weir, H.E. Klei, Environ. Progress., 1989, 8, 6-11.
[10] R. Andreozzi, V. Caprio, A. Insola, R. Marotta, Catal. Today., 1999, 53, 51-59.
[11] A. Kavitha, K. Palanivelu, Water Res., 2005, 39, 3062-3072.
[12] M.C. Venceslau, S. Tom, J.J. Simon, 1994, 15, 917-929.
[13] S. Gul, O. Ozcan-Yildirin, Chem. Eng. J., 2009, 155, 684-690.



